

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50403547 (ATROPEN | ATROPINE) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50027065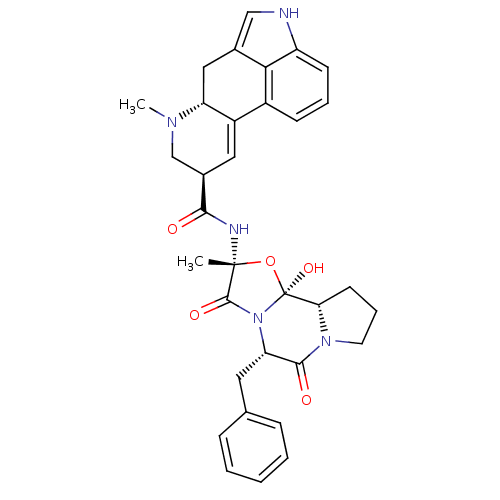 ((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50027065 ((5'alpha)-12'-hydroxy-2'-methyl-5'-(phenylmethyl)e...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT5A receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM22165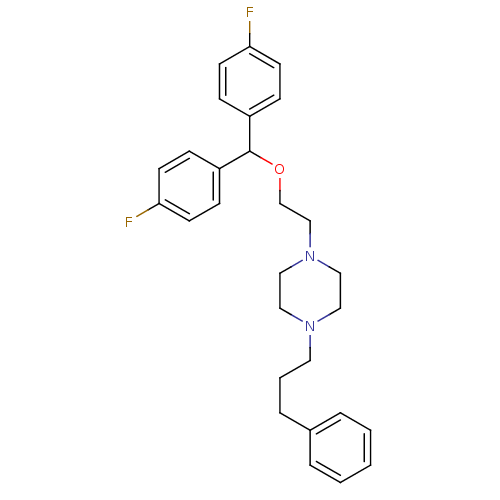 (1-{2-[bis(4-fluorophenyl)methoxy]ethyl}-4-(3-pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from DAT after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59098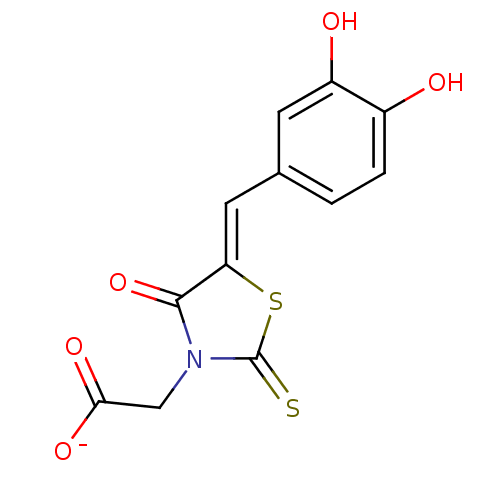 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT7 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50001888 ((chloropromazine) [3-(2-Chloro-phenothiazin-10-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59099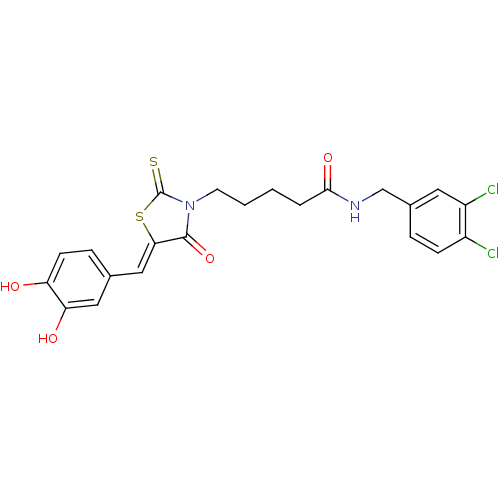 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50353902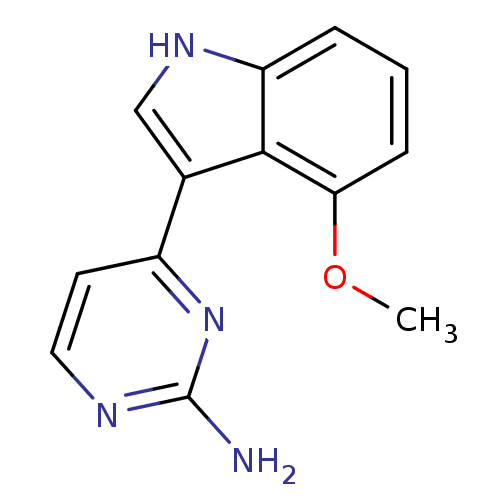 (CHEMBL1829959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT2B receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59101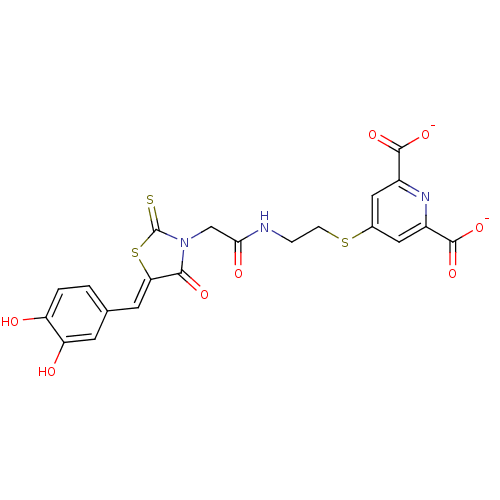 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50004923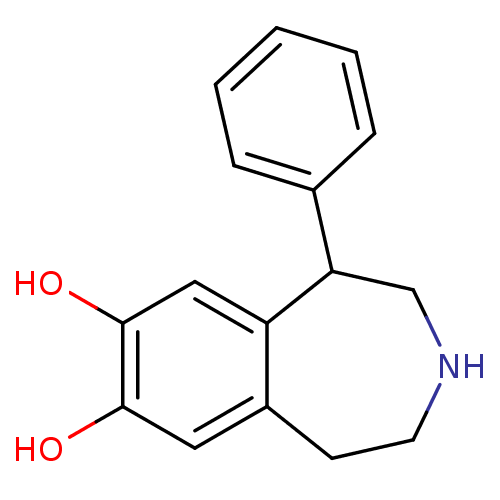 ((+/-)-SKF-38393 | 1-Phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM10838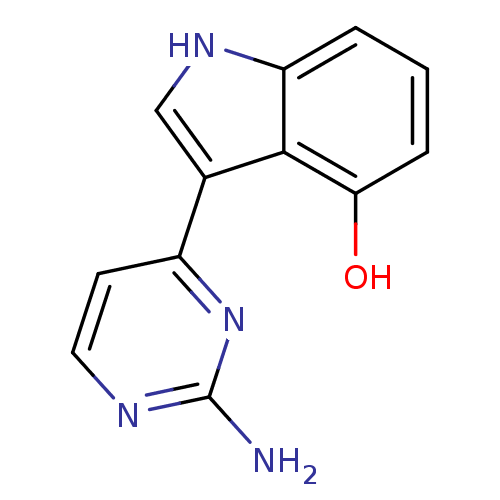 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT2B receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59100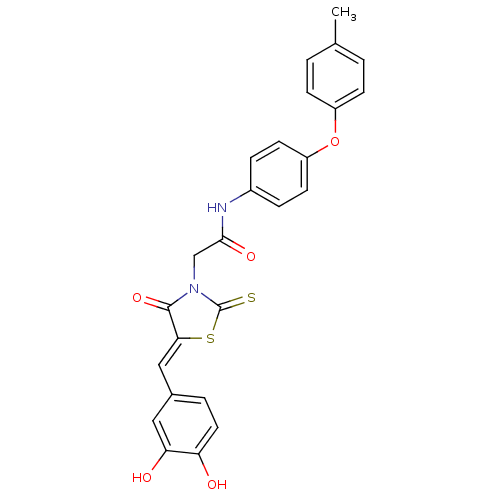 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 202 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 684 