

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidase 1 (GUINEA PIG) | BDBM50042574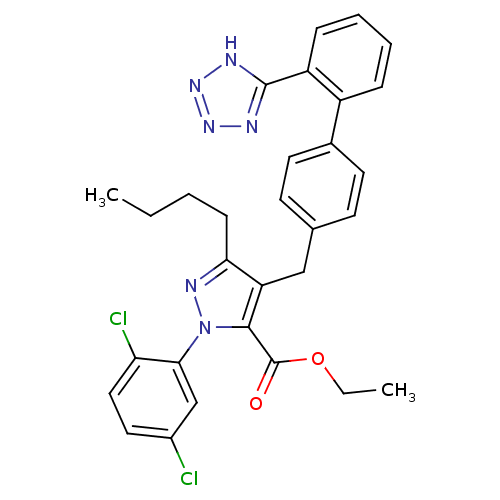 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042531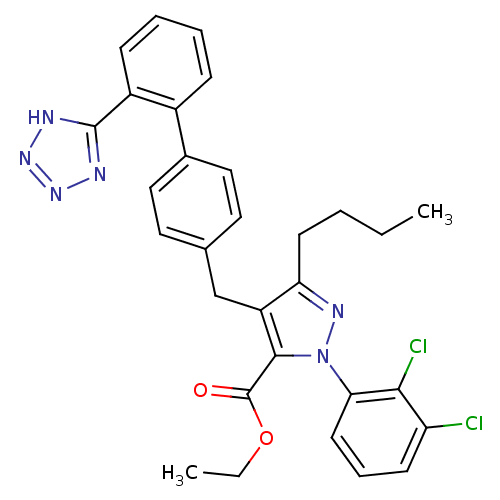 (5-Butyl-2-(2,3-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042561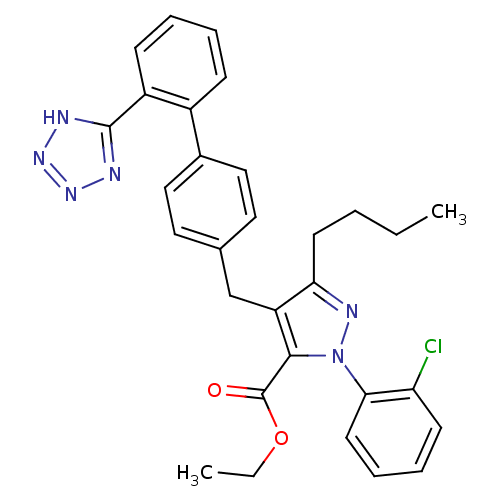 (5-Butyl-2-(2-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042579 (5-Butyl-2-phenyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042542 (5-Butyl-2-(2,3-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042559 (5-Butyl-2-(2-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042577 (5-Butyl-2-(2,5-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042564 (5-Butyl-2-(4-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042578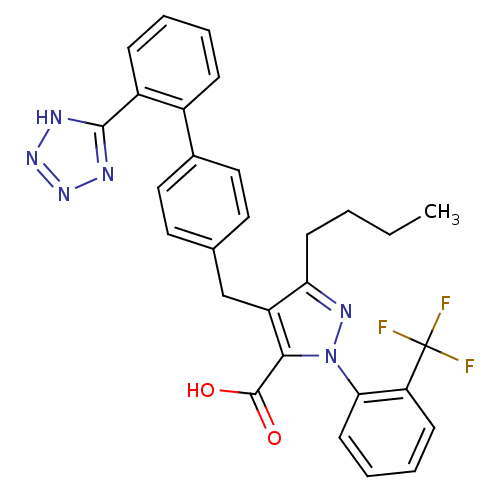 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042543 (5-Butyl-2-(2,4-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042558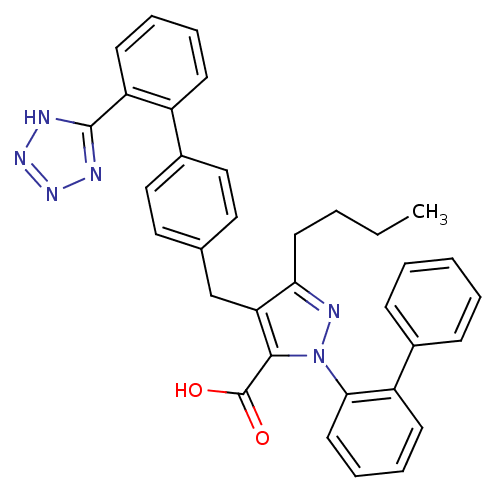 (2-Biphenyl-2-yl-5-butyl-4-[2'-(1H-tetrazol-5-yl)-b...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042535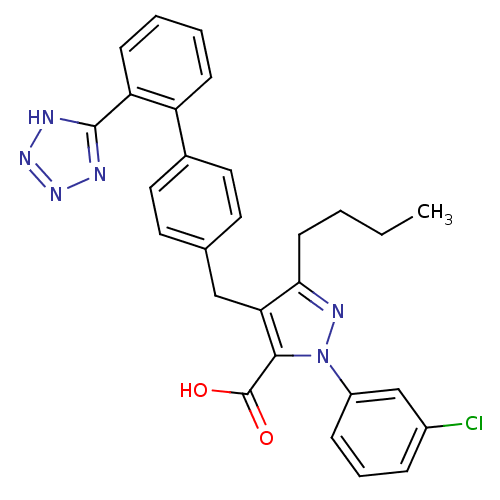 (5-Butyl-2-(3-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042556 (5-Butyl-2-(4-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042548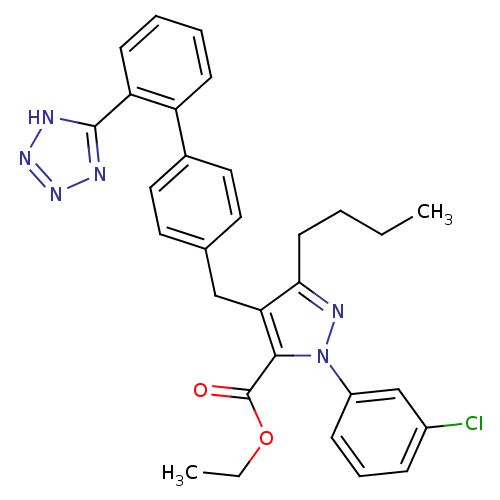 (5-Butyl-2-(3-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042528 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042569 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042568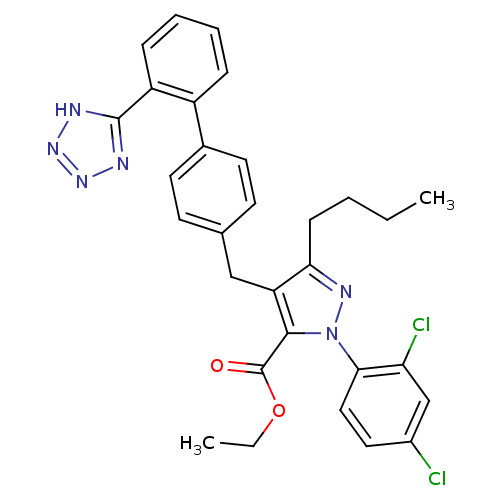 (5-Butyl-2-(2,4-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042565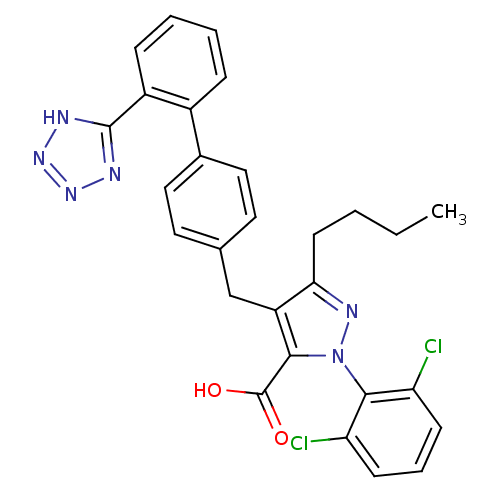 (5-Butyl-2-(2,6-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042555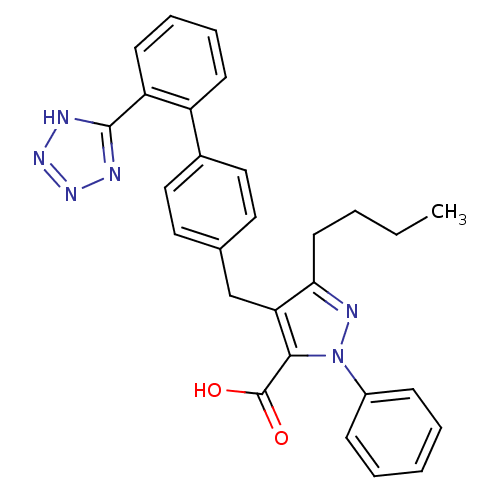 (5-Butyl-2-phenyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042544 (5-Butyl-2-(2-nitro-phenyl)-4-[2'-(1H-tetrazol-5-yl...