 Found 266 hits with Last Name = 'bandyala' and Initial = 'tr'
Found 266 hits with Last Name = 'bandyala' and Initial = 'tr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50318633

(3-benzenesulfonyl-8-piperazin-1-ylquinoline | CHEM...)Show InChI InChI=1S/C19H19N3O2S/c23-25(24,16-6-2-1-3-7-16)17-13-15-5-4-8-18(19(15)21-14-17)22-11-9-20-10-12-22/h1-8,13-14,20H,9-12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50483134
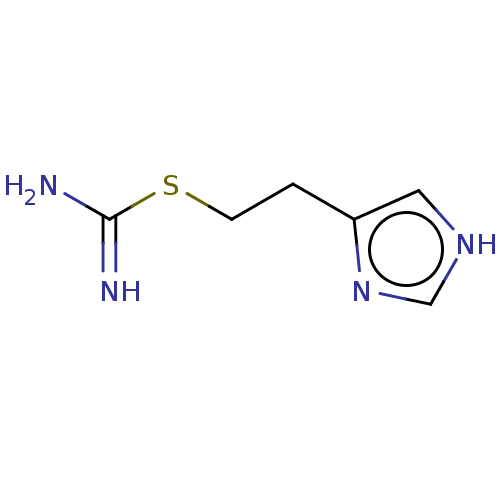
(CHEBI:64156 | Imetit)Show InChI InChI=1S/C6H10N4S/c7-6(8)11-2-1-5-3-9-4-10-5/h3-4H,1-2H2,(H3,7,8)(H,9,10) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50019754
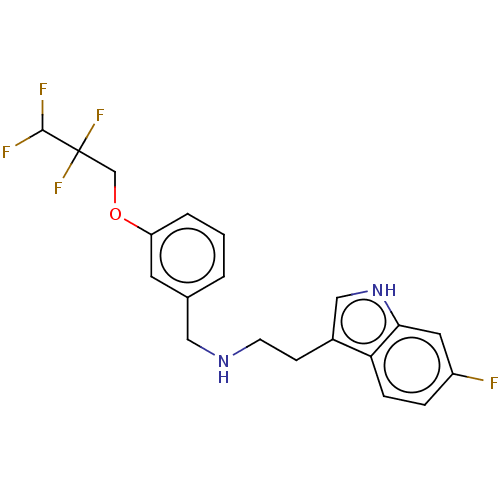
(IDALOPIRDINE | LU-AE58054)Show SMILES FC(F)C(F)(F)COc1cccc(CNCCc2c[nH]c3cc(F)ccc23)c1 Show InChI InChI=1S/C20H19F5N2O/c21-15-4-5-17-14(11-27-18(17)9-15)6-7-26-10-13-2-1-3-16(8-13)28-12-20(24,25)19(22)23/h1-5,8-9,11,19,26-27H,6-7,10,12H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at 5-HT6 receptor (unknown origin) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517508
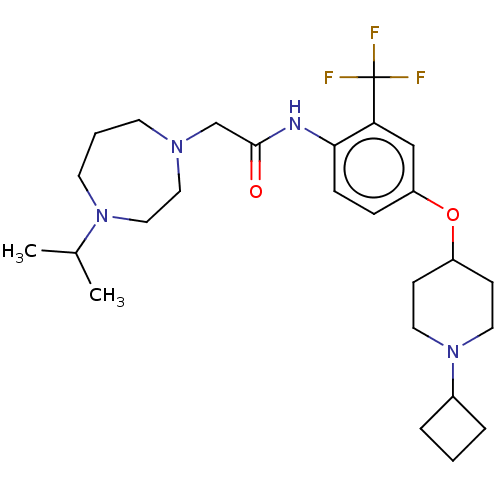
(CHEMBL4594107)Show SMILES CC(C)N1CCCN(CC(=O)Nc2ccc(OC3CCN(CC3)C3CCC3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C26H39F3N4O2/c1-19(2)32-12-4-11-31(15-16-32)18-25(34)30-24-8-7-22(17-23(24)26(27,28)29)35-21-9-13-33(14-10-21)20-5-3-6-20/h7-8,17,19-21H,3-6,9-16,18H2,1-2H3,(H,30,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395858
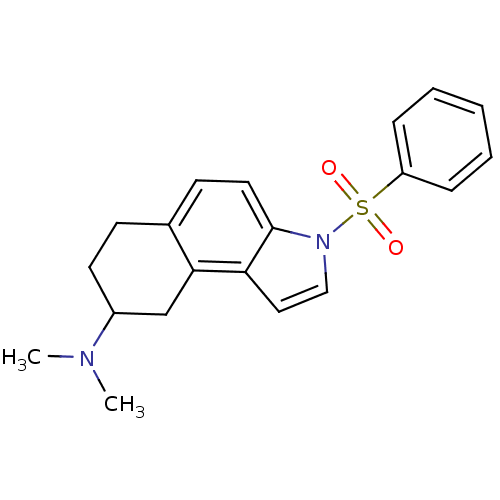
(CHEMBL2165519)Show SMILES CN(C)C1CCc2ccc3n(ccc3c2C1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H22N2O2S/c1-21(2)16-10-8-15-9-11-20-18(19(15)14-16)12-13-22(20)25(23,24)17-6-4-3-5-7-17/h3-7,9,11-13,16H,8,10,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HeLa cells after 120 mins by scintillation counter in presence of methiothepin |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517531
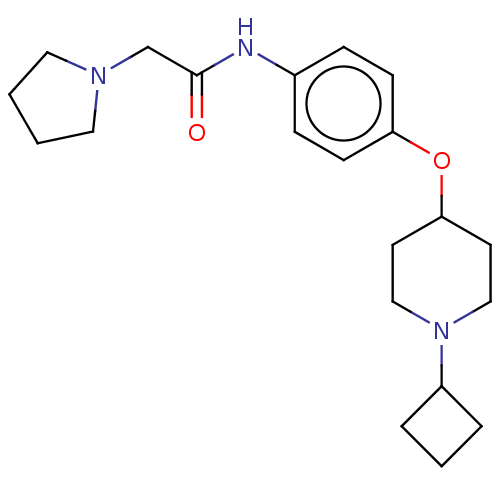
(CHEMBL4445638)Show SMILES Cl.Cl.O=C(CN1CCCC1)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C21H31N3O2/c25-21(16-23-12-1-2-13-23)22-17-6-8-19(9-7-17)26-20-10-14-24(15-11-20)18-4-3-5-18/h6-9,18,20H,1-5,10-16H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517534
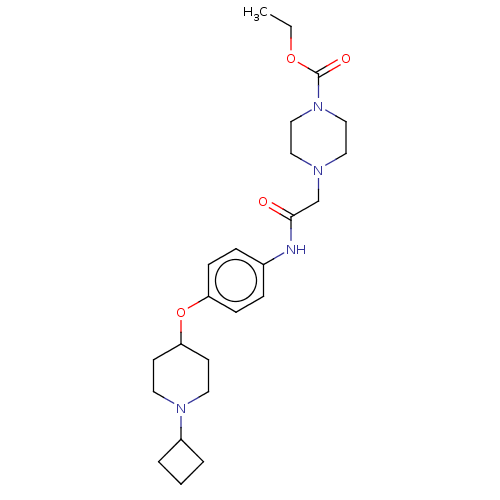
(CHEMBL4476066)Show SMILES Cl.Cl.CCOC(=O)N1CCN(CC(=O)Nc2ccc(OC3CCN(CC3)C3CCC3)cc2)CC1 Show InChI InChI=1S/C24H36N4O4/c1-2-31-24(30)28-16-14-26(15-17-28)18-23(29)25-19-6-8-21(9-7-19)32-22-10-12-27(13-11-22)20-4-3-5-20/h6-9,20,22H,2-5,10-18H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517529
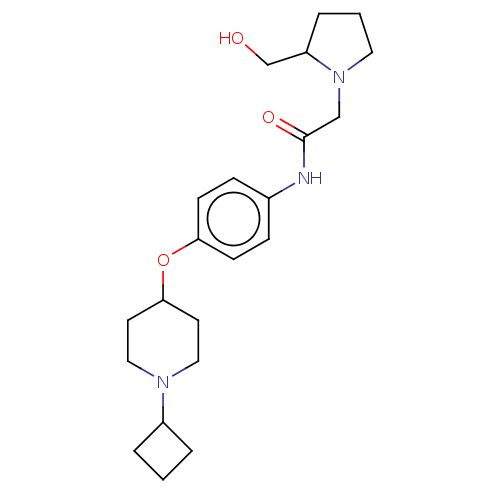
(CHEMBL4587541)Show SMILES Cl.Cl.OCC1CCCN1CC(=O)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C22H33N3O3.2ClH/c26-16-19-5-2-12-25(19)15-22(27)23-17-6-8-20(9-7-17)28-21-10-13-24(14-11-21)18-3-1-4-18;;/h6-9,18-19,21,26H,1-5,10-16H2,(H,23,27);2*1H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236846
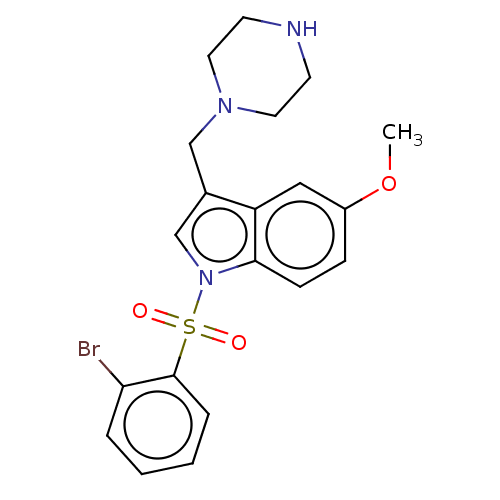
(CHEMBL4080401)Show SMILES O.O.CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCNCC3)c2c1)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C20H22BrN3O3S/c1-27-16-6-7-19-17(12-16)15(13-23-10-8-22-9-11-23)14-24(19)28(25,26)20-5-3-2-4-18(20)21/h2-7,12,14,22H,8-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mammalian Geranylgeranyl transferase type I expressed in baculovirus |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50044616
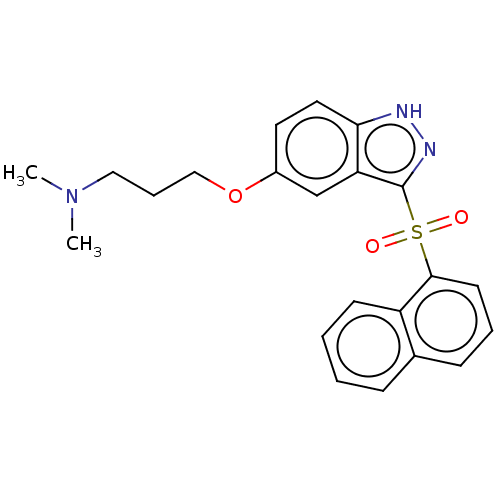
(Cerlapirdine | PF-05212365 | SAM-531 | WAY-262531)Show SMILES CN(C)CCCOc1ccc2[nH]nc(c2c1)S(=O)(=O)c1cccc2ccccc12 Show InChI InChI=1S/C22H23N3O3S/c1-25(2)13-6-14-28-17-11-12-20-19(15-17)22(24-23-20)29(26,27)21-10-5-8-16-7-3-4-9-18(16)21/h3-5,7-12,15H,6,13-14H2,1-2H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for agonistic activity at Metabotropic glutamate receptor 2 |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50163069
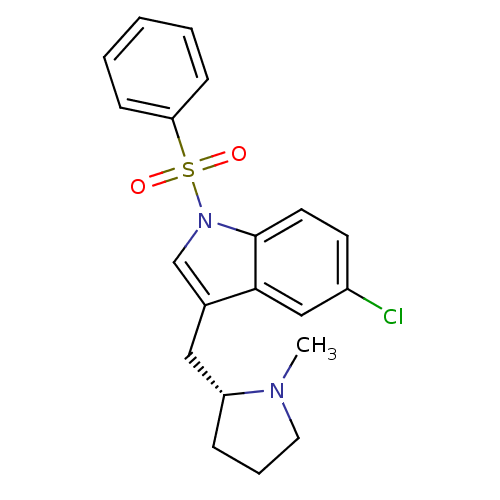
(1-Benzenesulfonyl-5-chloro-3-((R)-1-methyl-pyrroli...)Show SMILES CN1CCC[C@@H]1Cc1cn(c2ccc(Cl)cc12)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H21ClN2O2S/c1-22-11-5-6-17(22)12-15-14-23(20-10-9-16(21)13-19(15)20)26(24,25)18-7-3-2-4-8-18/h2-4,7-10,13-14,17H,5-6,11-12H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT6 receptor |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395860

(CHEMBL2165520)Show InChI InChI=1S/C17H16N2O2S/c18-14-9-12-5-4-8-16-17(12)13(10-14)11-19(16)22(20,21)15-6-2-1-3-7-15/h1-8,11,14H,9-10,18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT6 receptor |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236844
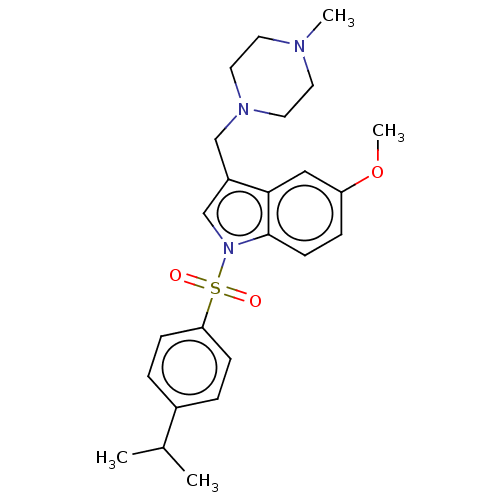
(CHEMBL4100484)Show SMILES CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C24H31N3O3S/c1-18(2)19-5-8-22(9-6-19)31(28,29)27-17-20(16-26-13-11-25(3)12-14-26)23-15-21(30-4)7-10-24(23)27/h5-10,15,17-18H,11-14,16H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517507
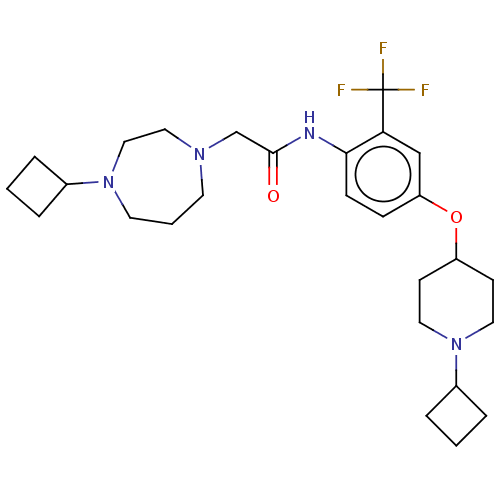
(CHEMBL4456037)Show SMILES FC(F)(F)c1cc(OC2CCN(CC2)C2CCC2)ccc1NC(=O)CN1CCCN(CC1)C1CCC1 Show InChI InChI=1S/C27H39F3N4O2/c28-27(29,30)24-18-23(36-22-10-14-34(15-11-22)21-6-2-7-21)8-9-25(24)31-26(35)19-32-12-3-13-33(17-16-32)20-4-1-5-20/h8-9,18,20-22H,1-7,10-17,19H2,(H,31,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236780
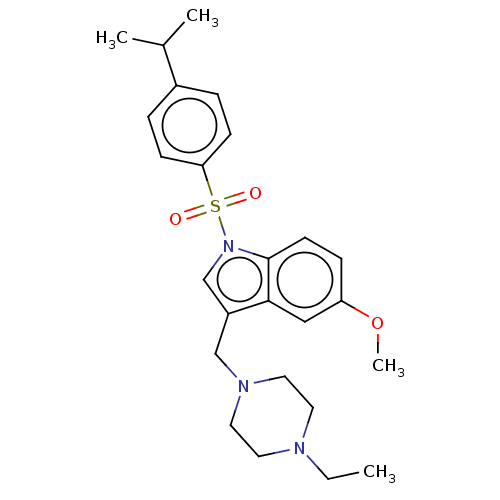
(CHEMBL4068232)Show SMILES CCN1CCN(Cc2cn(c3ccc(OC)cc23)S(=O)(=O)c2ccc(cc2)C(C)C)CC1 Show InChI InChI=1S/C25H33N3O3S/c1-5-26-12-14-27(15-13-26)17-21-18-28(25-11-8-22(31-4)16-24(21)25)32(29,30)23-9-6-20(7-10-23)19(2)3/h6-11,16,18-19H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395855

(CHEMBL2165524)Show SMILES CN(C)C1CCc2c(C1)c1cc(Br)ccc1n2S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C20H21BrN2O2S/c1-22(2)15-9-11-20-18(13-15)17-12-14(21)8-10-19(17)23(20)26(24,25)16-6-4-3-5-7-16/h3-8,10,12,15H,9,11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517523
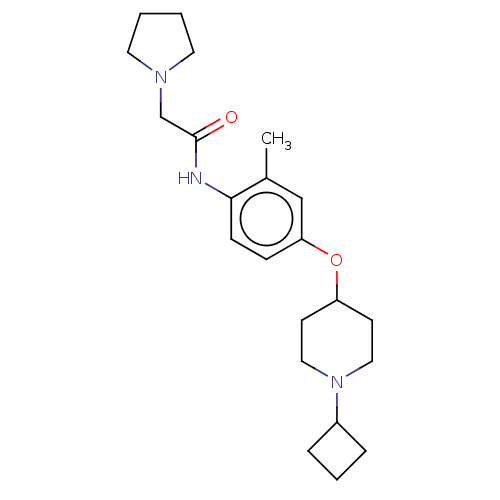
(CHEMBL4518550)Show SMILES Cl.Cl.Cc1cc(OC2CCN(CC2)C2CCC2)ccc1NC(=O)CN1CCCC1 Show InChI InChI=1S/C22H33N3O2/c1-17-15-20(27-19-9-13-25(14-10-19)18-5-4-6-18)7-8-21(17)23-22(26)16-24-11-2-3-12-24/h7-8,15,18-19H,2-6,9-14,16H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395838
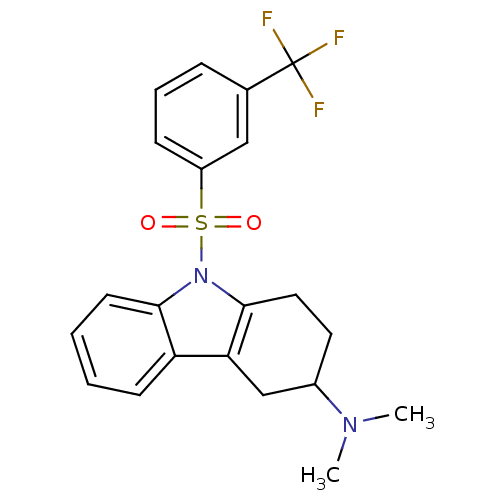
(CHEMBL2165541)Show SMILES CN(C)C1CCc2c(C1)c1ccccc1n2S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C21H21F3N2O2S/c1-25(2)15-10-11-20-18(13-15)17-8-3-4-9-19(17)26(20)29(27,28)16-7-5-6-14(12-16)21(22,23)24/h3-9,12,15H,10-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236756
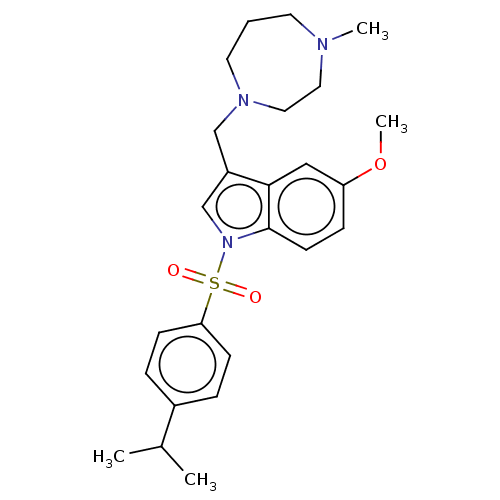
(CHEMBL4101284)Show SMILES Cl.Cl.COc1ccc2n(cc(CN3CCCN(C)CC3)c2c1)S(=O)(=O)c1ccc(cc1)C(C)C Show InChI InChI=1S/C25H33N3O3S/c1-19(2)20-6-9-23(10-7-20)32(29,30)28-18-21(17-27-13-5-12-26(3)14-15-27)24-16-22(31-4)8-11-25(24)28/h6-11,16,18-19H,5,12-15,17H2,1-4H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236754
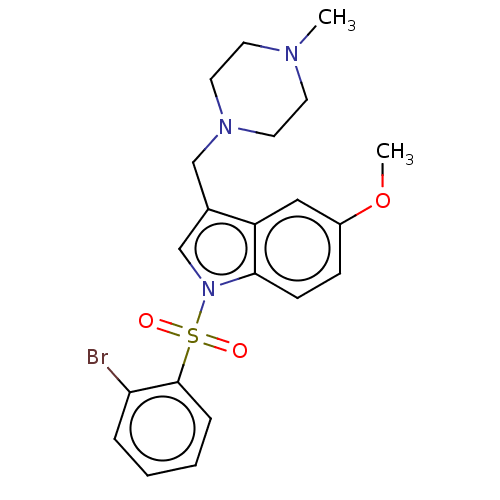
(CHEMBL4082473)Show SMILES O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C21H24BrN3O3S/c1-23-9-11-24(12-10-23)14-16-15-25(20-8-7-17(28-2)13-18(16)20)29(26,27)21-6-4-3-5-19(21)22/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Effective concentration required for partial agonistic activity at Metabotropic glutamate receptor 2; Partial agonist |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236773
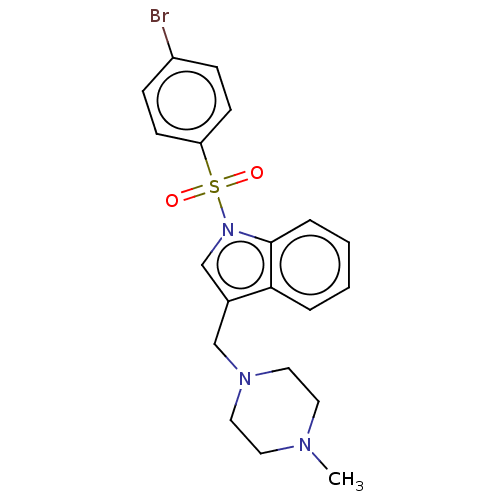
(CHEMBL4065437)Show SMILES CN1CCN(Cc2cn(c3ccccc23)S(=O)(=O)c2ccc(Br)cc2)CC1 Show InChI InChI=1S/C20H22BrN3O2S/c1-22-10-12-23(13-11-22)14-16-15-24(20-5-3-2-4-19(16)20)27(25,26)18-8-6-17(21)7-9-18/h2-9,15H,10-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50130274
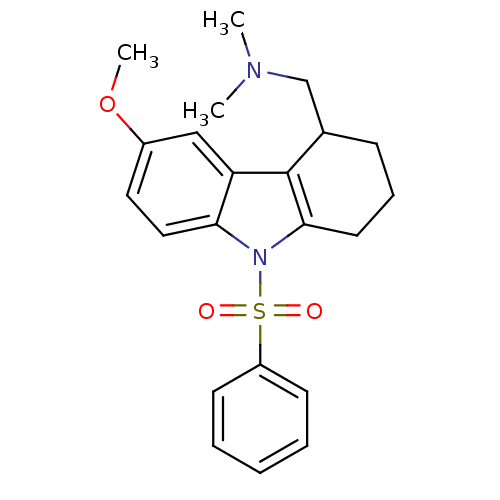
((9-Benzenesulfonyl-6-methoxy-2,3,4,9-tetrahydro-1H...)Show SMILES COc1ccc2n(c3CCCC(CN(C)C)c3c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C22H26N2O3S/c1-23(2)15-16-8-7-11-21-22(16)19-14-17(27-3)12-13-20(19)24(21)28(25,26)18-9-5-4-6-10-18/h4-6,9-10,12-14,16H,7-8,11,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT6 receptor |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM34141
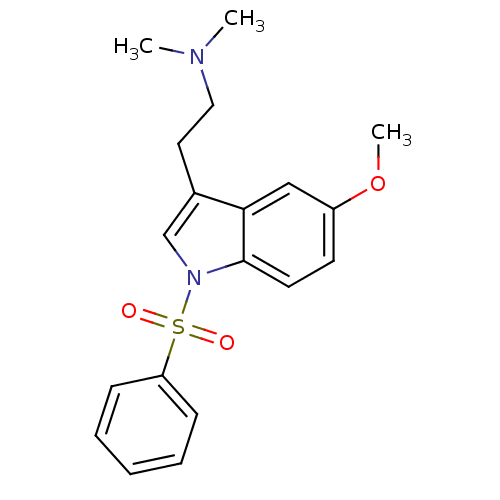
(CHEMBL76237 | MS-245)Show SMILES COc1ccc2n(cc(CCN(C)C)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C19H22N2O3S/c1-20(2)12-11-15-14-21(19-10-9-16(24-3)13-18(15)19)25(22,23)17-7-5-4-6-8-17/h4-10,13-14H,11-12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human 5-HT6 receptor |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517505
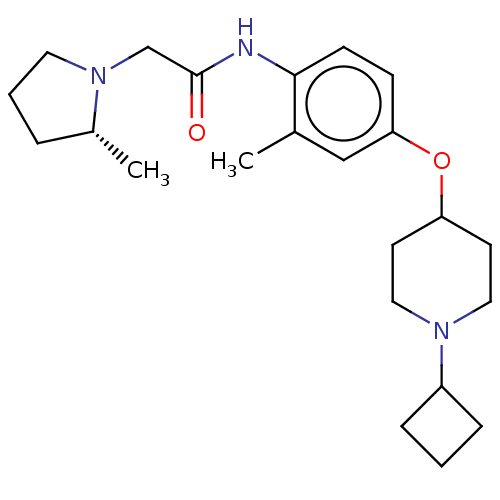
(CHEMBL4521909)Show SMILES C[C@@H]1CCCN1CC(=O)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1C |r| Show InChI InChI=1S/C23H35N3O2/c1-17-15-21(28-20-10-13-25(14-11-20)19-6-3-7-19)8-9-22(17)24-23(27)16-26-12-4-5-18(26)2/h8-9,15,18-20H,3-7,10-14,16H2,1-2H3,(H,24,27)/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517521
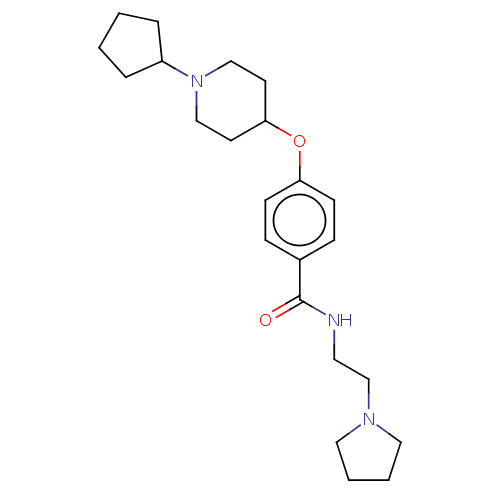
(CHEMBL4574463)Show SMILES O=C(NCCN1CCCC1)c1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C23H35N3O2/c27-23(24-13-18-25-14-3-4-15-25)19-7-9-21(10-8-19)28-22-11-16-26(17-12-22)20-5-1-2-6-20/h7-10,20,22H,1-6,11-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517522
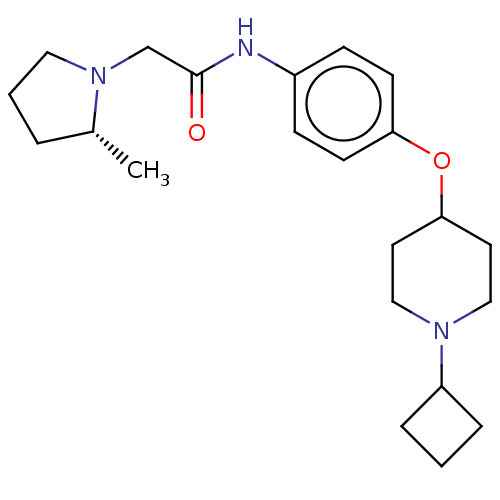
(CHEMBL4534815)Show SMILES C[C@@H]1CCCN1CC(=O)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C22H33N3O2/c1-17-4-3-13-25(17)16-22(26)23-18-7-9-20(10-8-18)27-21-11-14-24(15-12-21)19-5-2-6-19/h7-10,17,19,21H,2-6,11-16H2,1H3,(H,23,26)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517502
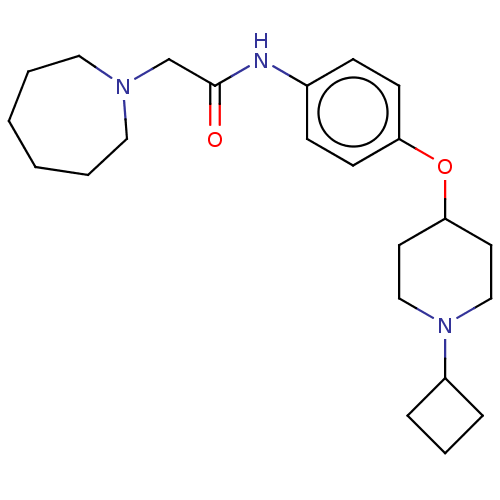
(CHEMBL4439392)Show SMILES O[C@H]([C@@H](O)C(O)=O)C(O)=O.O=C(CN1CCCCCC1)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C23H35N3O2/c27-23(18-25-14-3-1-2-4-15-25)24-19-8-10-21(11-9-19)28-22-12-16-26(17-13-22)20-6-5-7-20/h8-11,20,22H,1-7,12-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395839