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT5A receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]LSD from 5-HT7 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from DAT after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50353903 (CHEMBL1829961) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59101 (Bi-ligand, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50353903 (CHEMBL1829961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50353901 (CHEMBL1829958) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50353902 (CHEMBL1829959) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50353901 (CHEMBL1829958) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation counting | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59100 (Bi-ligand, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Escherichia coli) | BDBM59098 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 4-hydroxy-tetrahydrodipicolinate reductase (Escherichia coli) | BDBM59099 (Bi-ligand, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Quinone-dependent D-lactate dehydrogenase (Escherichia coli) | BDBM59098 (Bi-ligand, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Triad Therapeutics, Inc | Assay Description All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction. | Chem Biol 11: 185-94 (2004) Article DOI: 10.1016/j.chembiol.2004.02.012 BindingDB Entry DOI: 10.7270/Q2K9360M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50105417 (CHEMBL1089 | Nardil | PHENELZINE | Phenethyl-hydra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50105418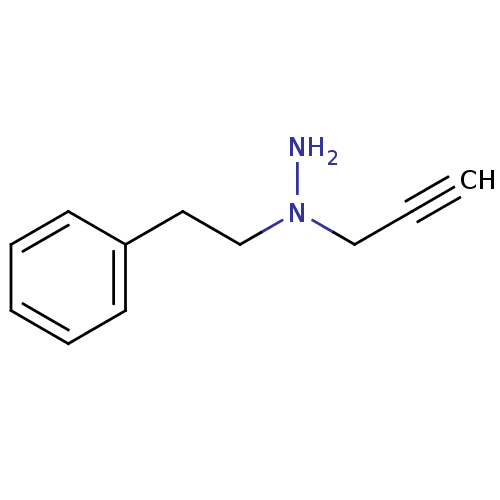 (CHEMBL90144 | N-Phenethyl-N-prop-2-ynyl-hydrazine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50105423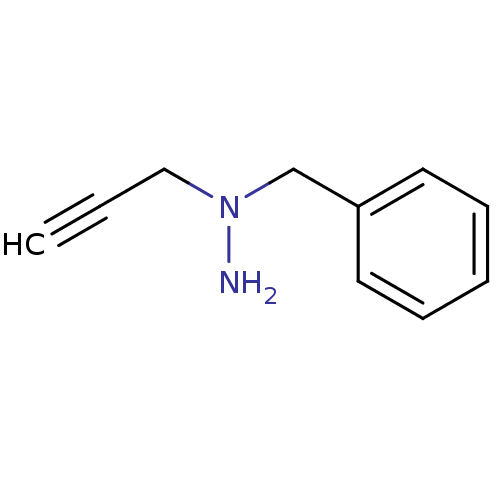 (CHEMBL90721 | N-Benzyl-N-prop-2-ynyl-hydrazine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105421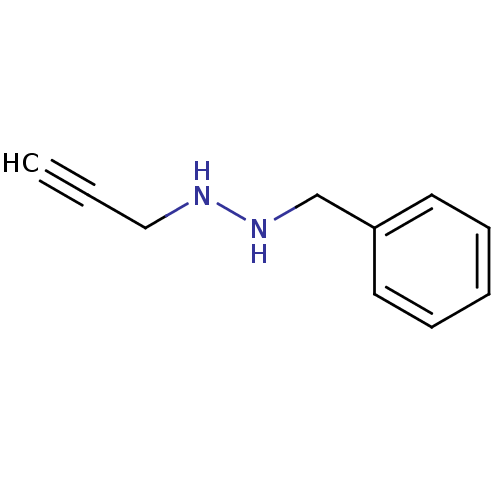 (CHEMBL89976 | N-Benzyl-N'-prop-2-ynyl-hydrazine) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM15579 (CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 516 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Rattus norvegicus (rat)) | BDBM50105422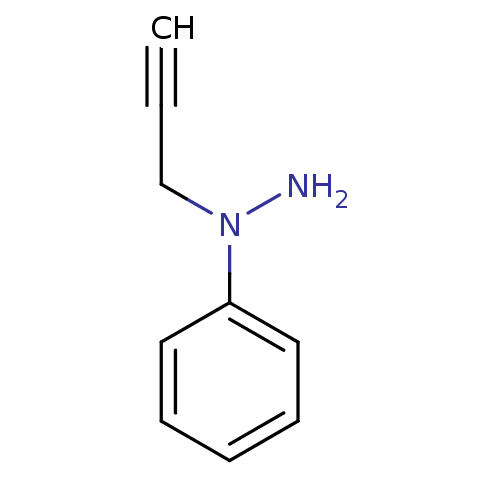 (CHEMBL328446 | N-Phenyl-N-prop-2-ynyl-hydrazine) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105416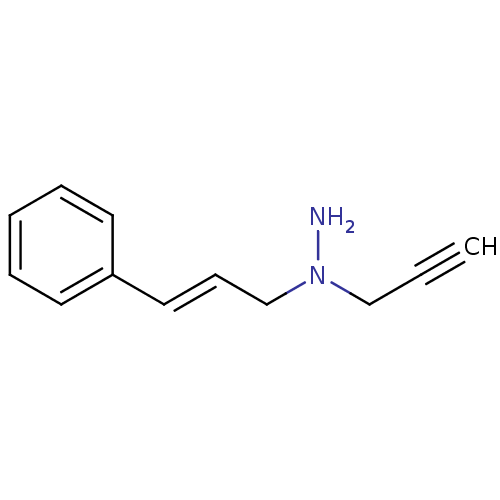 (CHEMBL328551 | N-((E)-3-Phenyl-allyl)-N-prop-2-yny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM10838 (3-(2-aminopyrimidin-4-yl)-1H-indol-4-ol | Meridian...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Florida Curated by ChEMBL | Assay Description Inhibition of GSK3-beta | Bioorg Med Chem 19: 5756-62 (2011) Article DOI: 10.1016/j.bmc.2011.08.033 BindingDB Entry DOI: 10.7270/Q25X29BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105423 (CHEMBL90721 | N-Benzyl-N-prop-2-ynyl-hydrazine) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105420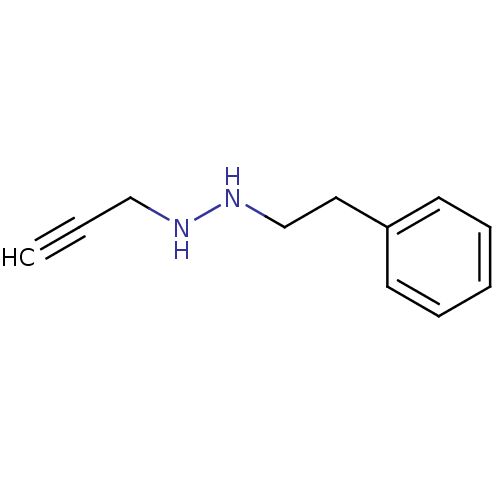 (CHEMBL90653 | N-Phenethyl-N'-prop-2-ynyl-hydrazine) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Rattus norvegicus (rat)) | BDBM50105418 (CHEMBL90144 | N-Phenethyl-N-prop-2-ynyl-hydrazine) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CV Technologies Inc. Curated by ChEMBL | Assay Description In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitro | Bioorg Med Chem Lett 11: 2715-7 (2001) BindingDB Entry DOI: 10.7270/Q2Z60NC5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |