 Found 483 hits with Last Name = 'andrésson' and Initial = 't'
Found 483 hits with Last Name = 'andrésson' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254766
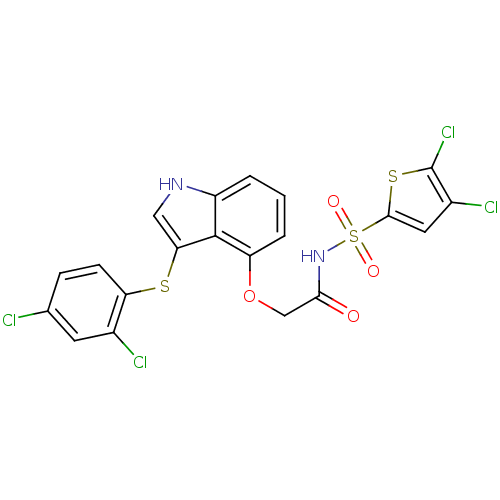
(2-(3-(2,4-dichlorophenylthio)-1H-indol-4-yloxy)-N-...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc(Cl)cc3Cl)c12 Show InChI InChI=1S/C20H12Cl4N2O4S3/c21-10-4-5-15(11(22)6-10)31-16-8-25-13-2-1-3-14(19(13)16)30-9-17(27)26-33(28,29)18-7-12(23)20(24)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254687
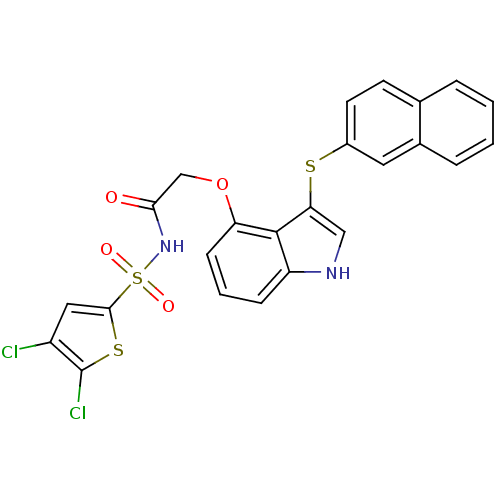
(CHEMBL464032 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C24H16Cl2N2O4S3/c25-17-11-22(34-24(17)26)35(30,31)28-21(29)13-32-19-7-3-6-18-23(19)20(12-27-18)33-16-9-8-14-4-1-2-5-15(14)10-16/h1-12,27H,13H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254841
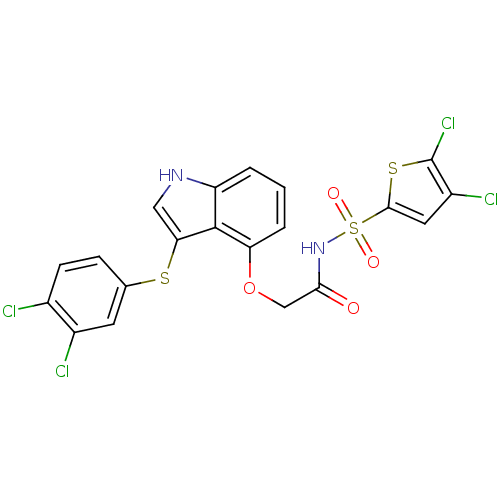
(2-(3-(3,4-dichlorophenylthio)-1H-indol-4-yloxy)-N-...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc(Cl)c(Cl)c3)c12 Show InChI InChI=1S/C20H12Cl4N2O4S3/c21-11-5-4-10(6-12(11)22)31-16-8-25-14-2-1-3-15(19(14)16)30-9-17(27)26-33(28,29)18-7-13(23)20(24)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor assessed as cAMP production by cell-based assay |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254883
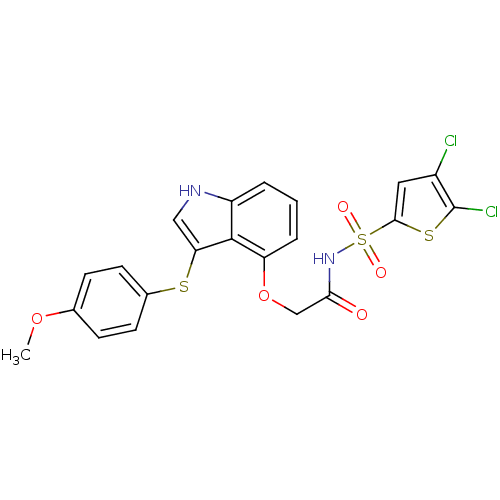
(CHEMBL479434 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES COc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)cc1 Show InChI InChI=1S/C21H16Cl2N2O5S3/c1-29-12-5-7-13(8-6-12)31-17-10-24-15-3-2-4-16(20(15)17)30-11-18(26)25-33(27,28)19-9-14(22)21(23)32-19/h2-10,24H,11H2,1H3,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254520
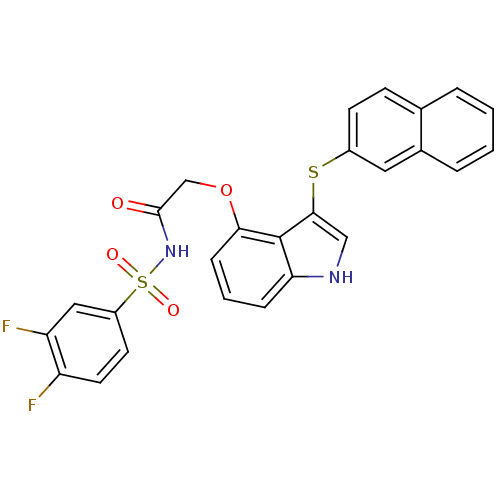
(CHEMBL466184 | N-(3,4-difluorophenylsulfonyl)-2-(3...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H18F2N2O4S2/c27-20-11-10-19(13-21(20)28)36(32,33)30-25(31)15-34-23-7-3-6-22-26(23)24(14-29-22)35-18-9-8-16-4-1-2-5-17(16)12-18/h1-14,29H,15H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254728
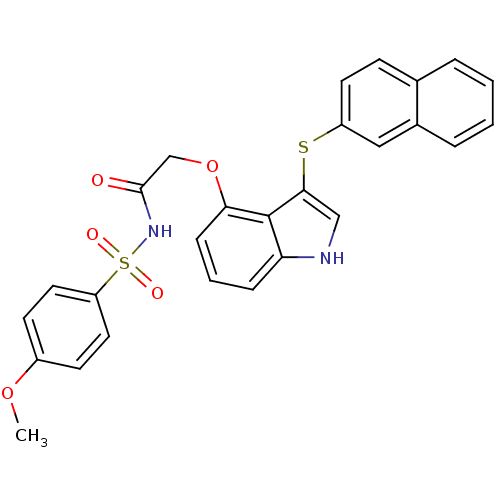
(CHEMBL465947 | N-(4-methoxyphenylsulfonyl)-2-(3-(n...)Show SMILES COc1ccc(cc1)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C27H22N2O5S2/c1-33-20-10-13-22(14-11-20)36(31,32)29-26(30)17-34-24-8-4-7-23-27(24)25(16-28-23)35-21-12-9-18-5-2-3-6-19(18)15-21/h2-16,28H,17H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311624
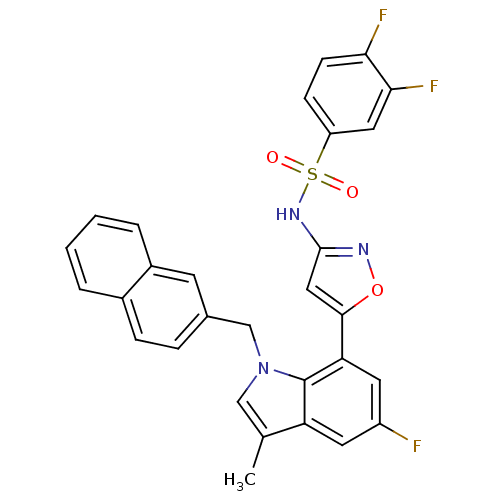
(3,4-difluoro-N-(5-(5-fluoro-3-methyl-1-(naphthalen...)Show SMILES Cc1cn(Cc2ccc3ccccc3c2)c2c(cc(F)cc12)-c1cc(NS(=O)(=O)c2ccc(F)c(F)c2)no1 Show InChI InChI=1S/C29H20F3N3O3S/c1-17-15-35(16-18-6-7-19-4-2-3-5-20(19)10-18)29-23(17)11-21(30)12-24(29)27-14-28(33-38-27)34-39(36,37)22-8-9-25(31)26(32)13-22/h2-15H,16H2,1H3,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303711
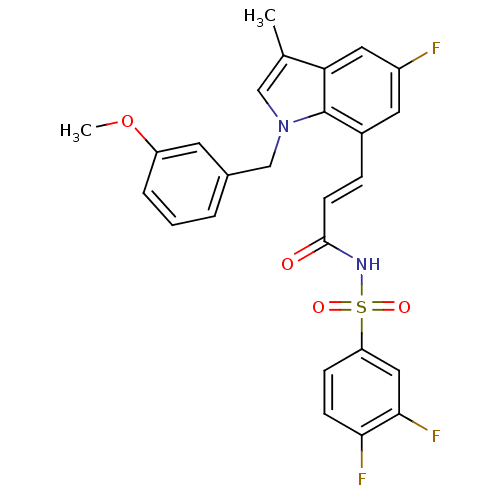
(3,4-Difluoro-N-{(E)-3-[5-fluoro-1-(3-methoxybenzyl...)Show SMILES COc1cccc(Cn2cc(C)c3cc(F)cc(\C=C\C(=O)NS(=O)(=O)c4ccc(F)c(F)c4)c23)c1 Show InChI InChI=1S/C26H21F3N2O4S/c1-16-14-31(15-17-4-3-5-20(10-17)35-2)26-18(11-19(27)12-22(16)26)6-9-25(32)30-36(33,34)21-7-8-23(28)24(29)13-21/h3-14H,15H2,1-2H3,(H,30,32)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254647
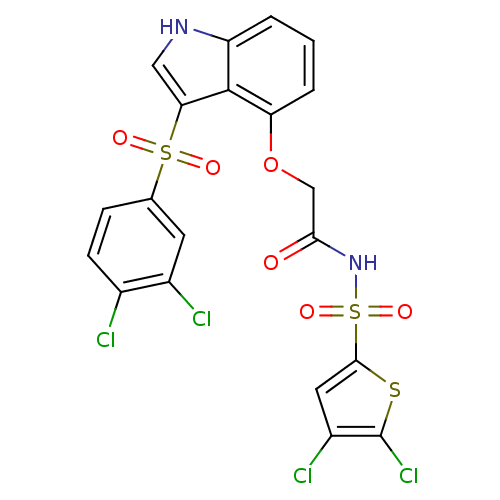
(2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(c12)S(=O)(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H12Cl4N2O6S3/c21-11-5-4-10(6-12(11)22)34(28,29)16-8-25-14-2-1-3-15(19(14)16)32-9-17(27)26-35(30,31)18-7-13(23)20(24)33-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor assessed as cAMP production by cell-based assay |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50317487

((S)-2-((4-(4-(thiophen-3-yl)phenoxy)phenoxy)methyl...)Show SMILES C(Oc1ccc(Oc2ccc(cc2)-c2ccsc2)cc1)[C@@H]1CCCCN1 |r| Show InChI InChI=1S/C22H23NO2S/c1-2-13-23-19(3-1)15-24-20-8-10-22(11-9-20)25-21-6-4-17(5-7-21)18-12-14-26-16-18/h4-12,14,16,19,23H,1-3,13,15H2/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human LTA4H hydrolysis assessed as inhibition of Ca2+ ionophore-stimulated LTB4 formation in human whole blood by ELISA |
Bioorg Med Chem Lett 20: 2851-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.047
BindingDB Entry DOI: 10.7270/Q2CC10VD |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311615
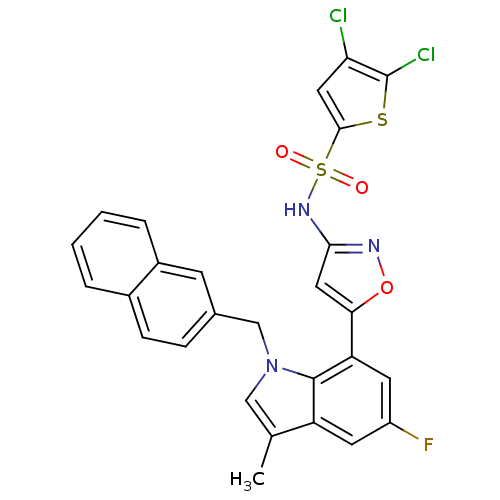
(4,5-dichloro-N-(5-(5-fluoro-3-methyl-1-(naphthalen...)Show SMILES Cc1cn(Cc2ccc3ccccc3c2)c2c(cc(F)cc12)-c1cc(NS(=O)(=O)c2cc(Cl)c(Cl)s2)no1 Show InChI InChI=1S/C27H18Cl2FN3O3S2/c1-15-13-33(14-16-6-7-17-4-2-3-5-18(17)8-16)26-20(15)9-19(30)10-21(26)23-12-24(31-36-23)32-38(34,35)25-11-22(28)27(29)37-25/h2-13H,14H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311621
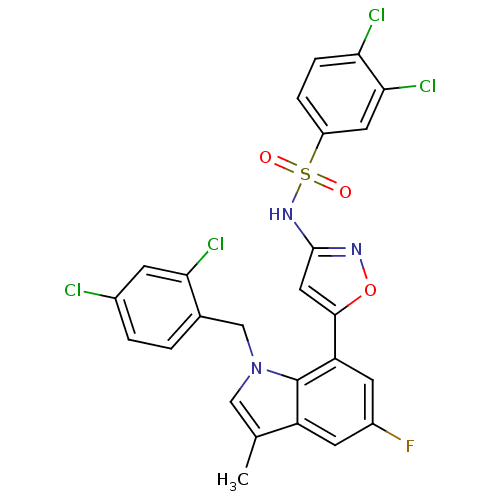
(3,4-dichloro-N-(5-(1-(2,4-dichlorobenzyl)-5-fluoro...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(cc(F)cc12)-c1cc(NS(=O)(=O)c2ccc(Cl)c(Cl)c2)no1 Show InChI InChI=1S/C25H16Cl4FN3O3S/c1-13-11-33(12-14-2-3-15(26)6-21(14)28)25-18(13)7-16(30)8-19(25)23-10-24(31-36-23)32-37(34,35)17-4-5-20(27)22(29)9-17/h2-11H,12H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311623
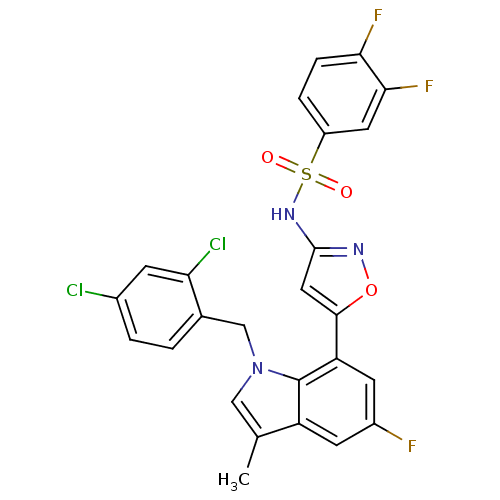
(CHEMBL1081186 | N-(5-(1-(2,4-dichlorobenzyl)-5-flu...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(cc(F)cc12)-c1cc(NS(=O)(=O)c2ccc(F)c(F)c2)no1 Show InChI InChI=1S/C25H16Cl2F3N3O3S/c1-13-11-33(12-14-2-3-15(26)6-20(14)27)25-18(13)7-16(28)8-19(25)23-10-24(31-36-23)32-37(34,35)17-4-5-21(29)22(30)9-17/h2-11H,12H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303675
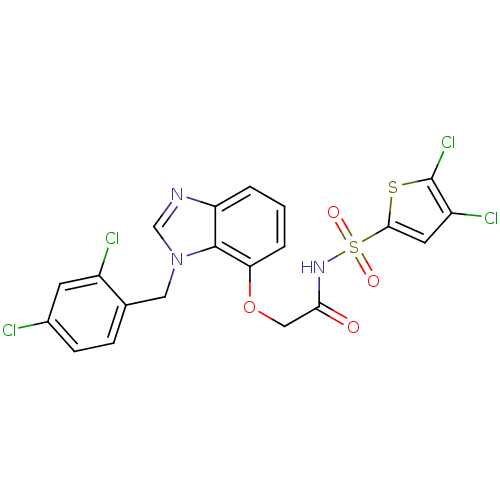
(2-(1-(2,4-dichlorobenzyl)-1H-benzo[d]imidazol-7-yl...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2ncn(Cc3ccc(Cl)cc3Cl)c12 Show InChI InChI=1S/C20H13Cl4N3O4S2/c21-12-5-4-11(13(22)6-12)8-27-10-25-15-2-1-3-16(19(15)27)31-9-17(28)26-33(29,30)18-7-14(23)20(24)32-18/h1-7,10H,8-9H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303675

(2-(1-(2,4-dichlorobenzyl)-1H-benzo[d]imidazol-7-yl...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2ncn(Cc3ccc(Cl)cc3Cl)c12 Show InChI InChI=1S/C20H13Cl4N3O4S2/c21-12-5-4-11(13(22)6-12)8-27-10-25-15-2-1-3-16(19(15)27)31-9-17(28)26-33(29,30)18-7-14(23)20(24)32-18/h1-7,10H,8-9H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 60 mins repeated washing by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254841

(2-(3-(3,4-dichlorophenylthio)-1H-indol-4-yloxy)-N-...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc(Cl)c(Cl)c3)c12 Show InChI InChI=1S/C20H12Cl4N2O4S3/c21-11-5-4-10(6-12(11)22)31-16-8-25-14-2-1-3-15(19(14)16)30-9-17(27)26-33(28,29)18-7-13(23)20(24)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254640
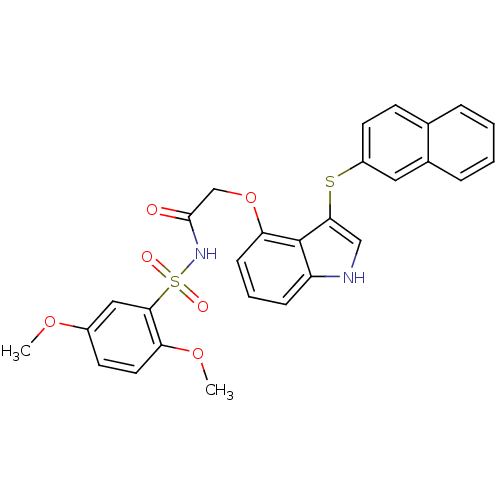
(CHEMBL482379 | N-(2,5-dimethoxyphenylsulfonyl)-2-(...)Show SMILES COc1ccc(OC)c(c1)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C28H24N2O6S2/c1-34-20-11-13-23(35-2)26(15-20)38(32,33)30-27(31)17-36-24-9-5-8-22-28(24)25(16-29-22)37-21-12-10-18-6-3-4-7-19(18)14-21/h3-16,29H,17H2,1-2H3,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254768
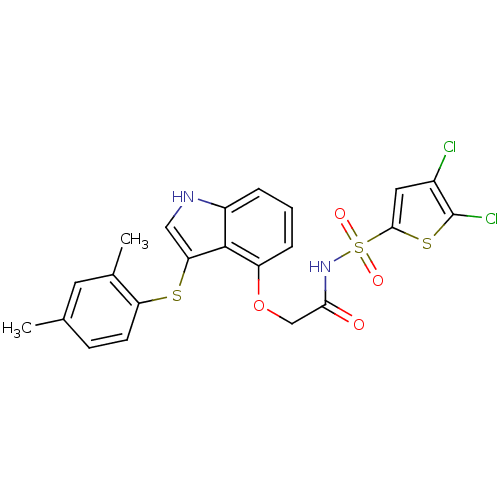
(CHEMBL518396 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Cc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)c(C)c1 Show InChI InChI=1S/C22H18Cl2N2O4S3/c1-12-6-7-17(13(2)8-12)31-18-10-25-15-4-3-5-16(21(15)18)30-11-19(27)26-33(28,29)20-9-14(23)22(24)32-20/h3-10,25H,11H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303663
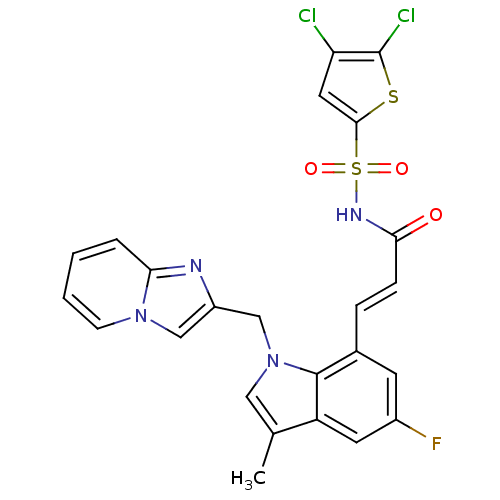
(4,5-Dichlorothiophene-2-sulfonic Acid[(E)-3-(5-Flu...)Show SMILES Cc1cn(Cc2cn3ccccc3n2)c2c(\C=C\C(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)cc(F)cc12 Show InChI InChI=1S/C24H17Cl2FN4O3S2/c1-14-11-31(13-17-12-30-7-3-2-4-20(30)28-17)23-15(8-16(27)9-18(14)23)5-6-21(32)29-36(33,34)22-10-19(25)24(26)35-22/h2-12H,13H2,1H3,(H,29,32)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254841

(2-(3-(3,4-dichlorophenylthio)-1H-indol-4-yloxy)-N-...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc(Cl)c(Cl)c3)c12 Show InChI InChI=1S/C20H12Cl4N2O4S3/c21-11-5-4-10(6-12(11)22)31-16-8-25-14-2-1-3-15(19(14)16)30-9-17(27)26-33(28,29)18-7-13(23)20(24)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254641
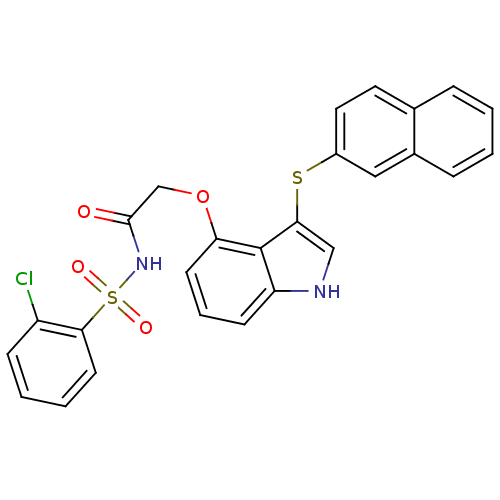
(CHEMBL482380 | N-(2-chlorophenylsulfonyl)-2-(3-(na...)Show SMILES Clc1ccccc1S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H19ClN2O4S2/c27-20-8-3-4-11-24(20)35(31,32)29-25(30)16-33-22-10-5-9-21-26(22)23(15-28-21)34-19-13-12-17-6-1-2-7-18(17)14-19/h1-15,28H,16H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254880
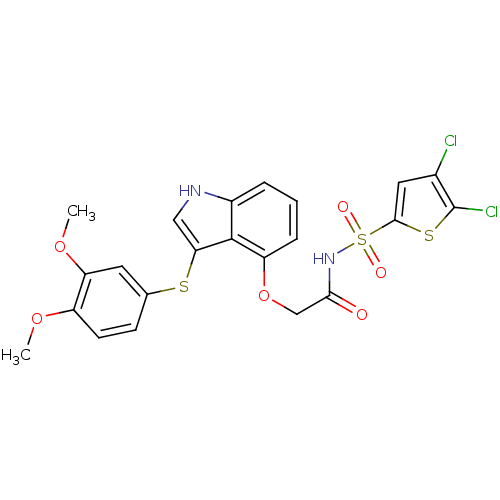
(CHEMBL520741 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES COc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)cc1OC Show InChI InChI=1S/C22H18Cl2N2O6S3/c1-30-15-7-6-12(8-17(15)31-2)33-18-10-25-14-4-3-5-16(21(14)18)32-11-19(27)26-35(28,29)20-9-13(23)22(24)34-20/h3-10,25H,11H2,1-2H3,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255058
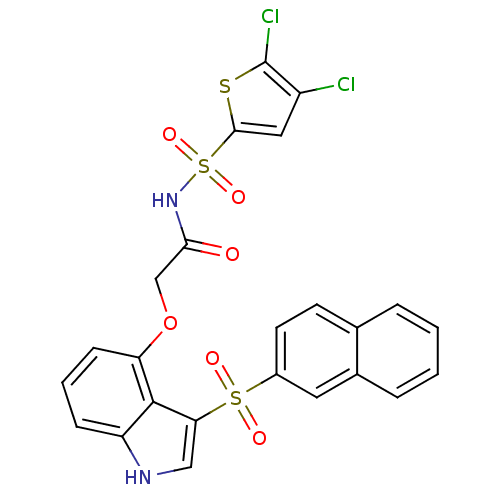
(CHEMBL479427 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(c12)S(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C24H16Cl2N2O6S3/c25-17-11-22(35-24(17)26)37(32,33)28-21(29)13-34-19-7-3-6-18-23(19)20(12-27-18)36(30,31)16-9-8-14-4-1-2-5-15(14)10-16/h1-12,27H,13H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255778
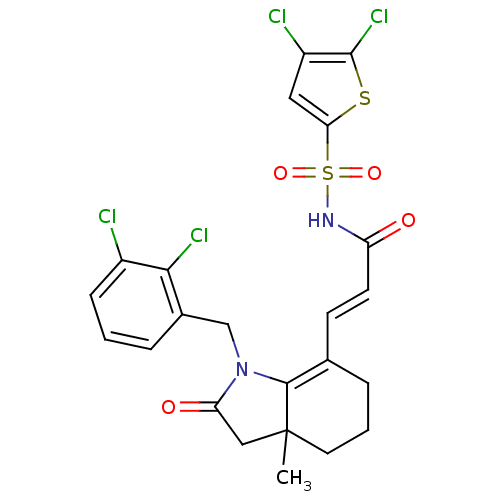
(3-(1-(2,3-dichlorobenzyl)-3a-methyl-2-oxo-2,3,3a,4...)Show SMILES CC12CC(=O)N(Cc3cccc(Cl)c3Cl)C1=C(CCC2)\C=C\C(=O)NS(=O)(=O)c1cc(Cl)c(Cl)s1 |c:17| Show InChI InChI=1S/C23H20Cl4N2O4S2/c1-23-9-3-5-13(7-8-17(30)28-35(32,33)19-10-16(25)22(27)34-19)21(23)29(18(31)11-23)12-14-4-2-6-15(24)20(14)26/h2,4,6-8,10H,3,5,9,11-12H2,1H3,(H,28,30)/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP3 receptor expressed in CHO-K1 cells assessed as reversal of inhibition of forskolin-induced cAMP production |
Bioorg Med Chem Lett 19: 778-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.027
BindingDB Entry DOI: 10.7270/Q24M94DH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255343
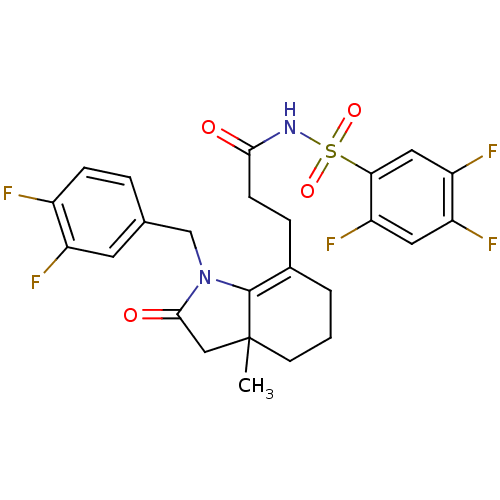
(3-(1-(3,4-difluorobenzyl)-3a-methyl-2-oxo-2,3,3a,4...)Show SMILES CC12CC(=O)N(Cc3ccc(F)c(F)c3)C1=C(CCC(=O)NS(=O)(=O)c1cc(F)c(F)cc1F)CCC2 |t:17| Show InChI InChI=1S/C25H23F5N2O4S/c1-25-8-2-3-15(24(25)32(23(34)12-25)13-14-4-6-16(26)17(27)9-14)5-7-22(33)31-37(35,36)21-11-19(29)18(28)10-20(21)30/h4,6,9-11H,2-3,5,7-8,12-13H2,1H3,(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 778-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.027
BindingDB Entry DOI: 10.7270/Q24M94DH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254797
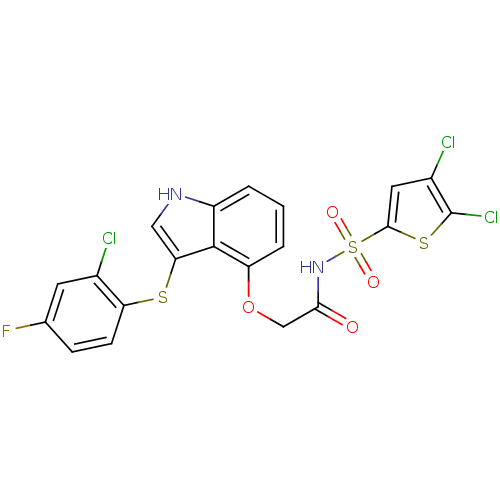
(2-(3-(2-chloro-4-fluorophenylthio)-1H-indol-4-ylox...)Show SMILES Fc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)c(Cl)c1 Show InChI InChI=1S/C20H12Cl3FN2O4S3/c21-11-6-10(24)4-5-15(11)31-16-8-25-13-2-1-3-14(19(13)16)30-9-17(27)26-33(28,29)18-7-12(22)20(23)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50248275
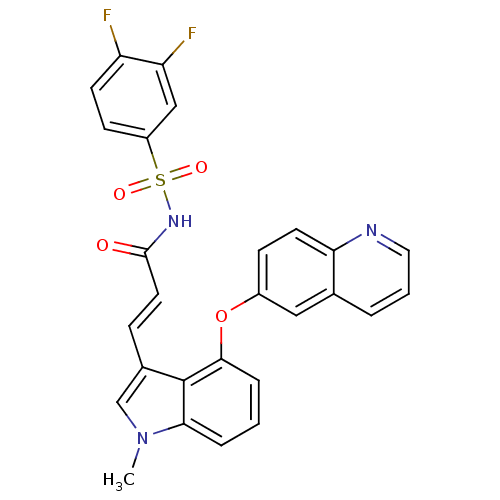
(CHEMBL474751 | N-(3,4-difluorophenylsulfonyl)-3-(1...)Show SMILES Cn1cc(\C=C\C(=O)NS(=O)(=O)c2ccc(F)c(F)c2)c2c(Oc3ccc4ncccc4c3)cccc12 Show InChI InChI=1S/C27H19F2N3O4S/c1-32-16-18(7-12-26(33)31-37(34,35)20-9-10-21(28)22(29)15-20)27-24(32)5-2-6-25(27)36-19-8-11-23-17(14-19)4-3-13-30-23/h2-16H,1H3,(H,31,33)/b12-7+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor at 20 uM in presence of normal buffer |
Bioorg Med Chem Lett 19: 1528-31 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.112
BindingDB Entry DOI: 10.7270/Q2JM29H0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254767
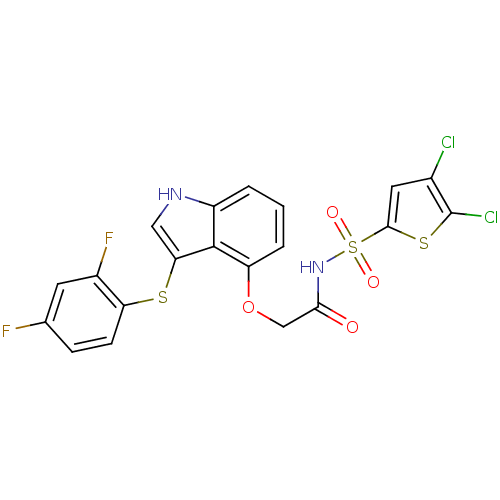
(CHEMBL464271 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Fc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)c(F)c1 Show InChI InChI=1S/C20H12Cl2F2N2O4S3/c21-11-7-18(32-20(11)22)33(28,29)26-17(27)9-30-14-3-1-2-13-19(14)16(8-25-13)31-15-5-4-10(23)6-12(15)24/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311610
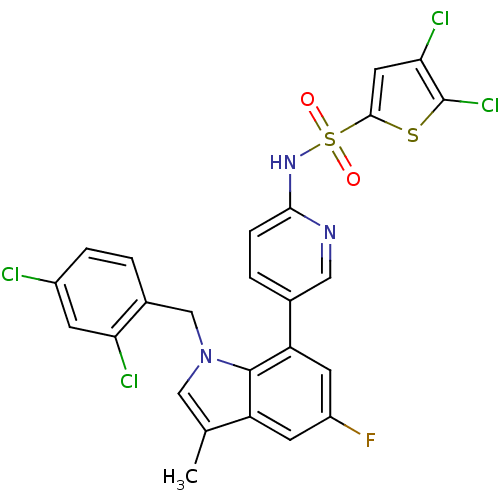
(4,5-dichloro-N-(5-(1-(2,4-dichlorobenzyl)-5-fluoro...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(cc(F)cc12)-c1ccc(NS(=O)(=O)c2cc(Cl)c(Cl)s2)nc1 Show InChI InChI=1S/C25H16Cl4FN3O2S2/c1-13-11-33(12-15-2-4-16(26)6-20(15)27)24-18(13)7-17(30)8-19(24)14-3-5-22(31-10-14)32-37(34,35)23-9-21(28)25(29)36-23/h2-11H,12H2,1H3,(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Mus musculus (Mouse)) | BDBM50254647

(2-(3-(3,4-dichlorophenylsulfonyl)-1H-indol-4-yloxy...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(c12)S(=O)(=O)c1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C20H12Cl4N2O6S3/c21-11-5-4-10(6-12(11)22)34(28,29)16-8-25-14-2-1-3-15(19(14)16)32-9-17(27)26-35(30,31)18-7-13(23)20(24)33-18/h1-8,25H,9H2,(H,26,27) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from mouse EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254686
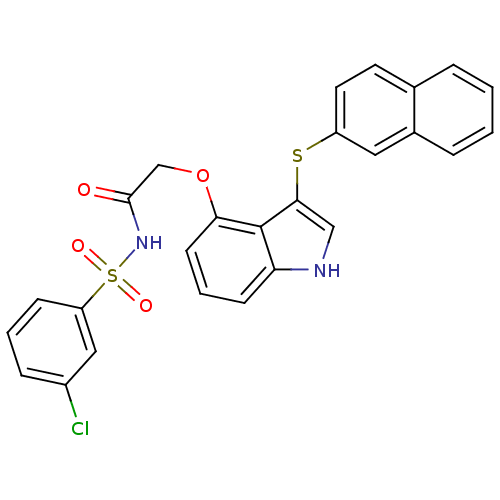
(CHEMBL464031 | N-(3-chlorophenylsulfonyl)-2-(3-(na...)Show SMILES Clc1cccc(c1)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H19ClN2O4S2/c27-19-7-3-8-21(14-19)35(31,32)29-25(30)16-33-23-10-4-9-22-26(23)24(15-28-22)34-20-12-11-17-5-1-2-6-18(17)13-20/h1-15,28H,16H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254520

(CHEMBL466184 | N-(3,4-difluorophenylsulfonyl)-2-(3...)Show SMILES Fc1ccc(cc1F)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H18F2N2O4S2/c27-20-11-10-19(13-21(20)28)36(32,33)30-25(31)15-34-23-7-3-6-22-26(23)24(14-29-22)35-18-9-8-16-4-1-2-5-17(16)12-18/h1-14,29H,15H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303660
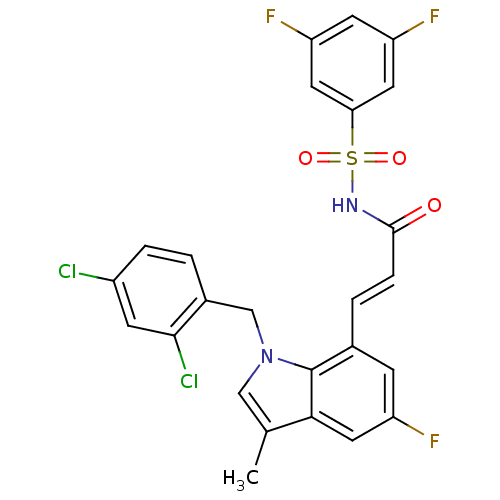
(CHEMBL566005 | N-{(E)-3-[1-(2,4-Dichlorobenzyl)-5-...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(\C=C\C(=O)NS(=O)(=O)c3cc(F)cc(F)c3)cc(F)cc12 Show InChI InChI=1S/C25H17Cl2F3N2O3S/c1-14-12-32(13-16-2-4-17(26)7-23(16)27)25-15(6-18(28)11-22(14)25)3-5-24(33)31-36(34,35)21-9-19(29)8-20(30)10-21/h2-12H,13H2,1H3,(H,31,33)/b5-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303695
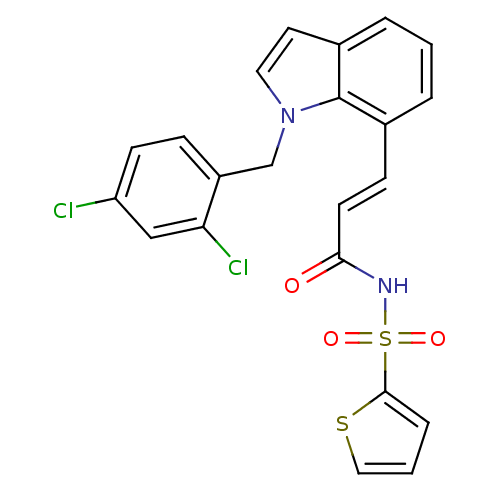
(CHEMBL585581 | Thiophene-2-sulfonic Acid{(E)-3-[1-...)Show SMILES Clc1ccc(Cn2ccc3cccc(\C=C\C(=O)NS(=O)(=O)c4cccs4)c23)c(Cl)c1 Show InChI InChI=1S/C22H16Cl2N2O3S2/c23-18-8-6-17(19(24)13-18)14-26-11-10-16-4-1-3-15(22(16)26)7-9-20(27)25-31(28,29)21-5-2-12-30-21/h1-13H,14H2,(H,25,27)/b9-7+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303695

(CHEMBL585581 | Thiophene-2-sulfonic Acid{(E)-3-[1-...)Show SMILES Clc1ccc(Cn2ccc3cccc(\C=C\C(=O)NS(=O)(=O)c4cccs4)c23)c(Cl)c1 Show InChI InChI=1S/C22H16Cl2N2O3S2/c23-18-8-6-17(19(24)13-18)14-26-11-10-16-4-1-3-15(22(16)26)7-9-20(27)25-31(28,29)21-5-2-12-30-21/h1-13H,14H2,(H,25,27)/b9-7+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 60 mins repeated washing by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50311619
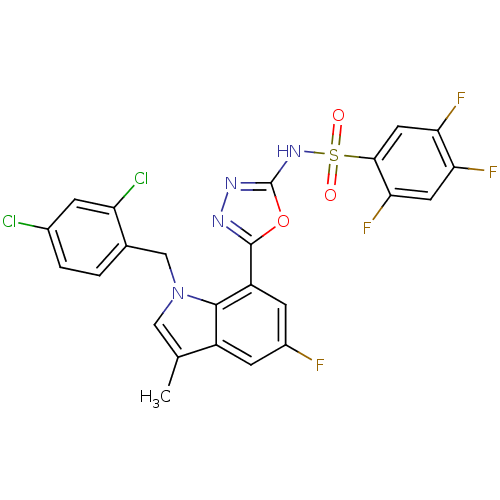
(CHEMBL1080106 | N-(5-(1-(2,4-dichlorobenzyl)-5-flu...)Show SMILES Cc1cn(Cc2ccc(Cl)cc2Cl)c2c(cc(F)cc12)-c1nnc(NS(=O)(=O)c2cc(F)c(F)cc2F)o1 Show InChI InChI=1S/C24H14Cl2F4N4O3S/c1-11-9-34(10-12-2-3-13(25)4-17(12)26)22-15(11)5-14(27)6-16(22)23-31-32-24(37-23)33-38(35,36)21-8-19(29)18(28)7-20(21)30/h2-9H,10H2,1H3,(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 6797-800 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.084
BindingDB Entry DOI: 10.7270/Q2833S51 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254730
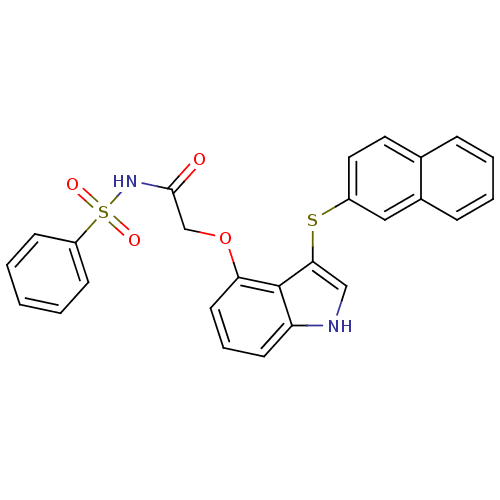
(2-(3-(naphthalen-2-ylthio)-1H-indol-4-yloxy)-N-(ph...)Show SMILES O=C(COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C26H20N2O4S2/c29-25(28-34(30,31)21-9-2-1-3-10-21)17-32-23-12-6-11-22-26(23)24(16-27-22)33-20-14-13-18-7-4-5-8-19(18)15-20/h1-16,27H,17H2,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255780
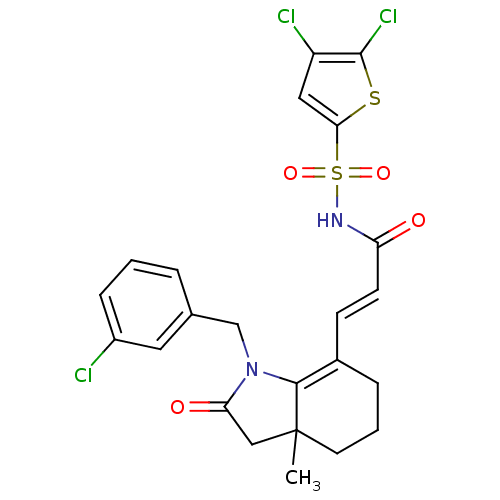
(3-(1-(3-chlorobenzyl)-3a-methyl-2-oxo-2,3,3a,4,5,6...)Show SMILES CC12CC(=O)N(Cc3cccc(Cl)c3)C1=C(CCC2)\C=C\C(=O)NS(=O)(=O)c1cc(Cl)c(Cl)s1 |c:16| Show InChI InChI=1S/C23H21Cl3N2O4S2/c1-23-9-3-5-15(7-8-18(29)27-34(31,32)20-11-17(25)22(26)33-20)21(23)28(19(30)12-23)13-14-4-2-6-16(24)10-14/h2,4,6-8,10-11H,3,5,9,12-13H2,1H3,(H,27,29)/b8-7+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 778-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.027
BindingDB Entry DOI: 10.7270/Q24M94DH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254686

(CHEMBL464031 | N-(3-chlorophenylsulfonyl)-2-(3-(na...)Show SMILES Clc1cccc(c1)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H19ClN2O4S2/c27-19-7-3-8-21(14-19)35(31,32)29-25(30)16-33-23-10-4-9-22-26(23)24(15-28-22)34-20-12-11-17-5-1-2-6-18(17)13-20/h1-15,28H,16H2,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303677