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidase 1 (GUINEA PIG) | BDBM50042576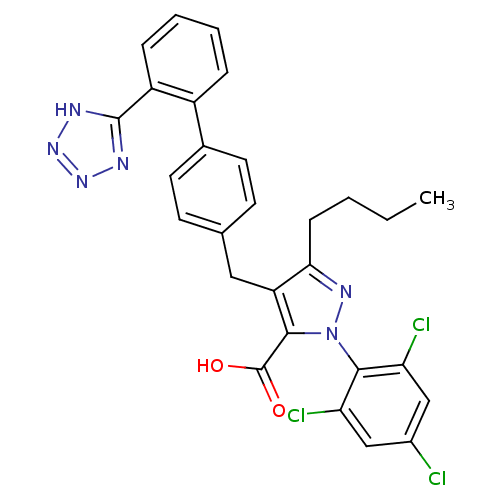 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro apparent inhibition constant of porcine renal Dehydropeptidase-1. | Bioorg Med Chem Lett 9: 2741-6 (1999) BindingDB Entry DOI: 10.7270/Q24Q7T6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035445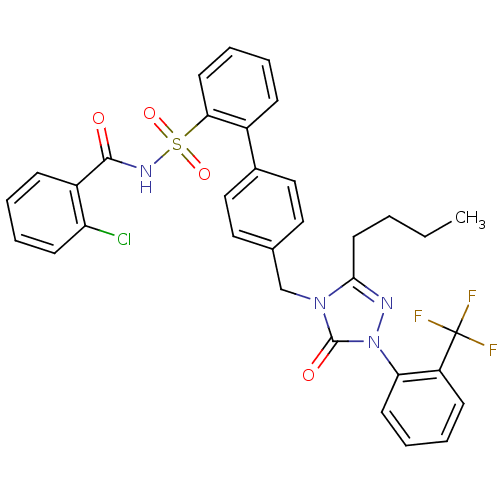 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039862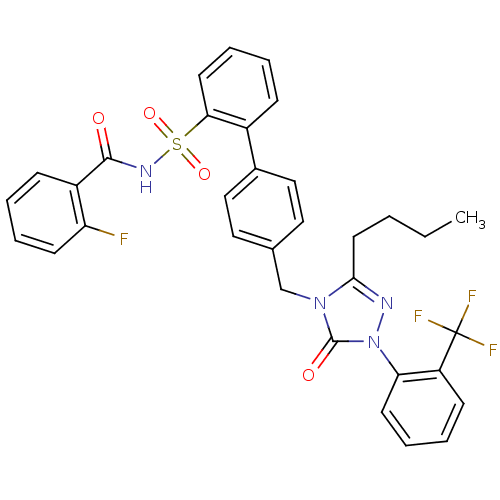 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039880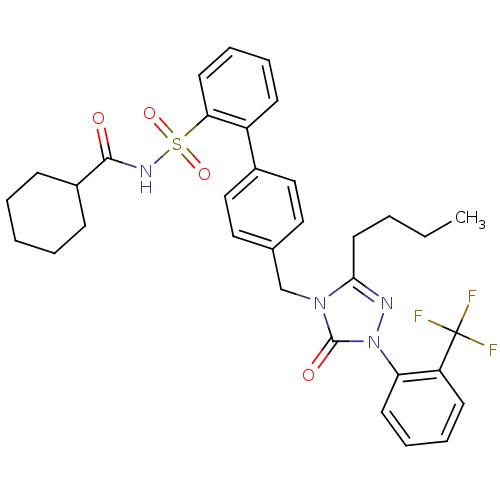 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039929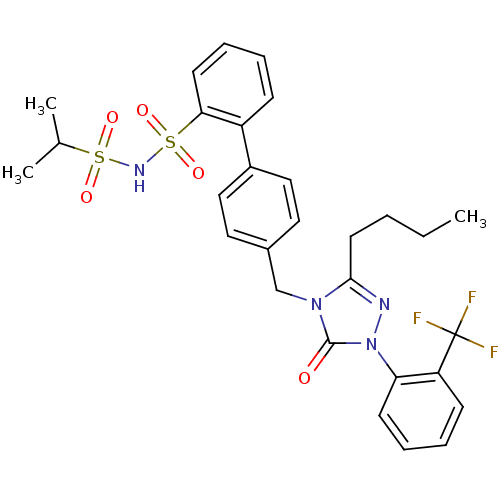 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039882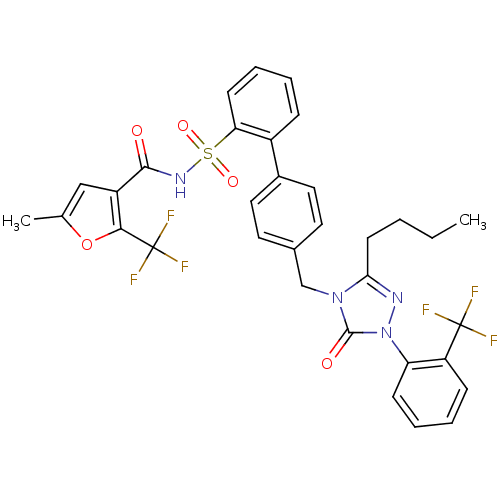 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039887 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042565 (5-Butyl-2-(2,6-dichloro-phenyl)-4-[2'-(1H-tetrazol...