 Found 1327 hits with Last Name = 'green' and Initial = 'l'
Found 1327 hits with Last Name = 'green' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B2 bradykinin receptor
(Cavia porcellus) | BDBM50406750

(Firazyr | HOE-140 | Icatibant)Show SMILES [H][C@]12C[C@H](N(C(=O)[C@H]3Cc4ccccc4CN3C(=O)[C@H](CO)NC(=O)[C@H](Cc3cccs3)NC(=O)CNC(=O)[C@@H]3C[C@@H](O)CN3C(=O)[C@@H]3CCCN3C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](N)CCCNC(N)=N)[C@@]1([H])CCCC2)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O Show InChI InChI=1S/C59H89N19O13S/c60-37(14-5-19-67-57(61)62)48(82)72-38(15-6-20-68-58(63)64)52(86)75-22-8-18-43(75)54(88)77-30-35(80)26-44(77)50(84)70-28-47(81)71-40(27-36-13-9-23-92-36)49(83)74-41(31-79)53(87)76-29-34-12-2-1-10-32(34)24-46(76)55(89)78-42-17-4-3-11-33(42)25-45(78)51(85)73-39(56(90)91)16-7-21-69-59(65)66/h1-2,9-10,12-13,23,33,35,37-46,79-80H,3-8,11,14-22,24-31,60H2,(H,70,84)(H,71,81)(H,72,82)(H,73,85)(H,74,83)(H,90,91)(H4,61,62,67)(H4,63,64,68)(H4,65,66,69)/t33-,35+,37+,38-,39-,40-,41-,42-,43-,44-,45-,46+/m0/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. |
J Med Chem 36: 1450-60 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1QS1 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Cavia porcellus) | BDBM50406751
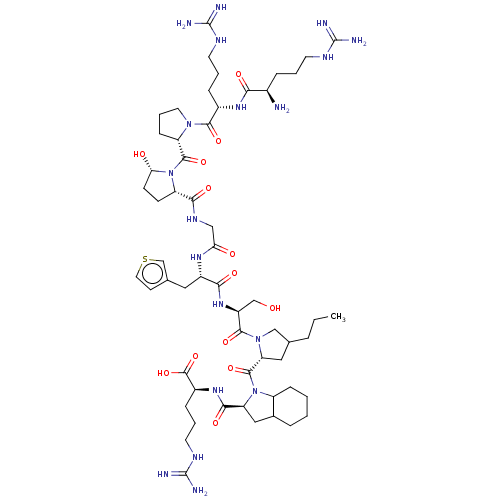
(CHEMBL2369941)Show SMILES [#6]-[#6]-[#6]-[#6]-1-[#6]-[#6@@H](-[#7](-[#6]-1)-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccsc1)-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6@H](-[#8])-[#7]-1-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6@@H](-[#6]-[#6]-2-[#6]-[#6]-[#6]-[#6]-[#6]-1-2)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#8])=O |r| Show InChI InChI=1S/C57H93N19O13S/c1-2-9-31-25-43(53(87)75-39-14-4-3-10-33(39)26-42(75)49(83)71-36(54(88)89)13-7-21-67-57(63)64)74(28-31)51(85)38(29-77)72-47(81)37(24-32-18-23-90-30-32)69-44(78)27-68-48(82)40-16-17-45(79)76(40)52(86)41-15-8-22-73(41)50(84)35(12-6-20-66-56(61)62)70-46(80)34(58)11-5-19-65-55(59)60/h18,23,30-31,33-43,45,77,79H,2-17,19-22,24-29,58H2,1H3,(H,68,82)(H,69,78)(H,70,80)(H,71,83)(H,72,81)(H,88,89)(H4,59,60,65)(H4,61,62,66)(H4,63,64,67)/t31?,33?,34-,35+,36+,37+,38+,39?,40+,41+,42+,43-,45+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. |
J Med Chem 36: 1450-60 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1QS1 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Cavia porcellus) | BDBM50406749
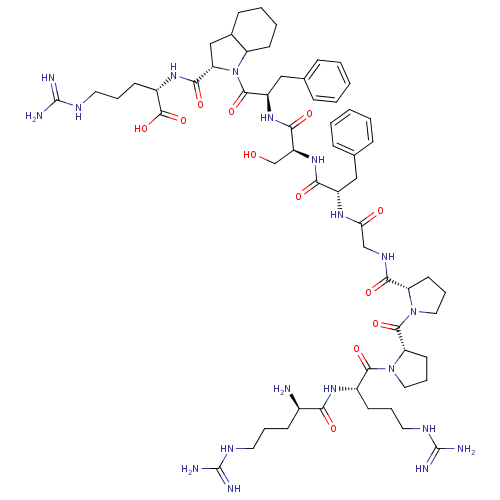
(CHEMBL2028979)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N1CCC[C@H]1C(=O)NCC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@H](Cc1ccccc1)C(=O)N1[C@@H](CC2CCCCC12)C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C60H91N19O12/c61-38(19-9-25-68-58(62)63)49(82)73-39(20-10-26-69-59(64)65)54(87)78-29-13-24-46(78)56(89)77-28-12-23-45(77)52(85)71-33-48(81)72-41(30-35-14-3-1-4-15-35)50(83)76-43(34-80)51(84)75-42(31-36-16-5-2-6-17-36)55(88)79-44-22-8-7-18-37(44)32-47(79)53(86)74-40(57(90)91)21-11-27-70-60(66)67/h1-6,14-17,37-47,80H,7-13,18-34,61H2,(H,71,85)(H,72,81)(H,73,82)(H,74,86)(H,75,84)(H,76,83)(H,90,91)(H4,62,63,68)(H4,64,65,69)(H4,66,67,70)/t37?,38-,39+,40+,41+,42-,43+,44?,45+,46+,47+/m1/s1 | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Scios Nova Inc.
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum. |
J Med Chem 36: 1450-60 (1993)
BindingDB Entry DOI: 10.7270/Q2PG1QS1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50223637