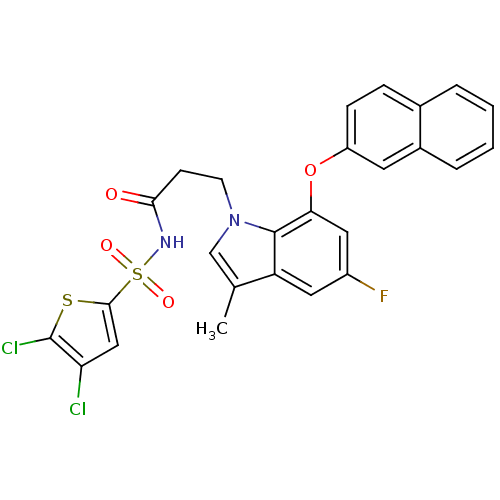
(CHEMBL566016 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Cc1cn(CCC(=O)NS(=O)(=O)c2cc(Cl)c(Cl)s2)c2c(Oc3ccc4ccccc4c3)cc(F)cc12 Show InChI InChI=1S/C26H19Cl2FN2O4S2/c1-15-14-31(9-8-23(32)30-37(33,34)24-13-21(27)26(28)36-24)25-20(15)11-18(29)12-22(25)35-19-7-6-16-4-2-3-5-17(16)10-19/h2-7,10-14H,8-9H2,1H3,(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255779
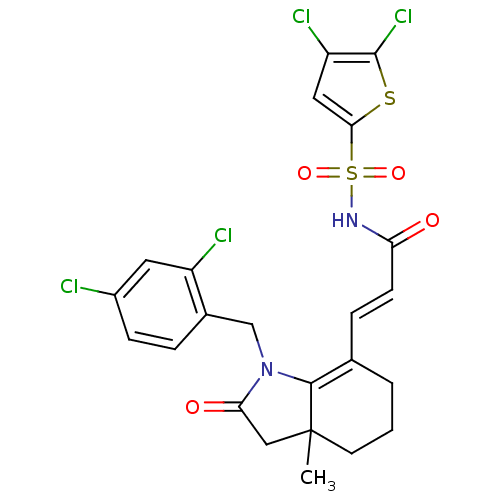
(3-(1-(2,4-dichlorobenzyl)-3a-methyl-2-oxo-2,3,3a,4...)Show SMILES CC12CC(=O)N(Cc3ccc(Cl)cc3Cl)C1=C(CCC2)\C=C\C(=O)NS(=O)(=O)c1cc(Cl)c(Cl)s1 |c:17| Show InChI InChI=1S/C23H20Cl4N2O4S2/c1-23-8-2-3-13(5-7-18(30)28-35(32,33)20-10-17(26)22(27)34-20)21(23)29(19(31)11-23)12-14-4-6-15(24)9-16(14)25/h4-7,9-10H,2-3,8,11-12H2,1H3,(H,28,30)/b7-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 778-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.027
BindingDB Entry DOI: 10.7270/Q24M94DH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303709
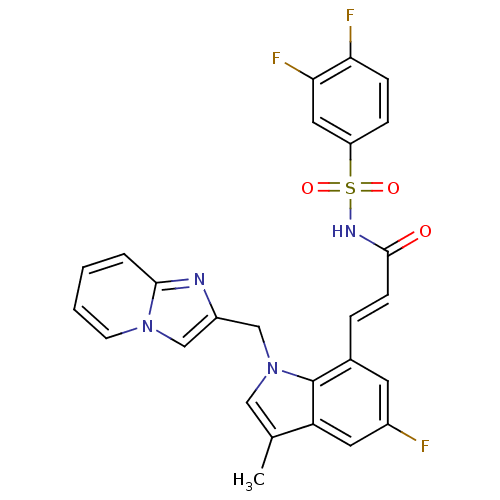
(3,4-Difluoro-N-[(E)-3-(5-fluoro-1-imidazo[1,2-a]py...)Show SMILES Cc1cn(Cc2cn3ccccc3n2)c2c(\C=C\C(=O)NS(=O)(=O)c3ccc(F)c(F)c3)cc(F)cc12 Show InChI InChI=1S/C26H19F3N4O3S/c1-16-13-33(15-19-14-32-9-3-2-4-24(32)30-19)26-17(10-18(27)11-21(16)26)5-8-25(34)31-37(35,36)20-6-7-22(28)23(29)12-20/h2-14H,15H2,1H3,(H,31,34)/b8-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254643
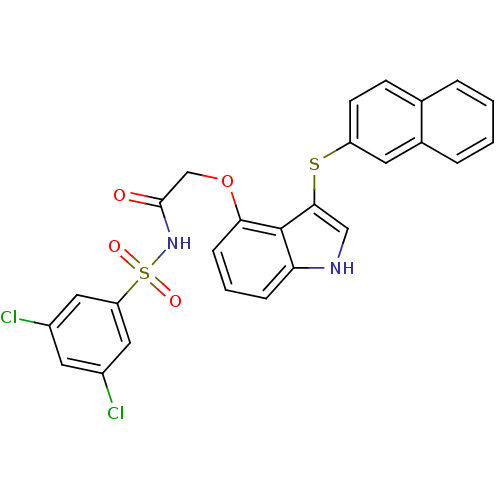
(CHEMBL481589 | N-(3,5-dichlorophenylsulfonyl)-2-(3...)Show SMILES Clc1cc(Cl)cc(c1)S(=O)(=O)NC(=O)COc1cccc2[nH]cc(Sc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H18Cl2N2O4S2/c27-18-11-19(28)13-21(12-18)36(32,33)30-25(31)15-34-23-7-3-6-22-26(23)24(14-29-22)35-20-9-8-16-4-1-2-5-17(16)10-20/h1-14,29H,15H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303680
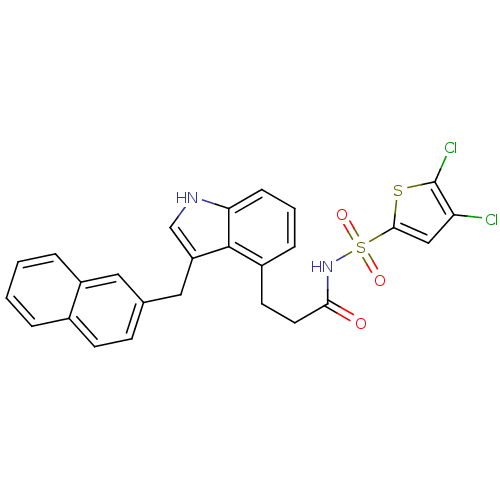
(4,5-Dichlorothiophene-2-sulfonic Acid[3-(3-Naphtha...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)CCc1cccc2[nH]cc(Cc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H20Cl2N2O3S2/c27-21-14-24(34-26(21)28)35(32,33)30-23(31)11-10-18-6-3-7-22-25(18)20(15-29-22)13-16-8-9-17-4-1-2-5-19(17)12-16/h1-9,12,14-15,29H,10-11,13H2,(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50255125
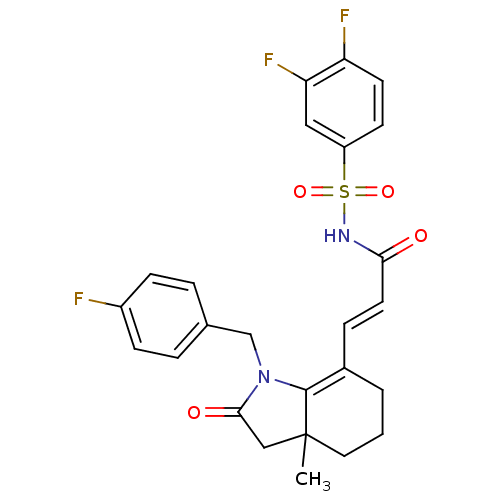
(CHEMBL481823 | N-(3,4-difluorophenylsulfonyl)-3-(1...)Show SMILES CC12CC(=O)N(Cc3ccc(F)cc3)C1=C(CCC2)\C=C\C(=O)NS(=O)(=O)c1ccc(F)c(F)c1 |c:16| Show InChI InChI=1S/C25H23F3N2O4S/c1-25-12-2-3-17(24(25)30(23(32)14-25)15-16-4-7-18(26)8-5-16)6-11-22(31)29-35(33,34)19-9-10-20(27)21(28)13-19/h4-11,13H,2-3,12,14-15H2,1H3,(H,29,31)/b11-6+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 778-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.027
