

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101832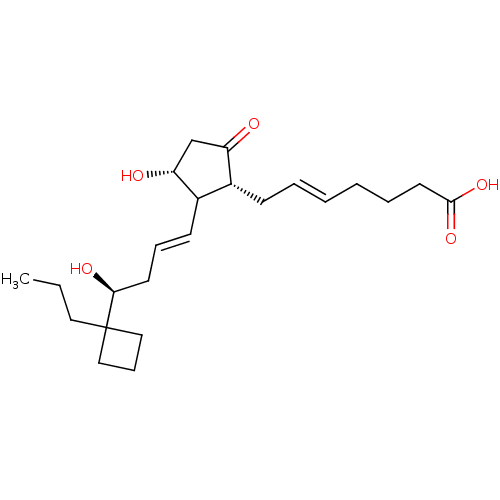 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101823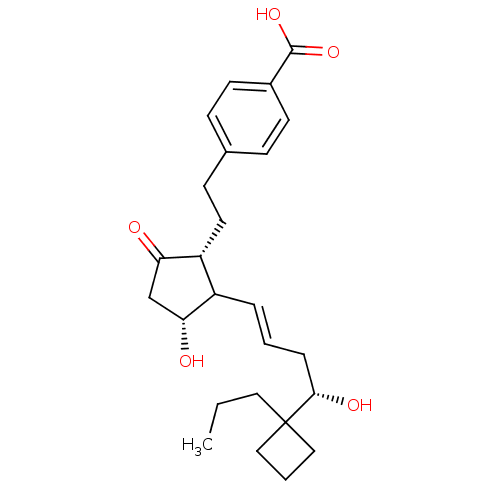 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101826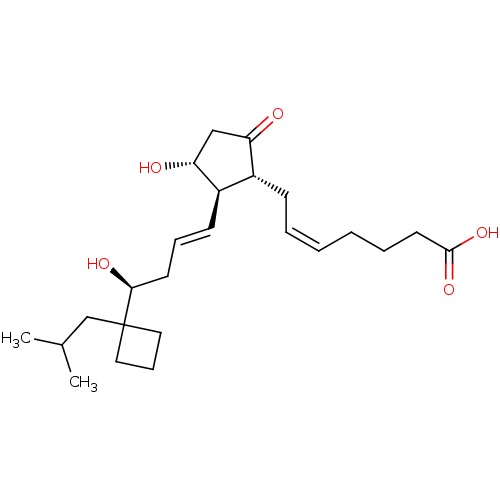 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101833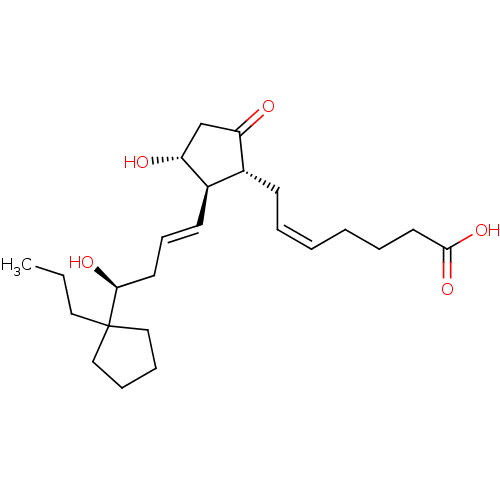 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101823 (4-(2-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostacyclin receptor (Homo sapiens (Human)) | BDBM50101832 ((E)-7-{(1R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4-(1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards human Prostanoid IP receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101827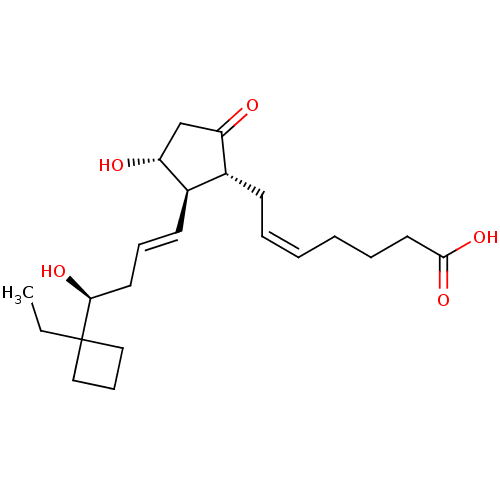 ((Z)-7-{(1R,2R,3R)-2-[(E)-(S)-4-(1-Ethyl-cyclobutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50101825 ((Z)-7-{(1R,2R,3R)-3-Hydroxy-2-[(E)-(S)-4-hydroxy-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >0.000100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Binding