(Alphacemethadone | Alphacetylmethadol)Show SMILES CC[C@@H](OC(C)=O)C(C[C@@H](C)N(C)C)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO2/c1-6-22(26-19(3)25)23(17-18(2)24(4)5,20-13-9-7-10-14-20)21-15-11-8-12-16-21/h7-16,18,22H,6,17H2,1-5H3/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50223633

(Levomethadone)Show SMILES CCC(=O)C(C[C@@H](C)N(C)C)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322986
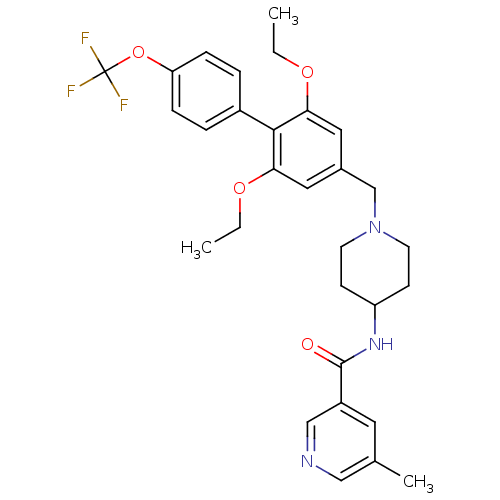
(CHEMBL1210141 | N-(1-((2,6-diethoxy-4'-(trifluorom...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H34F3N3O4/c1-4-38-26-15-21(16-27(39-5-2)28(26)22-6-8-25(9-7-22)40-30(31,32)33)19-36-12-10-24(11-13-36)35-29(37)23-14-20(3)17-34-18-23/h6-9,14-18,24H,4-5,10-13,19H2,1-3H3,(H,35,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322979

(CHEMBL1210209 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(NC)nc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H35FN4O3/c1-4-36-25-16-20(17-26(37-5-2)28(25)21-6-9-23(30)10-7-21)19-34-14-12-24(13-15-34)33-29(35)22-8-11-27(31-3)32-18-22/h6-11,16-18,24H,4-5,12-15,19H2,1-3H3,(H,31,32)(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50223637

(Alphacemethadone | Alphacetylmethadol)Show SMILES CC[C@@H](OC(C)=O)C(C[C@@H](C)N(C)C)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO2/c1-6-22(26-19(3)25)23(17-18(2)24(4)5,20-13-9-7-10-14-20)21-15-11-8-12-16-21/h7-16,18,22H,6,17H2,1-5H3/t18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the presence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322988

(CHEMBL1210084 | N-(1-(3,5-diethoxy-4-methylbenzyl)...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1C Show InChI InChI=1S/C24H33N3O3/c1-5-29-22-12-19(13-23(18(22)4)30-6-2)16-27-9-7-21(8-10-27)26-24(28)20-11-17(3)14-25-15-20/h11-15,21H,5-10,16H2,1-4H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322981
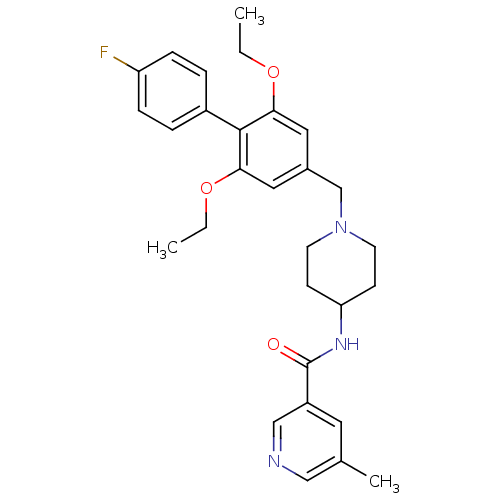
(CHEMBL1210207 | N-(1-((2,6-diethoxy-4'-fluorobiphe...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C29H34FN3O3/c1-4-35-26-15-21(16-27(36-5-2)28(26)22-6-8-24(30)9-7-22)19-33-12-10-25(11-13-33)32-29(34)23-14-20(3)17-31-18-23/h6-9,14-18,25H,4-5,10-13,19H2,1-3H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322987
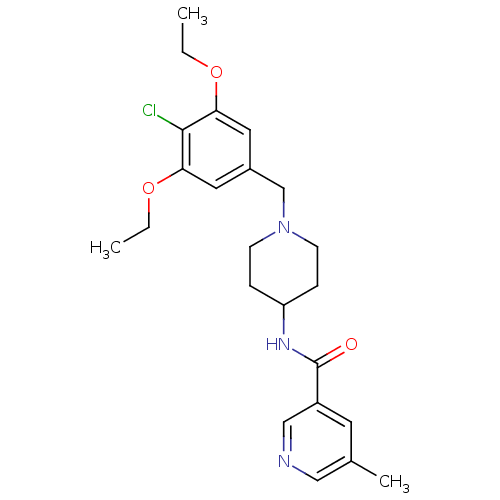
(CHEMBL1210085 | N-(1-(4-chloro-3,5-diethoxybenzyl)...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1Cl Show InChI InChI=1S/C23H30ClN3O3/c1-4-29-20-11-17(12-21(22(20)24)30-5-2)15-27-8-6-19(7-9-27)26-23(28)18-10-16(3)13-25-14-18/h10-14,19H,4-9,15H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322992