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039930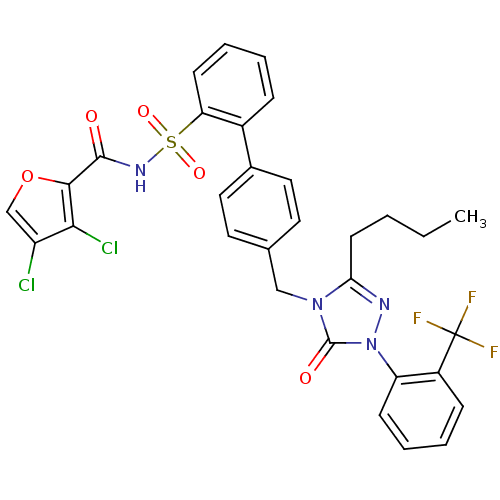 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042571 (2-Benzyl-5-butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039895 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039921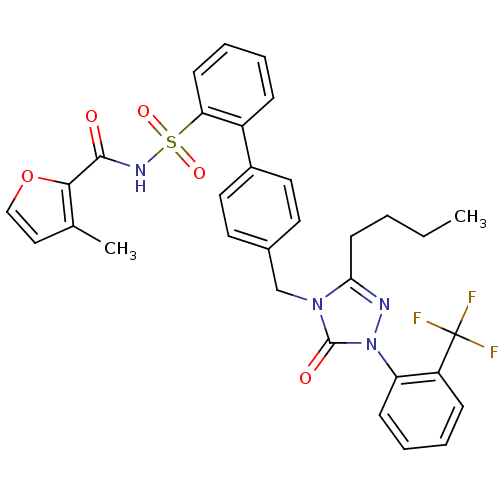 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039899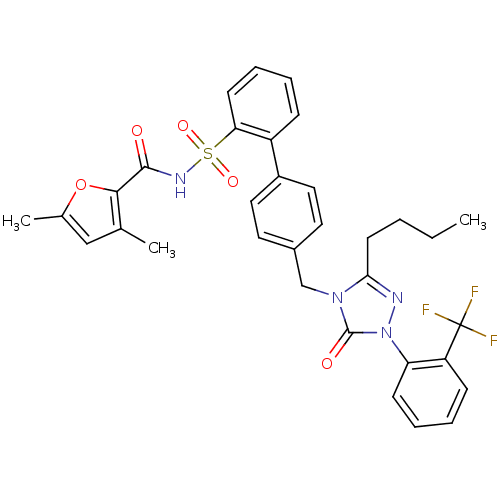 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039946 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042578 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042570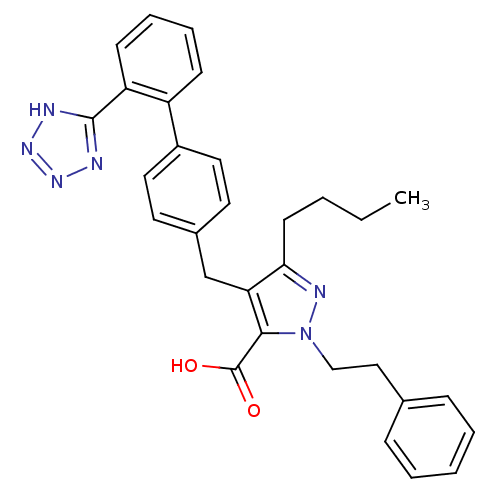 (5-Butyl-2-phenethyl-4-[2'-(1H-tetrazol-5-yl)-biphe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039922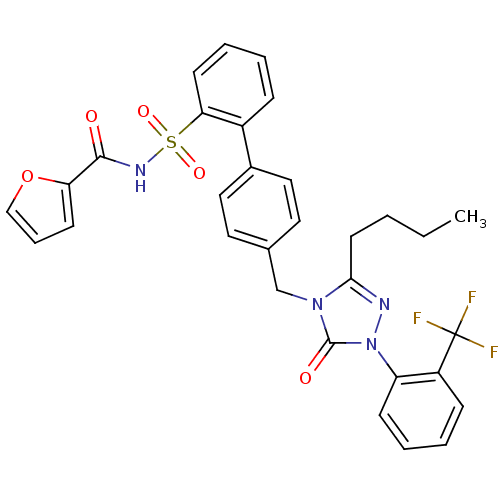 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039885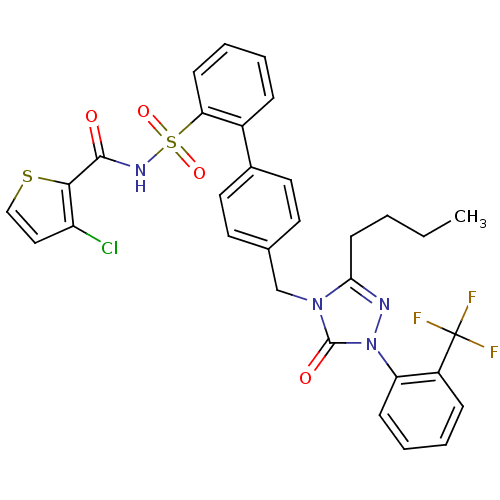 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039926 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50009718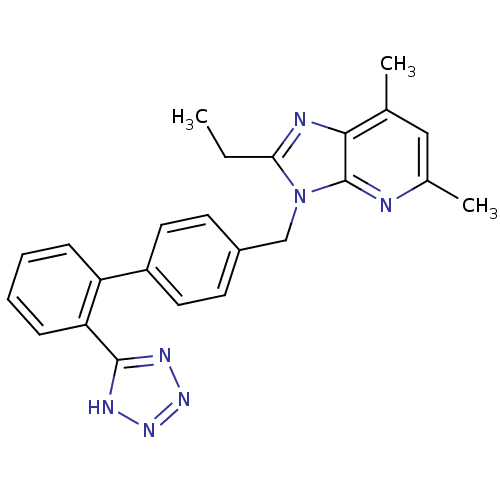 (2-Ethyl-5,7-dimethyl-3-[2'-(1H-tetrazol-5-yl)-biph...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-Sar1-Ile8-A II at the rabbit aorta angiotensin II receptor, type 1 | J Med Chem 36: 591-609 (1993) BindingDB Entry DOI: 10.7270/Q2P55MKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039904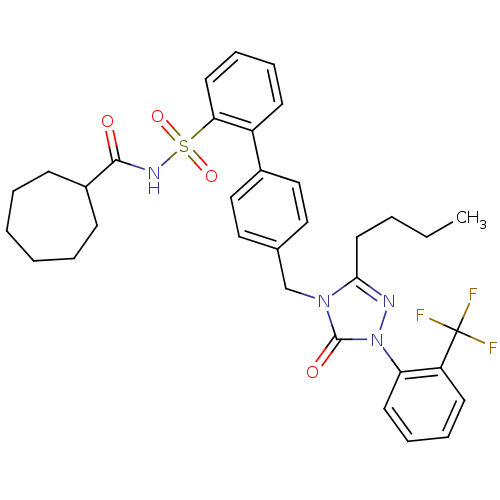 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039938 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039924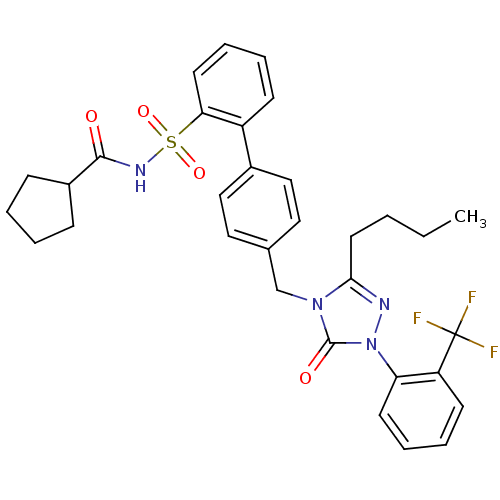 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50042559 (5-Butyl-2-(2-chloro-phenyl)-4-[2'-(1H-tetrazol-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Evaluation of Angiotensin II antagonistic activity by displacement of [125I]-Sar Ile-AII at the rabbit aorta Angiotensin