(CHEMBL2165540)Show SMILES CSc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C21H23ClN2O2S2/c1-23(2)15-7-9-20-18(12-15)19-13-16(27-3)8-10-21(19)24(20)28(25,26)17-6-4-5-14(22)11-17/h4-6,8,10-11,13,15H,7,9,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517540

(CHEMBL4452215)Show SMILES O=C(NCCN1CCOCC1)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C22H33N3O3/c26-22(23-10-13-24-14-16-27-17-15-24)18-4-6-20(7-5-18)28-21-8-11-25(12-9-21)19-2-1-3-19/h4-7,19,21H,1-3,8-17H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395886
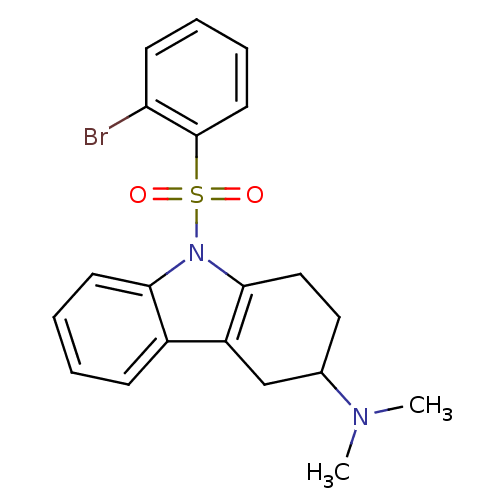
(CHEMBL2165545)Show SMILES CN(C)C1CCc2c(C1)c1ccccc1n2S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C20H21BrN2O2S/c1-22(2)14-11-12-19-16(13-14)15-7-3-5-9-18(15)23(19)26(24,25)20-10-6-4-8-17(20)21/h3-10,14H,11-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517527
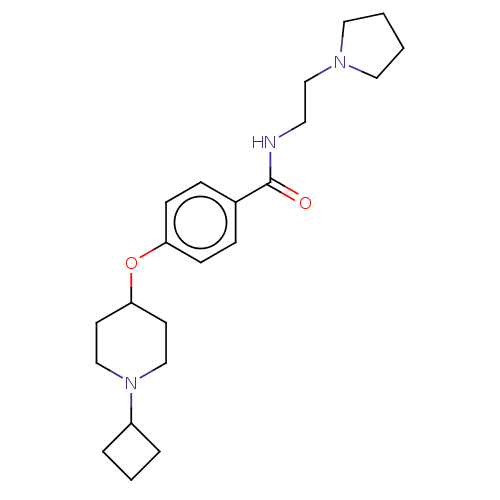
(CHEMBL4576724)Show InChI InChI=1S/C22H33N3O2/c26-22(23-12-17-24-13-1-2-14-24)18-6-8-20(9-7-18)27-21-10-15-25(16-11-21)19-4-3-5-19/h6-9,19,21H,1-5,10-17H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517491
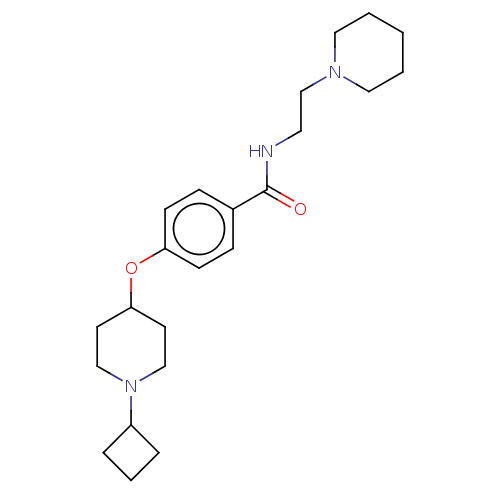
(CHEMBL4468778)Show SMILES O[C@H]([C@@H](O)C(O)=O)C(O)=O.O=C(NCCN1CCCCC1)c1ccc(OC2CCN(CC2)C2CCC2)cc1 |r| Show InChI InChI=1S/C23H35N3O2/c27-23(24-13-18-25-14-2-1-3-15-25)19-7-9-21(10-8-19)28-22-11-16-26(17-12-22)20-5-4-6-20/h7-10,20,22H,1-6,11-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517506
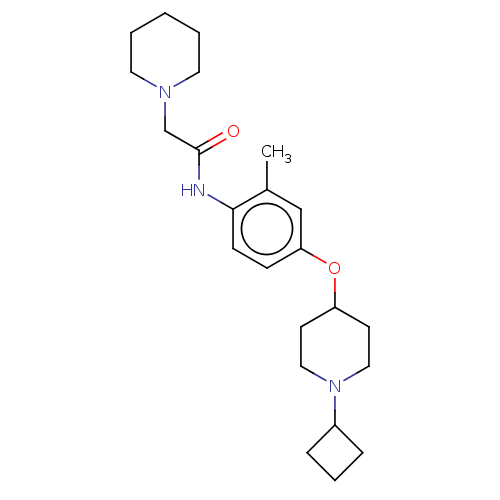
(CHEMBL4476420)Show SMILES Cl.Cl.Cc1cc(OC2CCN(CC2)C2CCC2)ccc1NC(=O)CN1CCCCC1 Show InChI InChI=1S/C23H35N3O2/c1-18-16-21(28-20-10-14-26(15-11-20)19-6-5-7-19)8-9-22(18)24-23(27)17-25-12-3-2-4-13-25/h8-9,16,19-20H,2-7,10-15,17H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395879
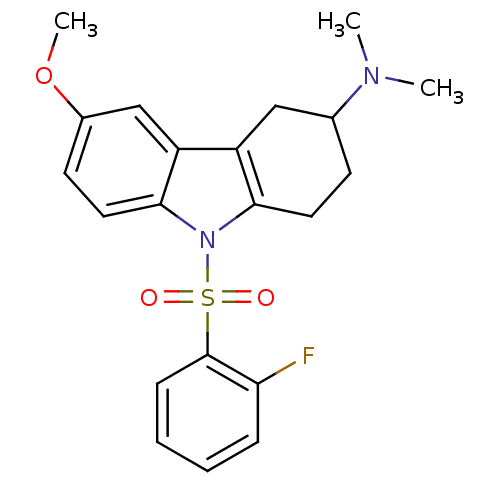
(CHEMBL2165552)Show SMILES COc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1ccccc1F Show InChI InChI=1S/C21H23FN2O3S/c1-23(2)14-8-10-19-16(12-14)17-13-15(27-3)9-11-20(17)24(19)28(25,26)21-7-5-4-6-18(21)22/h4-7,9,11,13-14H,8,10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395835