affinity towards mouse Prostanoid EP3 receptor in CHO cells. | Bioorg Med Chem Lett 11: 2025-8 (2001) BindingDB Entry DOI: 10.7270/Q2CV4H1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458766 (CHEMBL4212386) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19770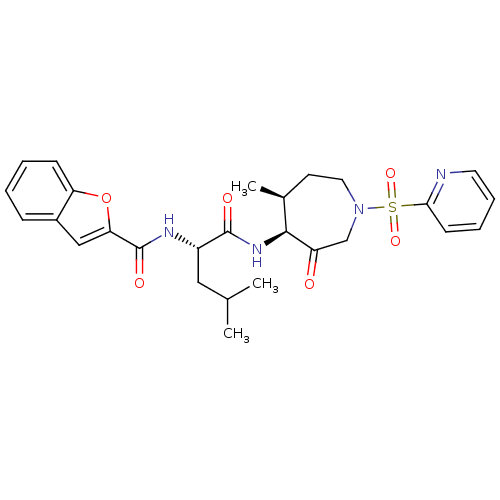 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.00990 | -62.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50184069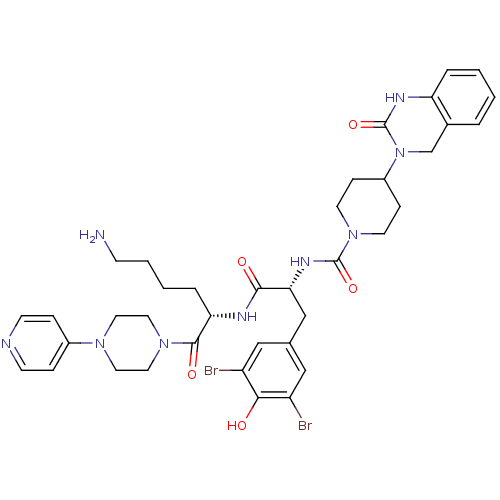 (CHEMBL207197 | N-((R)-1-((S)-6-amino-1-oxo-1-(4-(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447327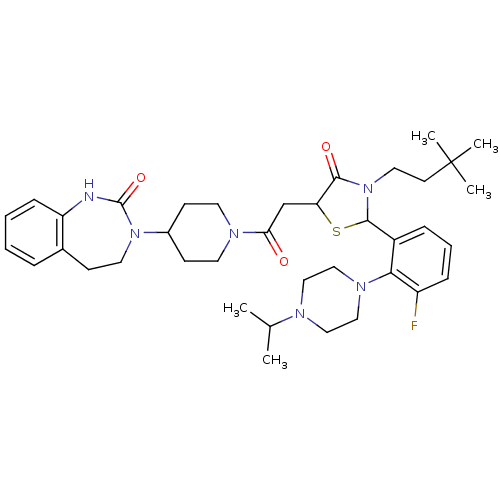 (CHEMBL3114495) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447325 (CHEMBL3114676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tolloid-like protein 1 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tolloid-like protein 2 (Homo sapiens) | BDBM50458766 (CHEMBL4212386) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem | PDB Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bone morphogenetic protein 1 (Homo sapiens (Human)) | BDBM50458771 (CHEMBL4214046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... | ACS Med Chem Lett 9: 736-740 (2018) Article DOI: 10.1021/acsmedchemlett.8b00173 BindingDB Entry DOI: 10.7270/Q2X35127 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19770 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0400 | -58.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0410 | -58.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cathepsin L2 (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50315980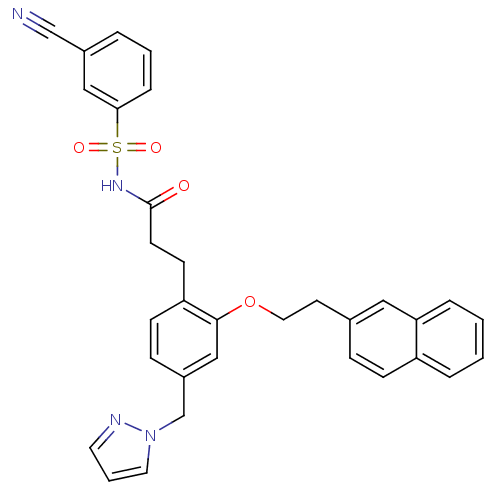 (3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter | Bioorg Med Chem Lett 20: 2639-43 (2010) Article DOI: 10.1016/j.bmcl.2010.02.034 BindingDB Entry DOI: 10.7270/Q23B6099 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19778 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0680 | -57.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447328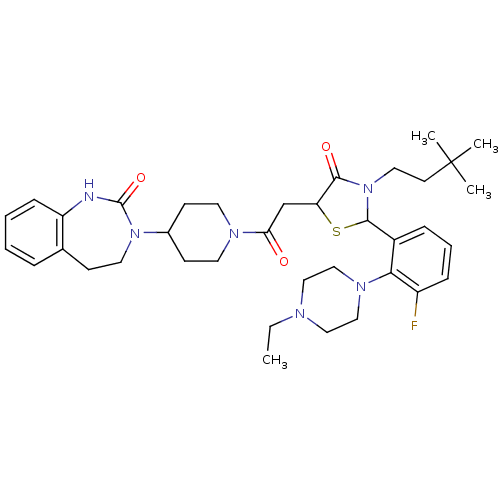 (CHEMBL3114494) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50315979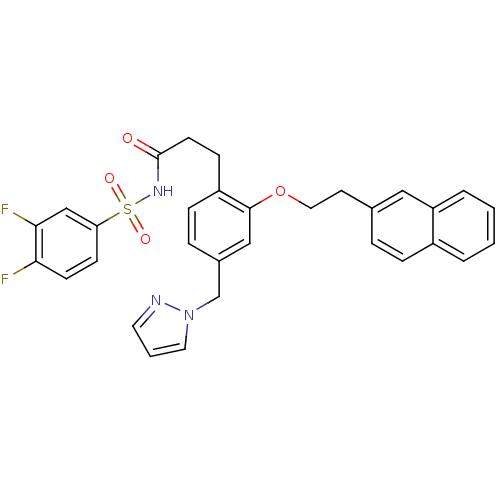 (3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(naphthalen-2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter | Bioorg Med Chem Lett 20: 2639-43 (2010) Article DOI: 10.1016/j.bmcl.2010.02.034 BindingDB Entry DOI: 10.7270/Q23B6099 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307436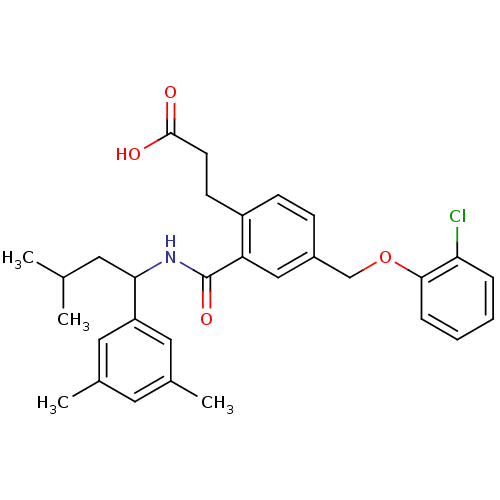 (3-[4-[(2-Chlorophenoxy)methyl]-2-({[1-(3,5-dimethy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50398821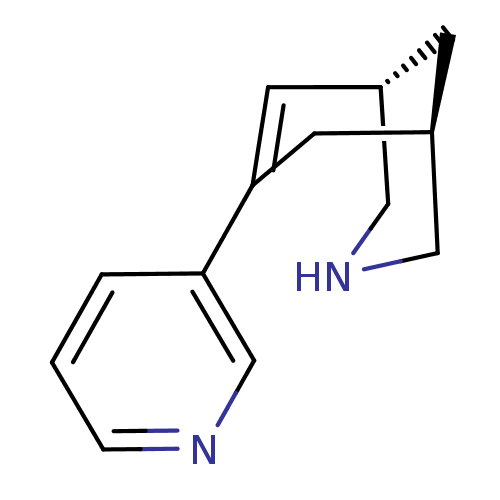 (CHEMBL2177537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells | J Med Chem 55: 9929-45 (2012) Article DOI: 10.1021/jm3011299 BindingDB Entry DOI: 10.7270/Q2474C0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calcitonin gene-related peptide type 1 receptor (Homo sapiens (Human)) | BDBM50447326 (CHEMBL3114496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Displacement of [125I]CGRP from CGRP receptor in human SK-N-MC cell membranes preincubated for 30 mins followed by radioligand addition measured afte... | Bioorg Med Chem Lett 24: 845-9 (2014) Article DOI: 10.1016/j.bmcl.2013.12.089 BindingDB Entry DOI: 10.7270/Q2FJ2J84 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4227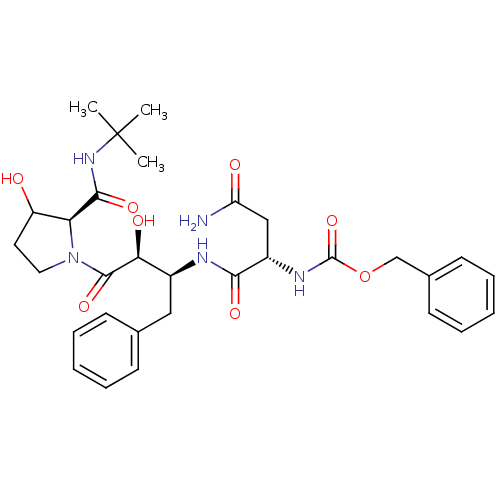 (AHPBA 35a | Z-Asn.