II receptor, type 1 | J Med Chem 36: 3595-605 (1994) BindingDB Entry DOI: 10.7270/Q25Q4V5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039894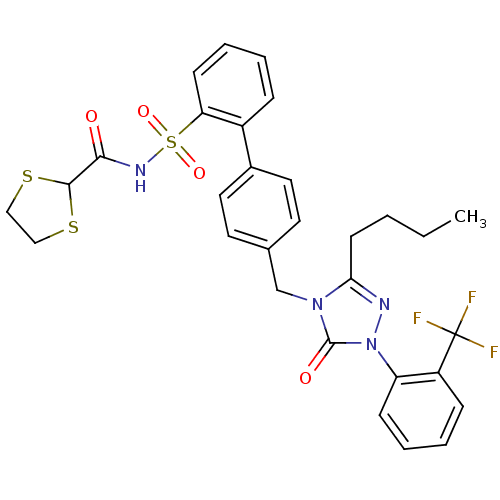 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039853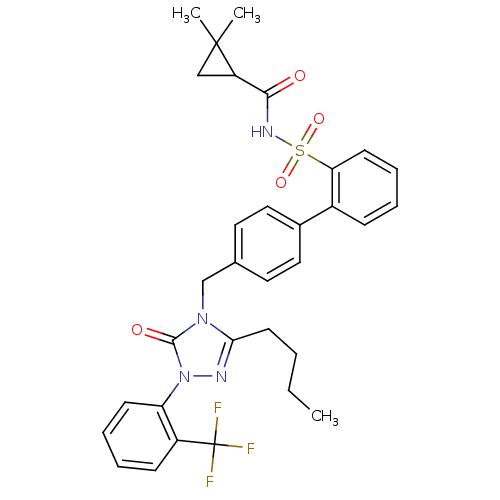 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039858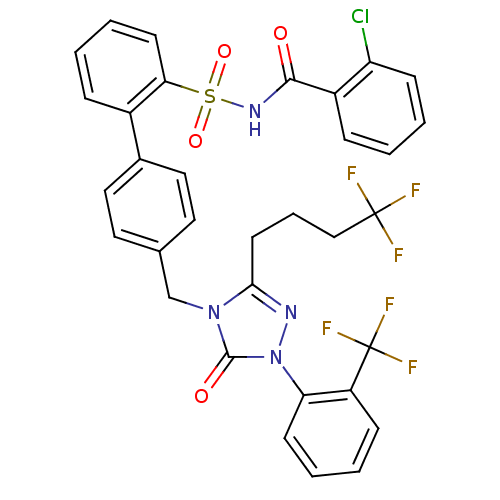 (4'-[5-Oxo-3-(4,4,4-trifluoro-butyl)-1-(2-trifluoro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039870 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039865 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50039863 (4'-[3-Butyl-5-oxo-1-(2-trifluoromethyl-phenyl)-1,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against angiotensin II rabbit aorta AT1 receptor using radioligand [125I]-Sar Ile-AII | J Med Chem 37: 2808-24 (1994) BindingDB Entry DOI: 10.7270/Q2M907QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 430 total ) | Next | Last >> |