(CHEMBL2165544)Show SMILES COc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C22H23F3N2O3S/c1-26(2)15-7-9-20-18(12-15)19-13-16(30-3)8-10-21(19)27(20)31(28,29)17-6-4-5-14(11-17)22(23,24)25/h4-6,8,10-11,13,15H,7,9,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517528

(CHEMBL4476745)Show SMILES Cl.Cl.CC(C)N1CCC(CC1)Oc1ccc(NC(=O)CN2CCCCC2)cc1 Show InChI InChI=1S/C21H33N3O2/c1-17(2)24-14-10-20(11-15-24)26-19-8-6-18(7-9-19)22-21(25)16-23-12-4-3-5-13-23/h6-9,17,20H,3-5,10-16H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517532
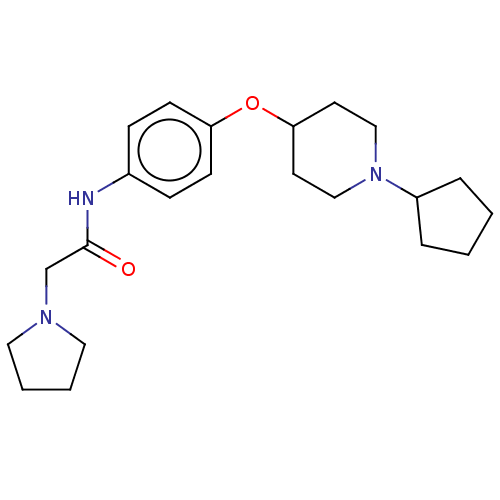
(CHEMBL4450961)Show InChI InChI=1S/C22H33N3O2/c26-22(17-24-13-3-4-14-24)23-18-7-9-20(10-8-18)27-21-11-15-25(16-12-21)19-5-1-2-6-19/h7-10,19,21H,1-6,11-17H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236832

(CHEMBL4093699)Show SMILES Cl.Cl.CC(C)c1ccc(cc1)S(=O)(=O)n1cc(CN2CCN(C)CC2)c2cc(F)ccc12 Show InChI InChI=1S/C23H28FN3O2S/c1-17(2)18-4-7-21(8-5-18)30(28,29)27-16-19(15-26-12-10-25(3)11-13-26)22-14-20(24)6-9-23(22)27/h4-9,14,16-17H,10-13,15H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517504
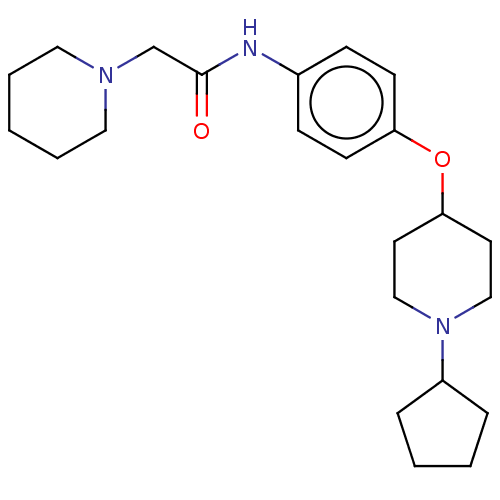
(CHEMBL4464101)Show SMILES Cl.Cl.O=C(CN1CCCCC1)Nc1ccc(OC2CCN(CC2)C2CCCC2)cc1 Show InChI InChI=1S/C23H35N3O2/c27-23(18-25-14-4-1-5-15-25)24-19-8-10-21(11-9-19)28-22-12-16-26(17-13-22)20-6-2-3-7-20/h8-11,20,22H,1-7,12-18H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517494

(CHEMBL4561912)Show SMILES OCC1CN(CC(=O)Nc2ccc(OC3CCN(CC3)C3CCC3)cc2)CCO1 Show InChI InChI=1S/C22H33N3O4/c26-16-21-14-24(12-13-28-21)15-22(27)23-17-4-6-19(7-5-17)29-20-8-10-25(11-9-20)18-2-1-3-18/h4-7,18,20-21,26H,1-3,8-16H2,(H,23,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395841
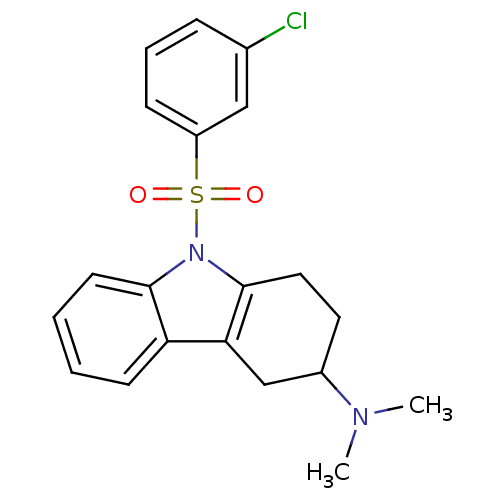
(CHEMBL2165538)Show SMILES CN(C)C1CCc2c(C1)c1ccccc1n2S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C20H21ClN2O2S/c1-22(2)15-10-11-20-18(13-15)17-8-3-4-9-19(17)23(20)26(24,25)16-7-5-6-14(21)12-16/h3-9,12,15H,10-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395883

(CHEMBL2165548)Show SMILES COc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1ccccc1Br Show InChI InChI=1S/C21H23BrN2O3S/c1-23(2)14-8-10-19-16(12-14)17-13-15(27-3)9-11-20(17)24(19)28(25,26)21-7-5-4-6-18(21)22/h4-7,9,11,13-14H,8,10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236806

(CHEMBL4100411)Show SMILES COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H25N3O3S/c1-22-10-12-23(13-11-22)15-17-16-24(21-9-8-18(27-2)14-20(17)21)28(25,26)19-6-4-3-5-7-19/h3-9,14,16H,10-13,15H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mammalian Geranylgeranyl transferase type I expressed in baculovirus |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236831