(2S,3S)-AHPBA-[3(R)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307408 (3-[4-[(2,5-Difluorophenoxy)methyl]-2-({[1-(3,5-dim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50397944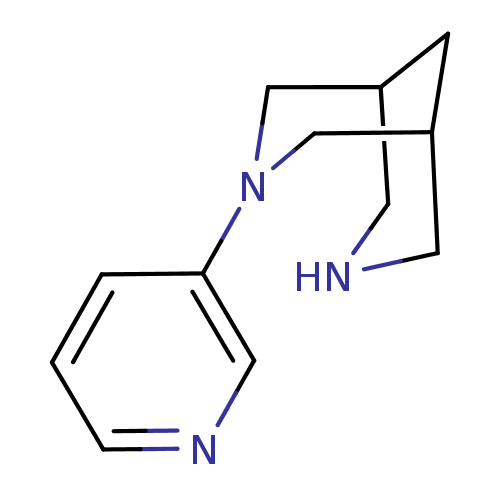 (CHEMBL2177512) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Targacept, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cells | J Med Chem 55: 9929-45 (2012) Article DOI: 10.1021/jm3011299 BindingDB Entry DOI: 10.7270/Q2474C0B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307434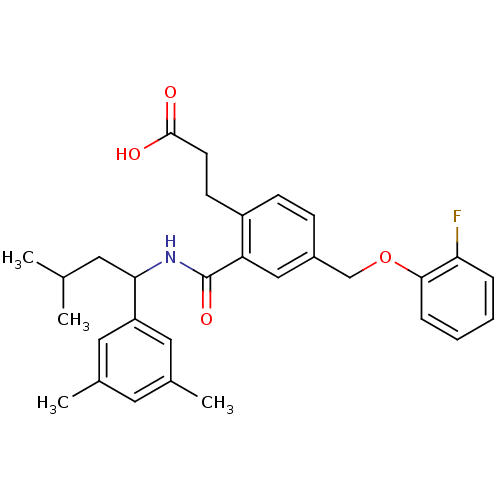 (3-{2-({[1-(3,5-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307441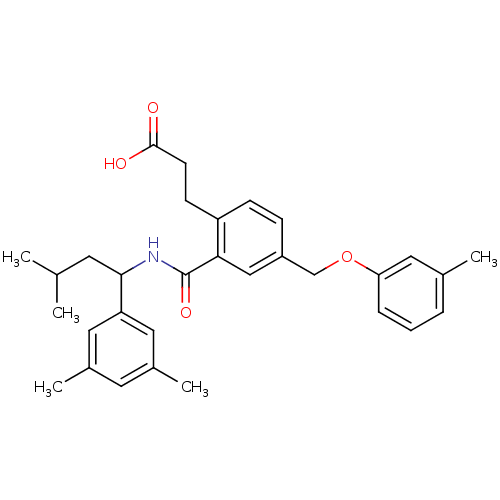 (3-{2-({[1-(3,5-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50315985 (3-(4-((1H-pyrazol-1-yl)methyl)-2-(2-(methyl(phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter | Bioorg Med Chem Lett 20: 2639-43 (2010) Article DOI: 10.1016/j.bmcl.2010.02.034 BindingDB Entry DOI: 10.7270/Q23B6099 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307406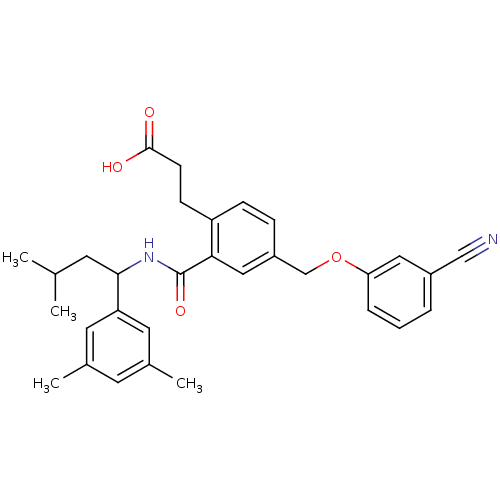 (3-[4-[(3-Cyanophenoxy)methyl]-2-({[1-(3,5-dimethyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307405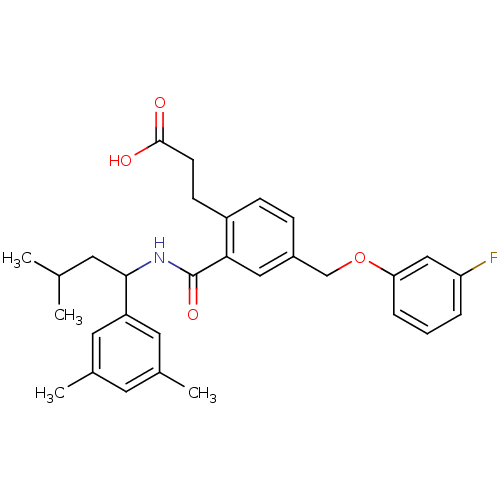 (3-{2-({[1-(3,5-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307414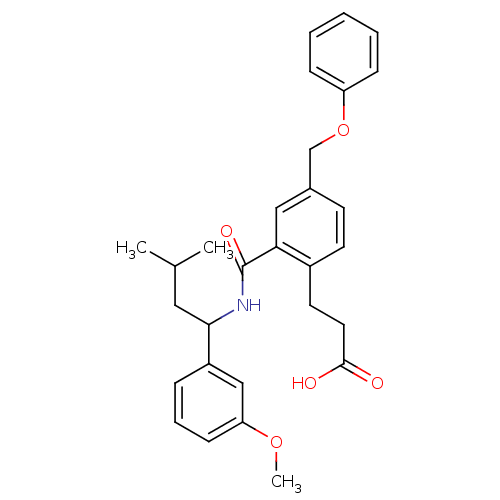 (3-[2-({[1-(3-Methoxyphenyl)-3-methylbutyl]amino}ca...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50315981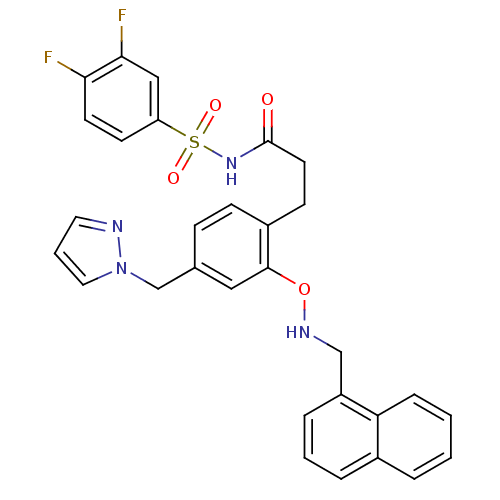 (3-(4-((1H-pyrazol-1-yl)methyl)-2-(naphthalen-1-ylm...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter | Bioorg Med Chem Lett 20: 2639-43 (2010) Article DOI: 10.1016/j.bmcl.2010.02.034 BindingDB Entry DOI: 10.7270/Q23B6099 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307445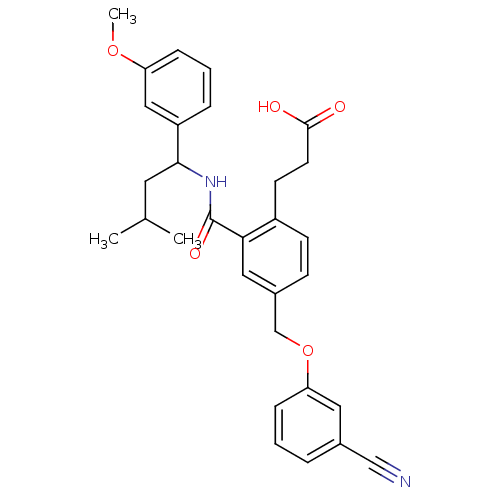 (3-[4-[(3-Cyanophenoxy)methyl]-2-({[1-(3-methoxyphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19775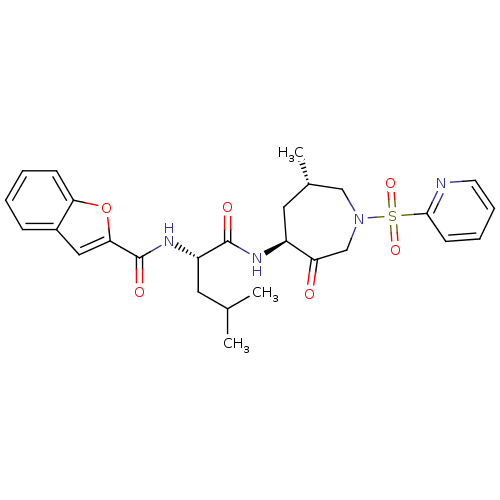 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | -55.