BindingDB Entry DOI: 10.7270/Q24M94DH |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303682
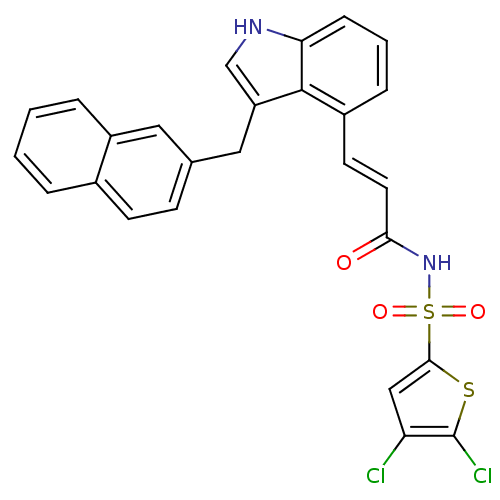
(4,5-Dichloro-thiophene-2-sulfonic acid{3-[5-fluoro...)Show SMILES Clc1cc(sc1Cl)S(=O)(=O)NC(=O)\C=C\c1cccc2[nH]cc(Cc3ccc4ccccc4c3)c12 Show InChI InChI=1S/C26H18Cl2N2O3S2/c27-21-14-24(34-26(21)28)35(32,33)30-23(31)11-10-18-6-3-7-22-25(18)20(15-29-22)13-16-8-9-17-4-1-2-5-19(17)12-16/h1-12,14-15,29H,13H2,(H,30,31)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254924
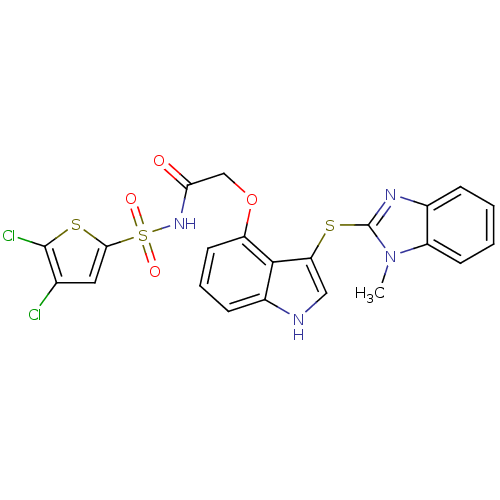
(CHEMBL465923 | N-(4,5-dichlorothiophen-2-ylsulfony...)Show SMILES Cn1c(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)nc2ccccc12 Show InChI InChI=1S/C22H16Cl2N4O4S3/c1-28-15-7-3-2-5-13(15)26-22(28)33-17-10-25-14-6-4-8-16(20(14)17)32-11-18(29)27-35(30,31)19-9-12(23)21(24)34-19/h2-10,25H,11H2,1H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50303679
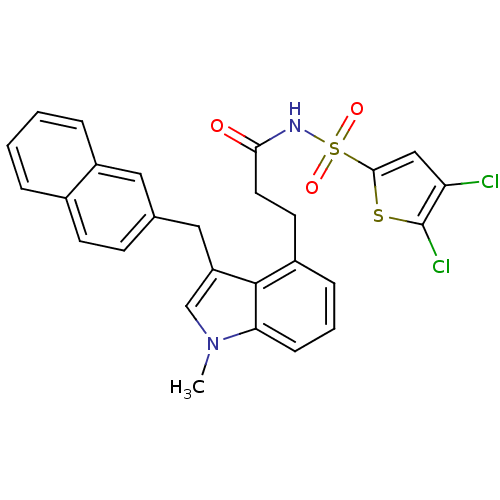
(4,5-Dichlorothiophene-2-sulfonic Acid[3-(1-Methyl-...)Show SMILES Cn1cc(Cc2ccc3ccccc3c2)c2c(CCC(=O)NS(=O)(=O)c3cc(Cl)c(Cl)s3)cccc12 Show InChI InChI=1S/C27H22Cl2N2O3S2/c1-31-16-21(14-17-9-10-18-5-2-3-6-20(18)13-17)26-19(7-4-8-23(26)31)11-12-24(32)30-36(33,34)25-15-22(28)27(29)35-25/h2-10,13,15-16H,11-12,14H2,1H3,(H,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor after 1 hr by liquid scintillation counting |
J Med Chem 53: 18-36 (2010)
Article DOI: 10.1021/jm9005912
BindingDB Entry DOI: 10.7270/Q2XP752H |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50248277
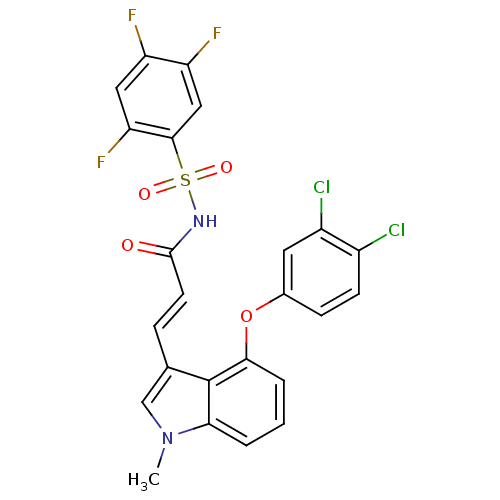
(3-(4-(3,4-dichlorophenoxy)-1-methyl-1H-indol-3-yl)...)Show SMILES Cn1cc(\C=C\C(=O)NS(=O)(=O)c2cc(F)c(F)cc2F)c2c(Oc3ccc(Cl)c(Cl)c3)cccc12 Show InChI InChI=1S/C24H15Cl2F3N2O4S/c1-31-12-13(5-8-23(32)30-36(33,34)22-11-18(28)17(27)10-19(22)29)24-20(31)3-2-4-21(24)35-14-6-7-15(25)16(26)9-14/h2-12H,1H3,(H,30,32)/b8-5+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor at 20 uM in presence of normal buffer |
Bioorg Med Chem Lett 19: 1528-31 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.112
BindingDB Entry DOI: 10.7270/Q2JM29H0 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50254797

(2-(3-(2-chloro-4-fluorophenylthio)-1H-indol-4-ylox...)Show SMILES Fc1ccc(Sc2c[nH]c3cccc(OCC(=O)NS(=O)(=O)c4cc(Cl)c(Cl)s4)c23)c(Cl)c1 Show InChI InChI=1S/C20H12Cl3FN2O4S3/c21-11-6-10(24)4-5-15(11)31-16-8-25-13-2-1-3-14(19(13)16)30-9-17(27)26-33(28,29)18-7-12(22)20(23)32-18/h1-8,25H,9H2,(H,26,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE Chemistry, Inc
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor in presence of 10% human serum |
Bioorg Med Chem Lett 19: 123-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.007
BindingDB Entry DOI: 10.7270/Q2JH3M1S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data