(CHEMBL4077537)Show SMILES Cl.Cl.Brc1ccccc1S(=O)(=O)n1cc(CN2CCNCC2)c2ccccc12 Show InChI InChI=1S/C19H20BrN3O2S/c20-17-6-2-4-8-19(17)26(24,25)23-14-15(13-22-11-9-21-10-12-22)16-5-1-3-7-18(16)23/h1-8,14,21H,9-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Herpes simplex virus type 1 thymidine kinase(HSV-1 TK) |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395851

(CHEMBL2165528)Show SMILES CSc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C21H24N2O2S2/c1-22(2)15-9-11-20-18(13-15)19-14-16(26-3)10-12-21(19)23(20)27(24,25)17-7-5-4-6-8-17/h4-8,10,12,14-15H,9,11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395881

(CHEMBL2165550)Show SMILES COc1ccc2n(c3CCC(Cc3c2c1)N(C)C)S(=O)(=O)c1ccccc1Cl Show InChI InChI=1S/C21H23ClN2O3S/c1-23(2)14-8-10-19-16(12-14)17-13-15(27-3)9-11-20(17)24(19)28(25,26)21-7-5-4-6-18(21)22/h4-7,9,11,13-14H,8,10,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517501

(CHEMBL4578297)Show SMILES Cl.Cl.O=C(CN1CCCCC1)Nc1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C22H33N3O2/c26-22(17-24-13-2-1-3-14-24)23-18-7-9-20(10-8-18)27-21-11-15-25(16-12-21)19-5-4-6-19/h7-10,19,21H,1-6,11-17H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50517519
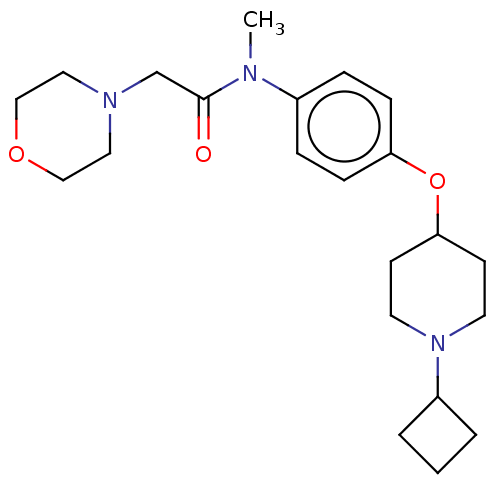
(CHEMBL4527873)Show SMILES CN(C(=O)CN1CCOCC1)c1ccc(OC2CCN(CC2)C2CCC2)cc1 Show InChI InChI=1S/C22H33N3O3/c1-23(22(26)17-24-13-15-27-16-14-24)18-5-7-20(8-6-18)28-21-9-11-25(12-10-21)19-3-2-4-19/h5-8,19,21H,2-4,9-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting |
J Med Chem 62: 1203-1217 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01280
BindingDB Entry DOI: 10.7270/Q2XD152G |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50236836
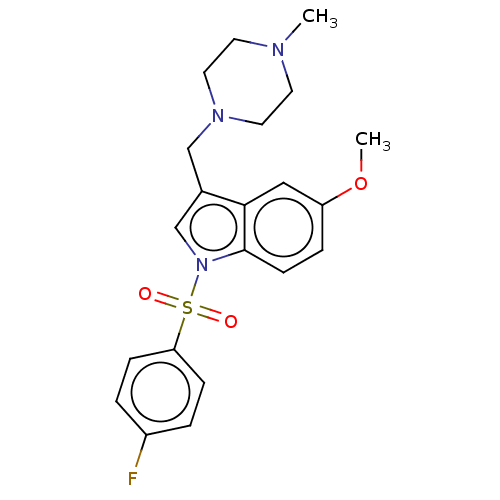
(CHEMBL4073586)Show SMILES CS(O)(=O)=O.CS(O)(=O)=O.COc1ccc2n(cc(CN3CCN(C)CC3)c2c1)S(=O)(=O)c1ccc(F)cc1 Show InChI InChI=1S/C21H24FN3O3S/c1-23-9-11-24(12-10-23)14-16-15-25(21-8-5-18(28-2)13-20(16)21)29(26,27)19-6-3-17(22)4-7-19/h3-8,13,15H,9-12,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]-LSD from recombinant human 5-HT6 receptor expressed in HEK293 cells after 60 mins |
J Med Chem 60: 1843-1859 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01662
BindingDB Entry DOI: 10.7270/Q27S7R1X |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 6
(Homo sapiens (Human)) | BDBM50395840
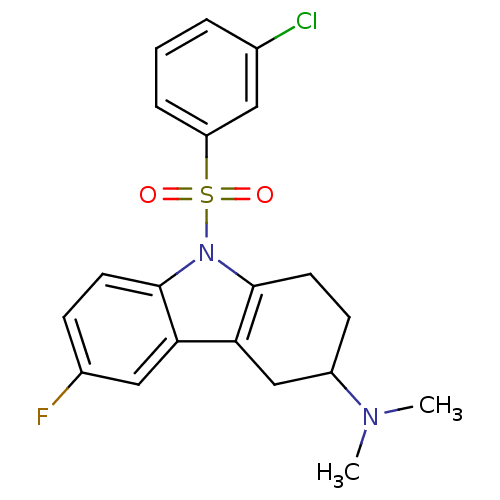
(CHEMBL2165539)Show SMILES CN(C)C1CCc2c(C1)c1cc(F)ccc1n2S(=O)(=O)c1cccc(Cl)c1 Show InChI InChI=1S/C20H20ClFN2O2S/c1-23(2)15-7-9-20-18(12-15)17-11-14(22)6-8-19(17)24(20)27(25,26)16-5-3-4-13(21)10-16/h3-6,8,10-11,15H,7,9,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd
Curated by ChEMBL
| Assay Description
Displacement of [3H]LSD from human cloned 5-HT6 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 22: 6980-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.002
BindingDB Entry DOI: 10.7270/Q2QZ2C3F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data