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307428 (3-[2-({[1-(3-Fluoro-4-methoxyphenyl)-3-methylbutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307449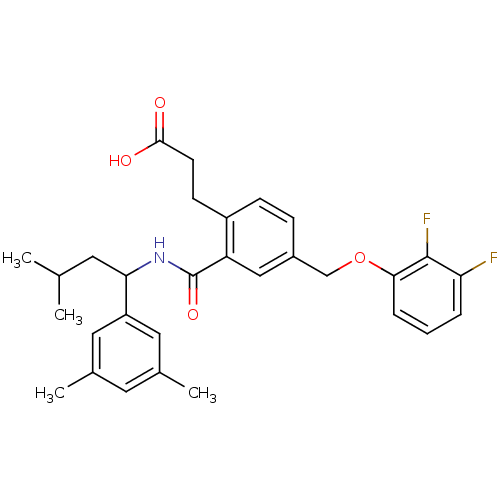 (3-[4-[(2,3-Difluorophenoxy)methyl]-2-({[1-(3,5-dim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307424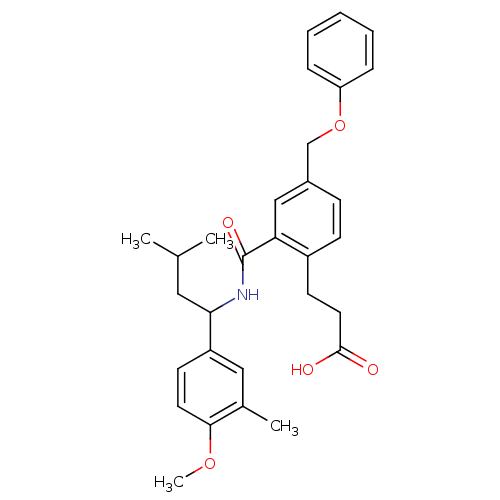 (3-[2-({[1-(4-Methoxy-3-methylphenyl)-3-methylbutyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19769 ((2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 22 |
GSK | Assay Description Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo... | J Med Chem 49: 1597-612 (2006) Article DOI: 10.1021/jm050915u BindingDB Entry DOI: 10.7270/Q2SQ8XP5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50315967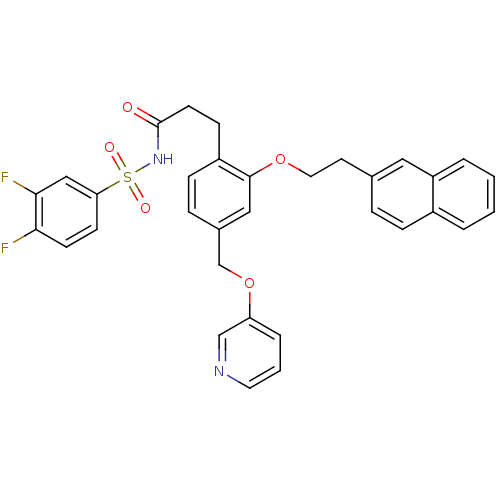 (CHEMBL1092432 | N-(3,4-difluorophenylsulfonyl)-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cells after 60 mins by scintillation counter | Bioorg Med Chem Lett 20: 2639-43 (2010) Article DOI: 10.1016/j.bmcl.2010.02.034 BindingDB Entry DOI: 10.7270/Q23B6099 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307403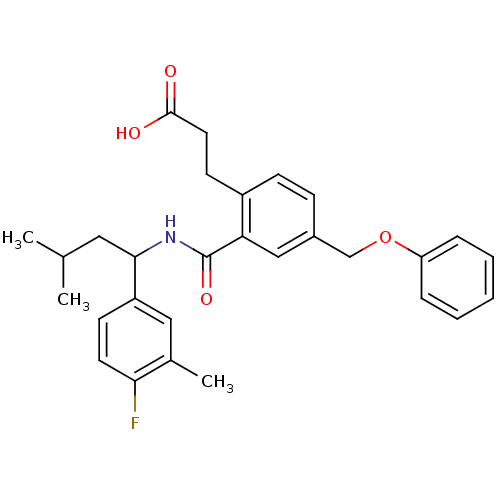 (3-[2-({[1-(4-Fluoro-3-methylphenyl)-3-methylbutyl]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307423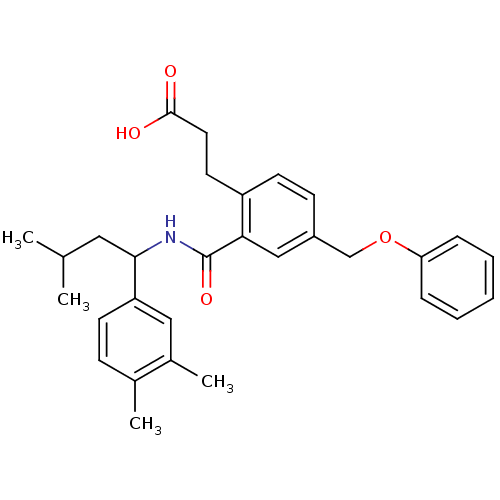 (3-[2-({[1-(3,4-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307446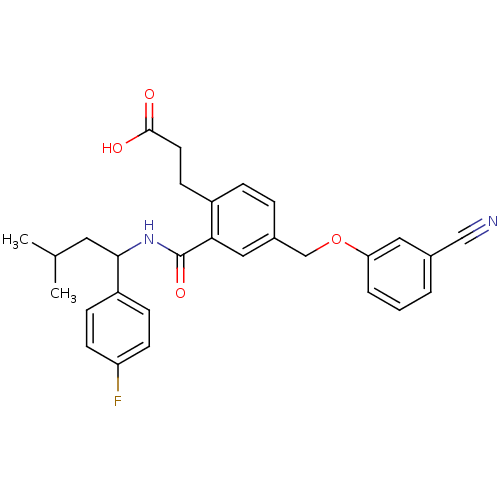 (3-[4-[(3-Cyanophenoxy)methyl]-2-({[1-(4-fluorophen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307407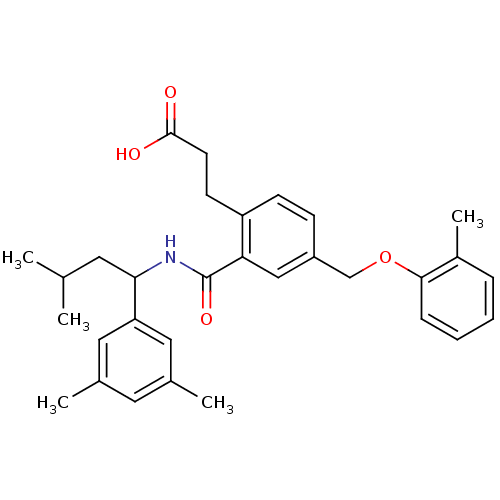 (3-{2-({[1-(3,5-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307419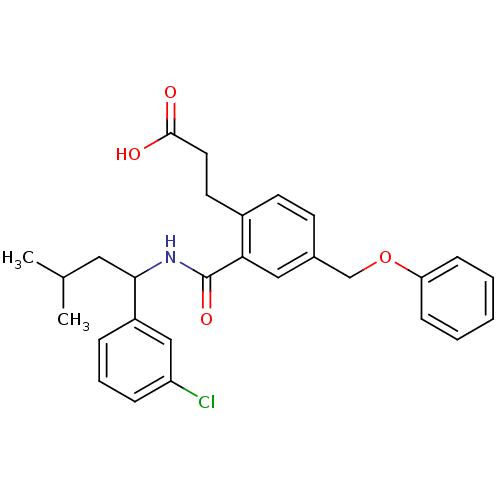 (3-[2-({[1-(3-Chlorophenyl)-3-methylbutyl]amino}car...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Mus musculus (Mouse)) | BDBM50307404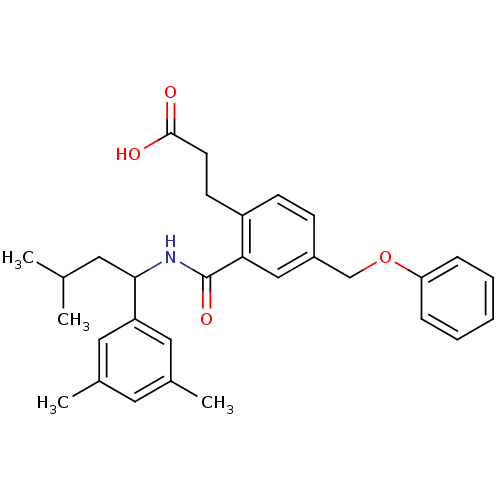 (3-[2-({[1-(3,5-Dimethylphenyl)-3-methylbutyl]amino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Minase Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from mouse EP3 receptor expressed in CHO cell membrane | Bioorg Med Chem 18: 1641-58 (2010) Article DOI: 10.1016/j.bmc.2009.12.068 BindingDB Entry DOI: 10.7270/Q29023W2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7895 total ) | Next | Last >> |