(CHEMBL1210030 | N-(1-((2-ethoxy-4'-fluorobiphenyl-...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)ccc1-c1ccc(F)cc1 Show InChI InChI=1S/C27H30FN3O2/c1-3-33-26-15-20(4-9-25(26)21-5-7-23(28)8-6-21)18-31-12-10-24(11-13-31)30-27(32)22-14-19(2)16-29-17-22/h4-9,14-17,24H,3,10-13,18H2,1-2H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322980
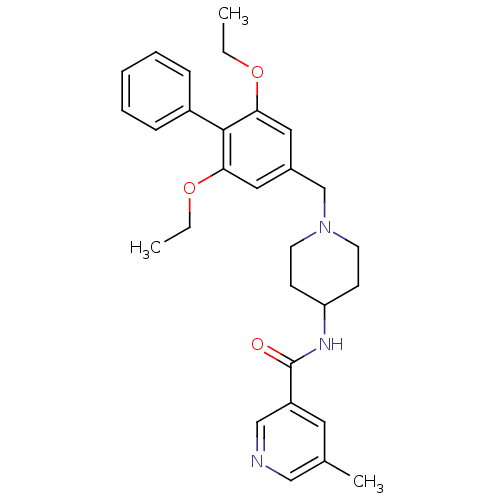
(CHEMBL1210208 | N-(1-((2,6-diethoxybiphenyl-4-yl)m...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccccc1 Show InChI InChI=1S/C29H35N3O3/c1-4-34-26-16-22(17-27(35-5-2)28(26)23-9-7-6-8-10-23)20-32-13-11-25(12-14-32)31-29(33)24-15-21(3)18-30-19-24/h6-10,15-19,25H,4-5,11-14,20H2,1-3H3,(H,31,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322984
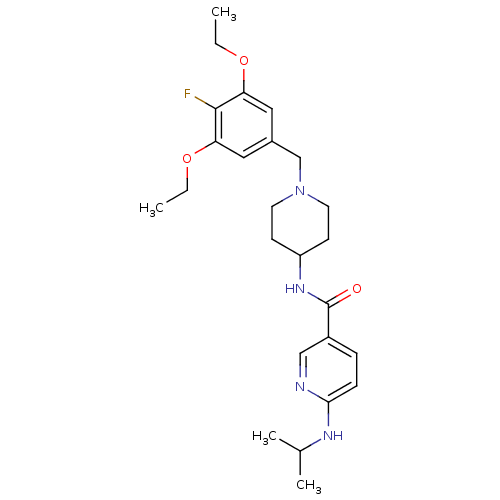
(CHEMBL1210143 | N-(1-(3,5-diethoxy-4-fluorobenzyl)...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(NC(C)C)nc2)cc(OCC)c1F Show InChI InChI=1S/C25H35FN4O3/c1-5-32-21-13-18(14-22(24(21)26)33-6-2)16-30-11-9-20(10-12-30)29-25(31)19-7-8-23(27-15-19)28-17(3)4/h7-8,13-15,17,20H,5-6,9-12,16H2,1-4H3,(H,27,28)(H,29,31) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322978

(6-amino-N-(1-((2-(benzyloxy)-6-ethoxy-4'-fluorobip...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(N)nc2)cc(OCc2ccccc2)c1-c1ccc(F)cc1 Show InChI InChI=1S/C33H35FN4O3/c1-2-40-29-18-24(21-38-16-14-28(15-17-38)37-33(39)26-10-13-31(35)36-20-26)19-30(41-22-23-6-4-3-5-7-23)32(29)25-8-11-27(34)12-9-25/h3-13,18-20,28H,2,14-17,21-22H2,1H3,(H2,35,36)(H,37,39) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322983

(CHEMBL1210144 | N-(1-((2,6-diethoxybiphenyl-4-yl)m...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(NC)nc2)cc(OCC)c1-c1ccccc1 Show InChI InChI=1S/C29H36N4O3/c1-4-35-25-17-21(18-26(36-5-2)28(25)22-9-7-6-8-10-22)20-33-15-13-24(14-16-33)32-29(34)23-11-12-27(30-3)31-19-23/h6-12,17-19,24H,4-5,13-16,20H2,1-3H3,(H,30,31)(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19455
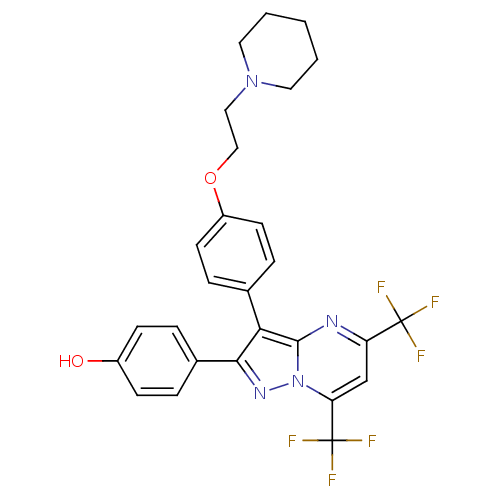
(4-(3-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...)Show SMILES Oc1ccc(cc1)-c1nn2c(cc(nc2c1-c1ccc(OCCN2CCCCC2)cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N4O2/c28-26(29,30)21-16-22(27(31,32)33)37-25(34-21)23(24(35-37)18-4-8-19(38)9-5-18)17-6-10-20(11-7-17)39-15-14-36-12-2-1-3-13-36/h4-11,16,38H,1-3,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | -42.6 | n/a | n/a | 90 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50336997
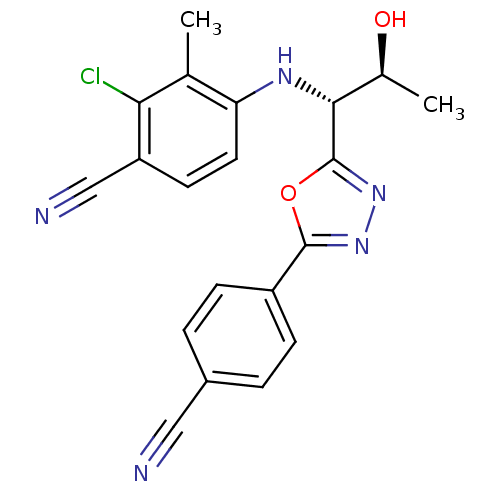
(2-chloro-4-((1R,2S)-1-(5-(4-cyanophenyl)-1,3,4-oxa...)Show SMILES C[C@H](O)[C@@H](Nc1ccc(C#N)c(Cl)c1C)c1nnc(o1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H16ClN5O2/c1-11-16(8-7-15(10-23)17(11)21)24-18(12(2)27)20-26-25-19(28-20)14-5-3-13(9-22)4-6-14/h3-8,12,18,24,27H,1-2H3/t12-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322985
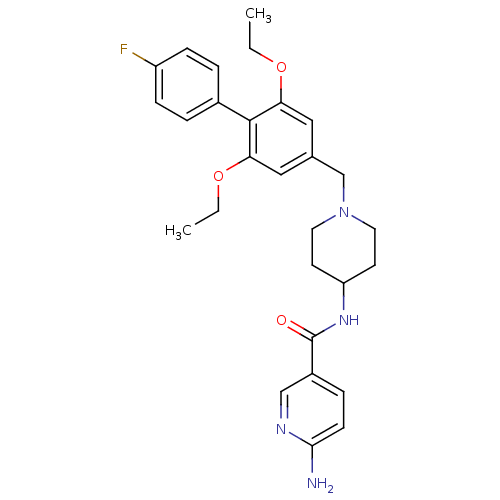
(6-amino-N-(1-((2,6-diethoxy-4'-fluorobiphenyl-4-yl...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(N)nc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C28H33FN4O3/c1-3-35-24-15-19(16-25(36-4-2)27(24)20-5-8-22(29)9-6-20)18-33-13-11-23(12-14-33)32-28(34)21-7-10-26(30)31-17-21/h5-10,15-17,23H,3-4,11-14,18H2,1-2H3,(H2,30,31)(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322982

(6-chloro-N-(1-((2,6-diethoxy-4'-fluorobiphenyl-4-y...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(Cl)nc2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C28H31ClFN3O3/c1-3-35-24-15-19(16-25(36-4-2)27(24)20-5-8-22(30)9-6-20)18-33-13-11-23(12-14-33)32-28(34)21-7-10-26(29)31-17-21/h5-10,15-17,23H,3-4,11-14,18H2,1-2H3,(H,32,34) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322971
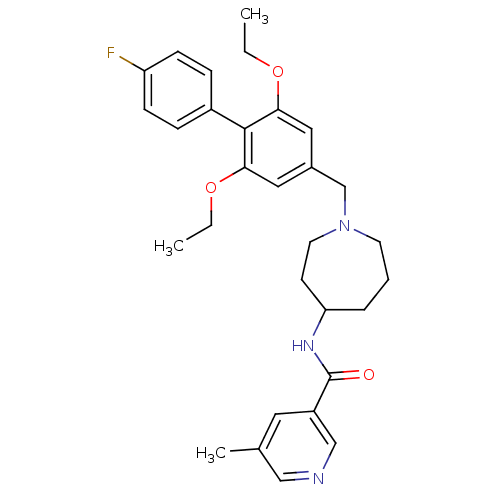
(CHEMBL1210375 | rac-N-(1-((2,6-diethoxy-4'-fluorob...)Show SMILES CCOc1cc(CN2CCCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-c1ccc(F)cc1 Show InChI InChI=1S/C30H36FN3O3/c1-4-36-27-16-22(17-28(37-5-2)29(27)23-8-10-25(31)11-9-23)20-34-13-6-7-26(12-14-34)33-30(35)24-15-21(3)18-32-19-24/h8-11,15-19,26H,4-7,12-14,20H2,1-3H3,(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322989

(CHEMBL1210083 | N-(1-(3,5-diethoxy-4-(1H-imidazol-...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1-n1ccnc1 Show InChI InChI=1S/C26H33N5O3/c1-4-33-23-13-20(14-24(34-5-2)25(23)31-11-8-27-18-31)17-30-9-6-22(7-10-30)29-26(32)21-12-19(3)15-28-16-21/h8,11-16,18,22H,4-7,9-10,17H2,1-3H3,(H,29,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322991
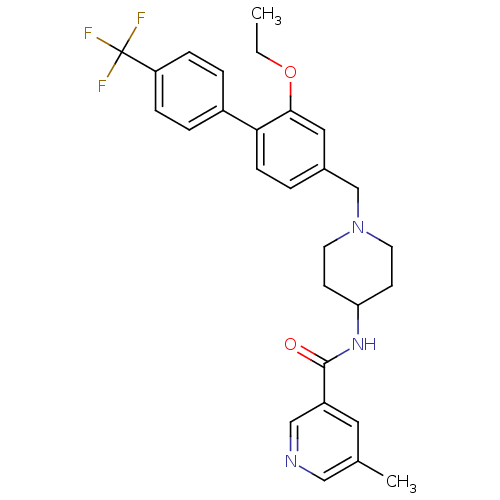
(CHEMBL1210031 | N-(1-((2-ethoxy-4'-(trifluoromethy...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)ccc1-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C28H30F3N3O2/c1-3-36-26-15-20(4-9-25(26)21-5-7-23(8-6-21)28(29,30)31)18-34-12-10-24(11-13-34)33-27(35)22-14-19(2)16-32-17-22/h4-9,14-17,24H,3,10-13,18H2,1-2H3,(H,33,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227099

(2-(1-(4-amino-3,5-diethoxybenzyl)piperidin-4-ylami...)Show SMILES CCOc1cc(CN2CCC(CC2)Nc2nc3cc(ccc3o2)S(N)(=O)=O)cc(OCC)c1N Show InChI InChI=1S/C23H31N5O5S/c1-3-31-20-11-15(12-21(22(20)24)32-4-2)14-28-9-7-16(8-10-28)26-23-27-18-13-17(34(25,29)30)5-6-19(18)33-23/h5-6,11-13,16H,3-4,7-10,14,24H2,1-2H3,(H,26,27)(H2,25,29,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]11-Tyr somatostatin-14 from human SST5R expressed in CHO cells |
J Med Chem 50: 6291-4 (2007)
Article DOI: 10.1021/jm701143p
BindingDB Entry DOI: 10.7270/Q2WS8T0K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227129

(CHEMBL241329 | N-(1-(4-chloro-3-ethoxybenzyl)piper...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)ccc1Cl Show InChI InChI=1S/C21H26ClN3O2/c1-3-27-20-11-16(4-5-19(20)22)14-25-8-6-18(7-9-25)24-21(26)17-10-15(2)12-23-13-17/h4-5,10-13,18H,3,6-9,14H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50223631

(Betacetylmethadol)Show SMILES CC[C@H](OC(C)=O)C(C[C@@H](C)N(C)C)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C23H31NO2/c1-6-22(26-19(3)25)23(17-18(2)24(4)5,20-13-9-7-10-14-20)21-15-11-8-12-16-21/h7-16,18,22H,6,17H2,1-5H3/t18-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the absence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227100
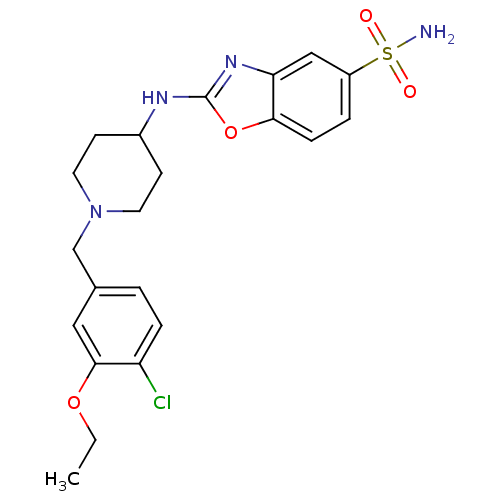
(2-(1-(4-chloro-3-ethoxybenzyl)piperidin-4-ylamino)...)Show SMILES CCOc1cc(CN2CCC(CC2)Nc2nc3cc(ccc3o2)S(N)(=O)=O)ccc1Cl Show InChI InChI=1S/C21H25ClN4O4S/c1-2-29-20-11-14(3-5-17(20)22)13-26-9-7-15(8-10-26)24-21-25-18-12-16(31(23,27)28)4-6-19(18)30-21/h3-6,11-12,15H,2,7-10,13H2,1H3,(H,24,25)(H2,23,27,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]11-Tyr somatostatin-14 from human SST5R expressed in CHO cells |
J Med Chem 50: 6291-4 (2007)
Article DOI: 10.1021/jm701143p
BindingDB Entry DOI: 10.7270/Q2WS8T0K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322977

(CHEMBL1210268 | N-(1-(3,5-diethoxy-4-fluorobenzyl)...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2ccc(NC)nc2)cc(OCC)c1F Show InChI InChI=1S/C23H31FN4O3/c1-4-30-19-12-16(13-20(22(19)24)31-5-2)15-28-10-8-18(9-11-28)27-23(29)17-6-7-21(25-3)26-14-17/h6-7,12-14,18H,4-5,8-11,15H2,1-3H3,(H,25,26)(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322976
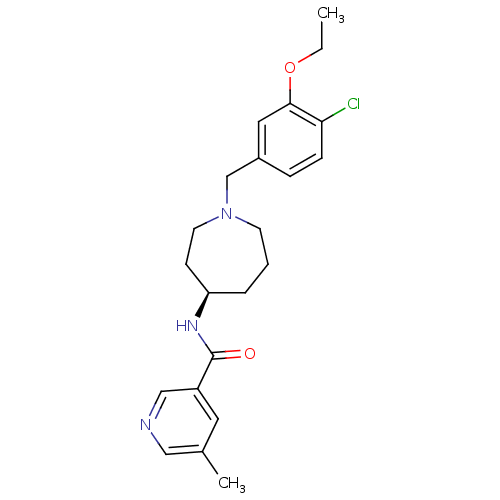
((R)-N-(1-(4-chloro-3-ethoxybenzyl)azepan-4-yl)-5-m...)Show SMILES CCOc1cc(CN2CCC[C@H](CC2)NC(=O)c2cncc(C)c2)ccc1Cl |r| Show InChI InChI=1S/C22H28ClN3O2/c1-3-28-21-12-17(6-7-20(21)23)15-26-9-4-5-19(8-10-26)25-22(27)18-11-16(2)13-24-14-18/h6-7,11-14,19H,3-5,8-10,15H2,1-2H3,(H,25,27)/t19-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322993
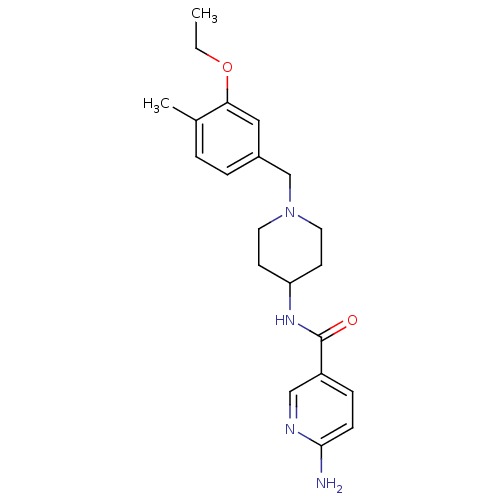
(6-amino-N-(1-(3-ethoxy-4-methylbenzyl)piperidin-4-...)Show InChI InChI=1S/C21H28N4O2/c1-3-27-19-12-16(5-4-15(19)2)14-25-10-8-18(9-11-25)24-21(26)17-6-7-20(22)23-13-17/h4-7,12-13,18H,3,8-11,14H2,1-2H3,(H2,22,23)(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227132
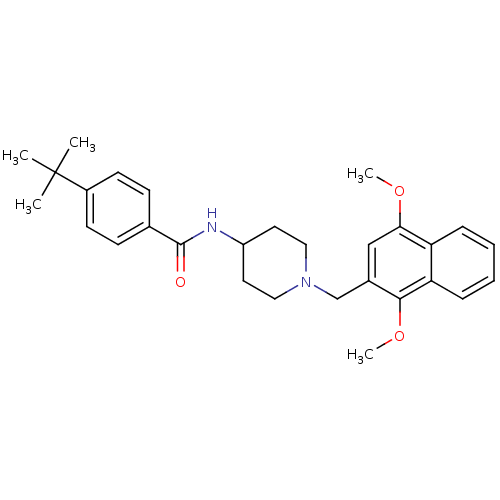
(4-tert-butyl-N-(1-((1,4-dimethoxynaphthalen-2-yl)m...)Show SMILES COc1cc(CN2CCC(CC2)NC(=O)c2ccc(cc2)C(C)(C)C)c(OC)c2ccccc12 Show InChI InChI=1S/C29H36N2O3/c1-29(2,3)22-12-10-20(11-13-22)28(32)30-23-14-16-31(17-15-23)19-21-18-26(33-4)24-8-6-7-9-25(24)27(21)34-5/h6-13,18,23H,14-17,19H2,1-5H3,(H,30,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human SST5R |
J Med Chem 50: 6295-8 (2007)
Article DOI: 10.1021/jm701144e
BindingDB Entry DOI: 10.7270/Q2S18273 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227129

(CHEMBL241329 | N-(1-(4-chloro-3-ethoxybenzyl)piper...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)ccc1Cl Show InChI InChI=1S/C21H26ClN3O2/c1-3-27-20-11-16(4-5-19(20)22)14-25-8-6-18(7-9-25)24-21(26)17-10-15(2)12-23-13-17/h4-5,10-13,18H,3,6-9,14H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human SST5R |
J Med Chem 50: 6295-8 (2007)
Article DOI: 10.1021/jm701144e
BindingDB Entry DOI: 10.7270/Q2S18273 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322994
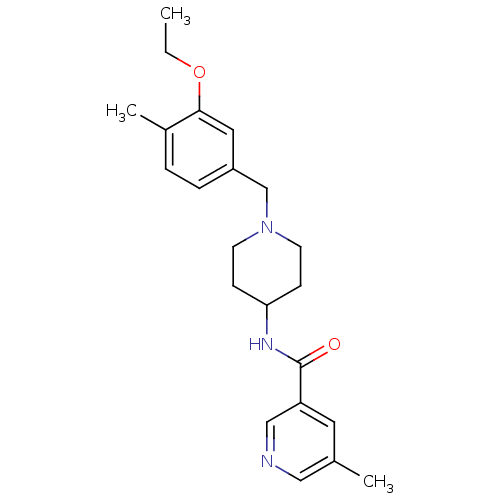
(CHEMBL1210028 | N-(1-(3-ethoxy-4-methylbenzyl)pipe...)Show InChI InChI=1S/C22H29N3O2/c1-4-27-21-12-18(6-5-17(21)3)15-25-9-7-20(8-10-25)24-22(26)19-11-16(2)13-23-14-19/h5-6,11-14,20H,4,7-10,15H2,1-3H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322974
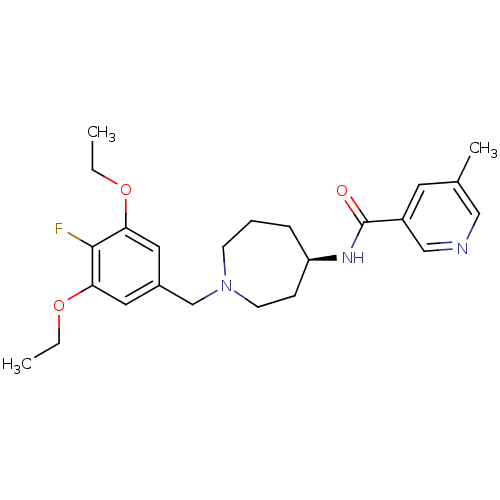
((R)-N-(1-(3,5-diethoxy-4-fluorobenzyl)azepan-4-yl)...)Show SMILES CCOc1cc(CN2CCC[C@H](CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1F |r| Show InChI InChI=1S/C24H32FN3O3/c1-4-30-21-12-18(13-22(23(21)25)31-5-2)16-28-9-6-7-20(8-10-28)27-24(29)19-11-17(3)14-26-15-19/h11-15,20H,4-10,16H2,1-3H3,(H,27,29)/t20-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322972
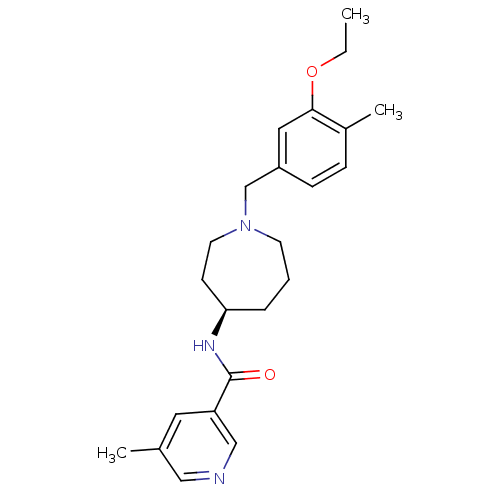
((R)-N-(1-(3-ethoxy-4-methylbenzyl)azepan-4-yl)-5-m...)Show SMILES CCOc1cc(CN2CCC[C@H](CC2)NC(=O)c2cncc(C)c2)ccc1C |r| Show InChI InChI=1S/C23H31N3O2/c1-4-28-22-13-19(8-7-18(22)3)16-26-10-5-6-21(9-11-26)25-23(27)20-12-17(2)14-24-15-20/h7-8,12-15,21H,4-6,9-11,16H2,1-3H3,(H,25,27)/t21-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227119
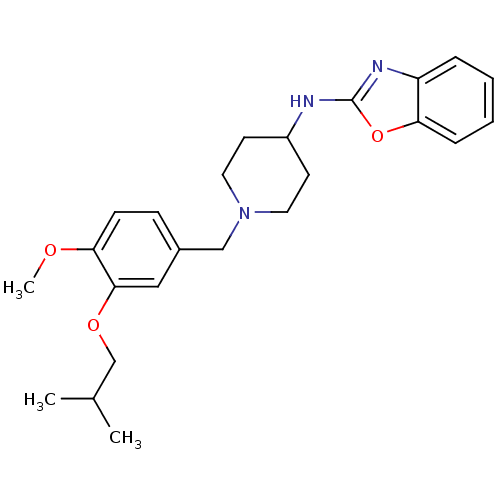
(CHEMBL236588 | N-(1-(3-isobutoxy-4-methoxybenzyl)p...)Show SMILES COc1ccc(CN2CCC(CC2)Nc2nc3ccccc3o2)cc1OCC(C)C Show InChI InChI=1S/C24H31N3O3/c1-17(2)16-29-23-14-18(8-9-22(23)28-3)15-27-12-10-19(11-13-27)25-24-26-20-6-4-5-7-21(20)30-24/h4-9,14,17,19H,10-13,15-16H2,1-3H3,(H,25,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]11-Tyr somatostatin-14 from human SST5R expressed in CHO cells |
J Med Chem 50: 6291-4 (2007)
Article DOI: 10.1021/jm701143p
BindingDB Entry DOI: 10.7270/Q2WS8T0K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50322973
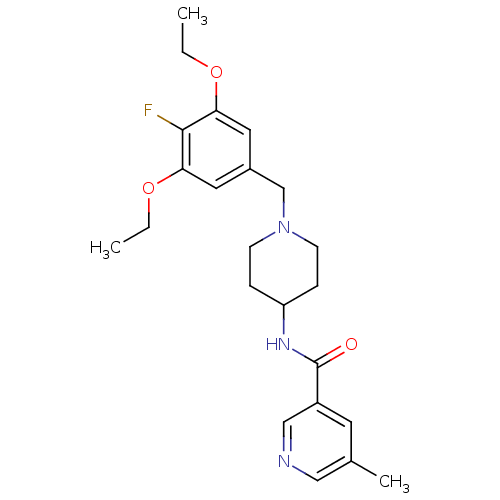
(CHEMBL1210373 | N-(1-(3,5-diethoxy-4-fluorobenzyl)...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)cc(OCC)c1F Show InChI InChI=1S/C23H30FN3O3/c1-4-29-20-11-17(12-21(22(20)24)30-5-2)15-27-8-6-19(7-9-27)26-23(28)18-10-16(3)13-25-14-18/h10-14,19H,4-9,15H2,1-3H3,(H,26,28) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 11 Tyr SST14 from human SST5 receptor expressed in CHO cells |
Bioorg Med Chem Lett 20: 4521-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.026
BindingDB Entry DOI: 10.7270/Q2J103BN |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19454
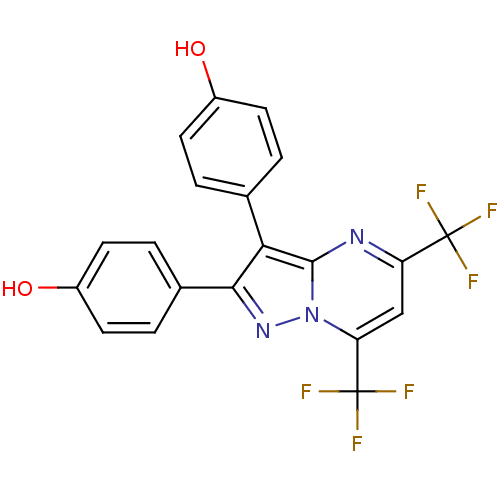
(4-[2-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)pyr...)Show SMILES Oc1ccc(cc1)-c1nn2c(cc(nc2c1-c1ccc(O)cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H11F6N3O2/c21-19(22,23)14-9-15(20(24,25)26)29-18(27-14)16(10-1-5-12(30)6-2-10)17(28-29)11-3-7-13(31)8-4-11/h1-9,30-31H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | -39.7 | n/a | n/a | 6.00E+3 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM8885
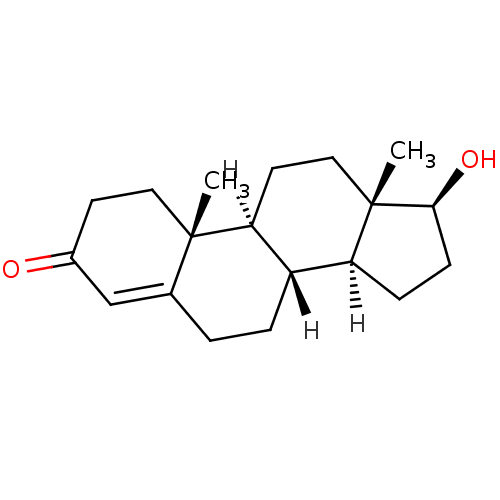
((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18| Show InChI InChI=1S/C19H28O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h11,14-17,21H,3-10H2,1-2H3/t14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of fluorescent-tagged R1881 from androgen receptor after 4 hrs by fluorometric assay |
ACS Med Chem Lett 2: 124-129 (2011)
Article DOI: 10.1021/ml1002508
BindingDB Entry DOI: 10.7270/Q2JQ119N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227117
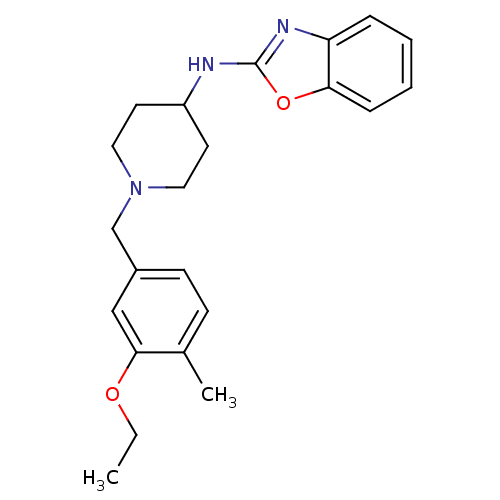
(CHEMBL236611 | N-(1-(3-ethoxy-4-methylbenzyl)piper...)Show InChI InChI=1S/C22H27N3O2/c1-3-26-21-14-17(9-8-16(21)2)15-25-12-10-18(11-13-25)23-22-24-19-6-4-5-7-20(19)27-22/h4-9,14,18H,3,10-13,15H2,1-2H3,(H,23,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]11-Tyr somatostatin-14 from human SST5R expressed in CHO cells |
J Med Chem 50: 6291-4 (2007)
Article DOI: 10.1021/jm701143p
BindingDB Entry DOI: 10.7270/Q2WS8T0K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227130
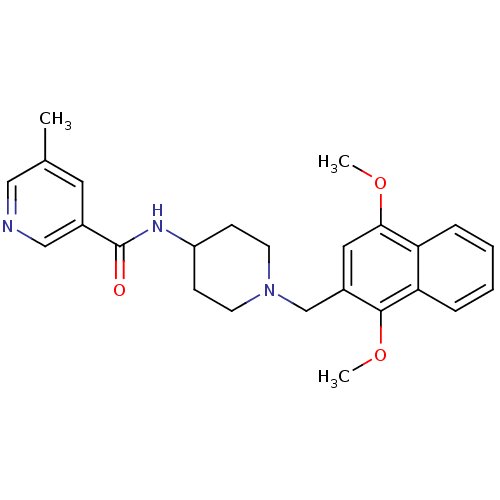
(CHEMBL241328 | N-(1-((1,4-dimethoxynaphthalen-2-yl...)Show SMILES COc1cc(CN2CCC(CC2)NC(=O)c2cncc(C)c2)c(OC)c2ccccc12 Show InChI InChI=1S/C25H29N3O3/c1-17-12-18(15-26-14-17)25(29)27-20-8-10-28(11-9-20)16-19-13-23(30-2)21-6-4-5-7-22(21)24(19)31-3/h4-7,12-15,20H,8-11,16H2,1-3H3,(H,27,29) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human SST5R |
J Med Chem 50: 6295-8 (2007)
Article DOI: 10.1021/jm701144e
BindingDB Entry DOI: 10.7270/Q2S18273 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19456

(4-(3-{4-[2-(dimethylamino)ethoxy]phenyl}-5,7-bis(t...)Show SMILES CN(C)CCOc1ccc(cc1)-c1c(nn2c(cc(nc12)C(F)(F)F)C(F)(F)F)-c1ccc(O)cc1 Show InChI InChI=1S/C24H20F6N4O2/c1-33(2)11-12-36-17-9-5-14(6-10-17)20-21(15-3-7-16(35)8-4-15)32-34-19(24(28,29)30)13-18(23(25,26)27)31-22(20)34/h3-10,13,35H,11-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | -38.3 | n/a | n/a | 1.00E+3 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19454

(4-[2-(4-hydroxyphenyl)-5,7-bis(trifluoromethyl)pyr...)Show SMILES Oc1ccc(cc1)-c1nn2c(cc(nc2c1-c1ccc(O)cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H11F6N3O2/c21-19(22,23)14-9-15(20(24,25)26)29-18(27-14)16(10-1-5-12(30)6-2-10)17(28-29)11-3-7-13(31)8-4-11/h1-9,30-31H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 48 | -38.3 | n/a | n/a | 600 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19453
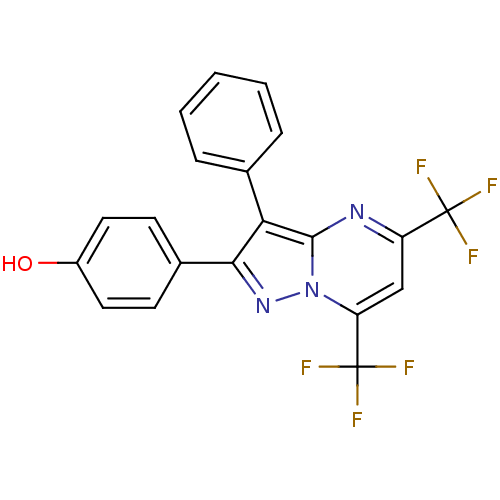
(4-[3-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a...)Show SMILES Oc1ccc(cc1)-c1nn2c(cc(nc2c1-c1ccccc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H11F6N3O/c21-19(22,23)14-10-15(20(24,25)26)29-18(27-14)16(11-4-2-1-3-5-11)17(28-29)12-6-8-13(30)9-7-12/h1-10,30H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 51 | -38.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227131

(CHEMBL241330 | N-(1-(4-chloro-3-ethoxybenzyl)piper...)Show SMILES CCOc1cc(CN2CCC(CC2)NC(=O)c2cccc(c2)S(C)(=O)=O)ccc1Cl Show InChI InChI=1S/C22H27ClN2O4S/c1-3-29-21-13-16(7-8-20(21)23)15-25-11-9-18(10-12-25)24-22(26)17-5-4-6-19(14-17)30(2,27)28/h4-8,13-14,18H,3,9-12,15H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Binding affinity to human SST5R |
J Med Chem 50: 6295-8 (2007)
Article DOI: 10.1021/jm701144e
BindingDB Entry DOI: 10.7270/Q2S18273 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 5
(Homo sapiens (Human)) | BDBM50227106

(4-((4-(benzo[d]oxazol-2-ylamino)piperidin-1-yl)met...)Show InChI InChI=1S/C21H25N3O3/c1-2-26-20-13-15(7-8-18(20)25)14-24-11-9-16(10-12-24)22-21-23-17-5-3-4-6-19(17)27-21/h3-8,13,16,25H,2,9-12,14H2,1H3,(H,22,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [125I]11-Tyr somatostatin-14 from human SST5R expressed in CHO cells |
J Med Chem 50: 6291-4 (2007)
Article DOI: 10.1021/jm701143p
BindingDB Entry DOI: 10.7270/Q2WS8T0K |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19455

(4-(3-{4-[2-(piperidin-1-yl)ethoxy]phenyl}-5,7-bis(...)Show SMILES Oc1ccc(cc1)-c1nn2c(cc(nc2c1-c1ccc(OCCN2CCCCC2)cc1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N4O2/c28-26(29,30)21-16-22(27(31,32)33)37-25(34-21)23(24(35-37)18-4-8-19(38)9-5-18)17-6-10-20(11-7-17)39-15-14-36-12-2-1-3-13-36/h4-11,16,38H,1-3,12-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 71 | -37.4 | n/a | n/a | 40 | n/a | n/a | 7.5 | 0 |
University of Illinois at Urbana
| Assay Description
Relative binding affinities were determined by a competitive radiometric binding assay using [3H]estradiol as tracer, and purified full-length human ... |
J Med Chem 50: 399-403 (2007)
Article DOI: 10.1021/jm061035y
BindingDB Entry DOI: 10.7270/Q2QZ288F |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor/Kappa-type opioid receptor/Mu-type opioid receptor/Sigma non-opioid intracellular receptor 1
(Rattus norvegicus (rat)-RAT) | BDBM50223633

(Levomethadone)Show SMILES CCC(=O)C(C[C@@H](C)N(C)C)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of [3H]- naloxone binding to Opioid receptors in rat brain membrane in the presence of Na |
J Med Chem 24: 903-6 (1981)
BindingDB Entry DOI: 10.7270/Q26W9D8D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data