

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Equus caballus (Horse)) | BDBM50393864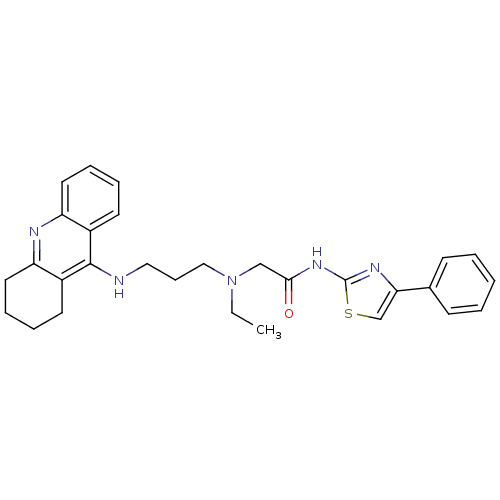 (CHEMBL2158116) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0447 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393868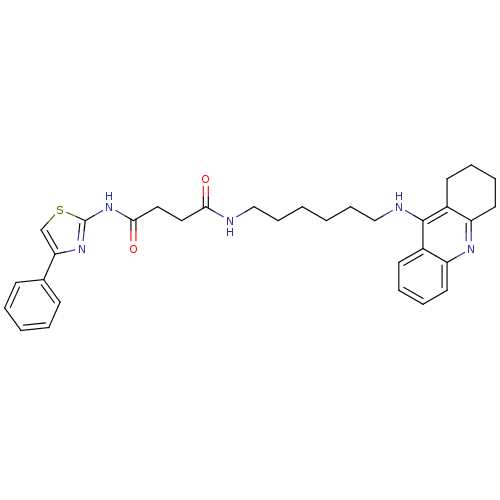 (CHEMBL2158112) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393858 (CHEMBL2158122) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393867 (CHEMBL2158113) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393870 (CHEMBL2158110) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.603 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27707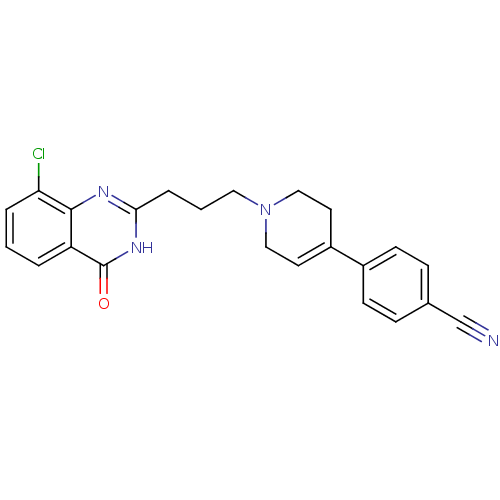 (4-{1-[3-(8-chloro-4-oxo-3,4-dihydroquinazolin-2-yl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165496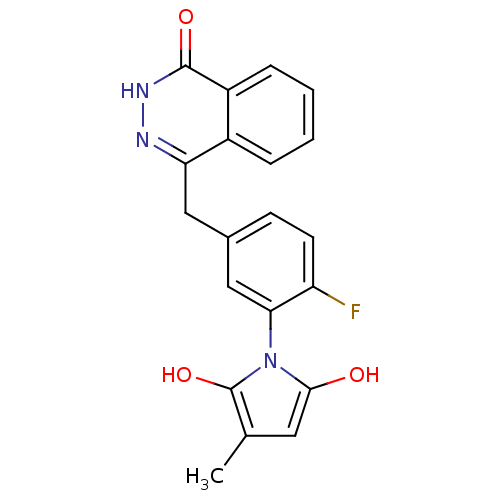 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum butyrylcholine esterase using butyrylcholine chloride as substrate incubated for 5 mins prior to substrate addition measur... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165488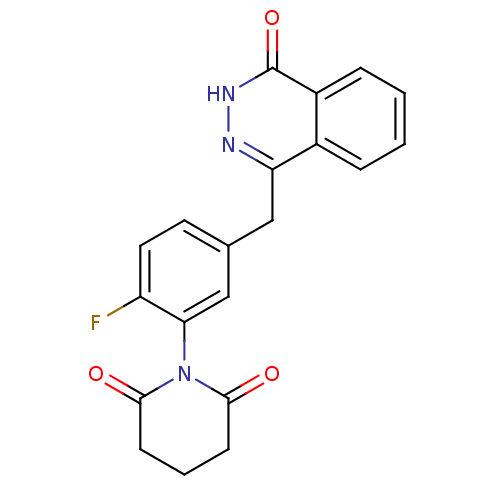 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393857 (CHEMBL2158123) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165486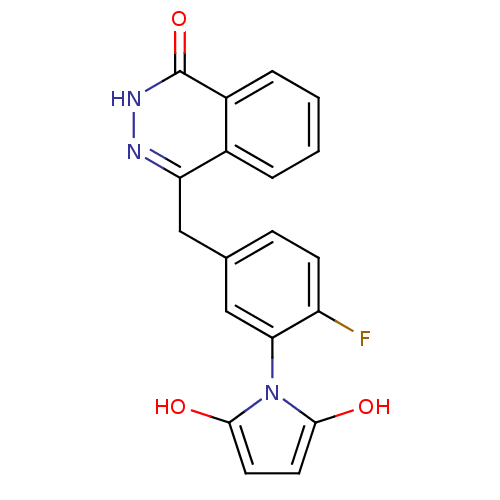 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393859 (CHEMBL2158121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393855 (CHEMBL2158125) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165498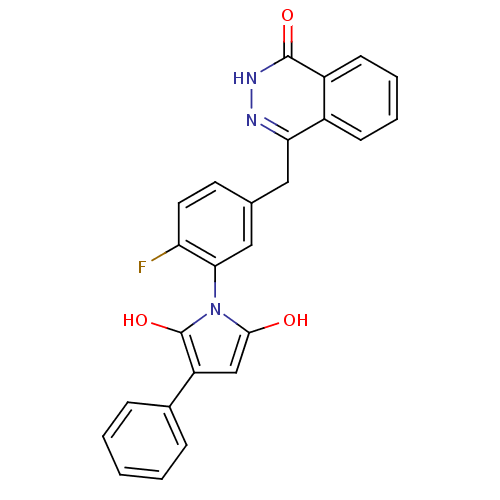 (1-(2-fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165478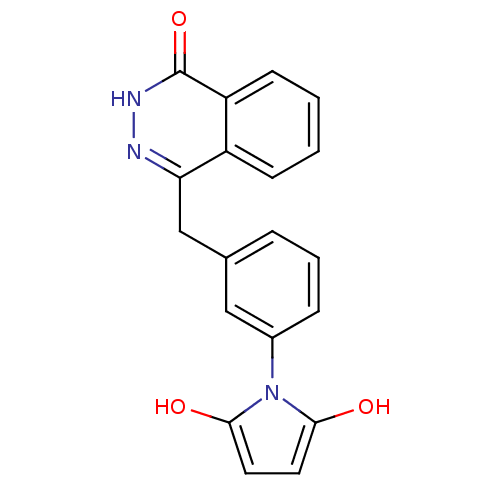 (1-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27708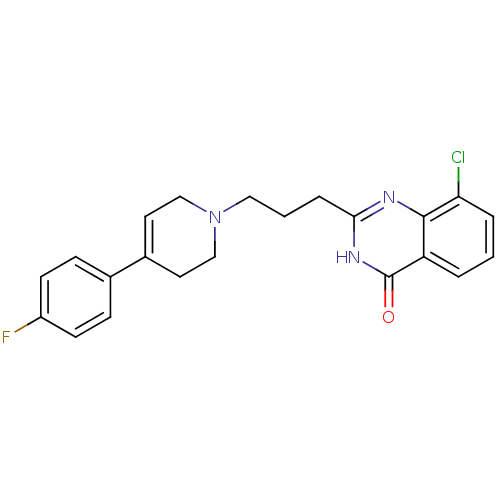 (8-chloro-2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393862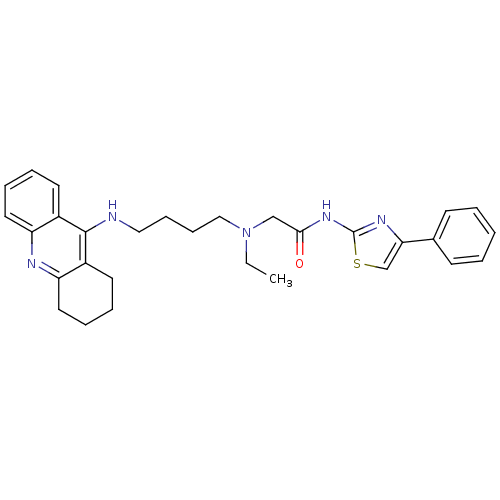 (CHEMBL2158118) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27709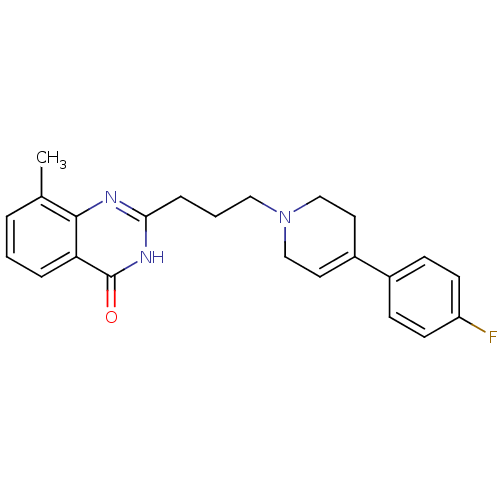 (2-{3-[4-(4-fluorophenyl)-1,2,3,6-tetrahydropyridin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393866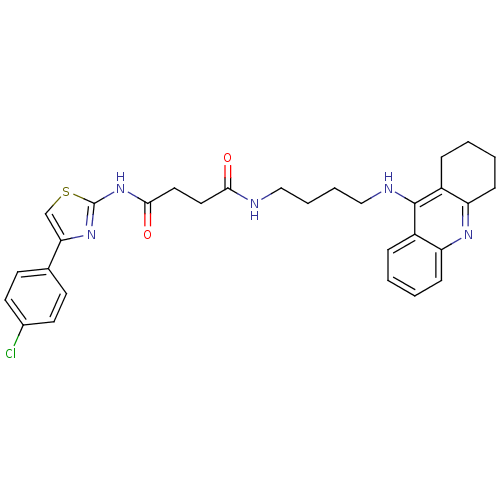 (CHEMBL2158114) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393856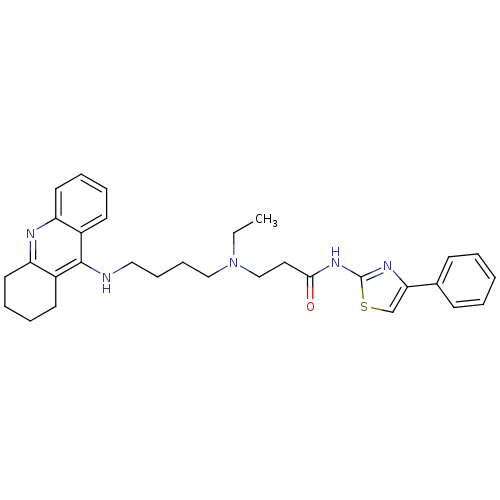 (CHEMBL2158124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165472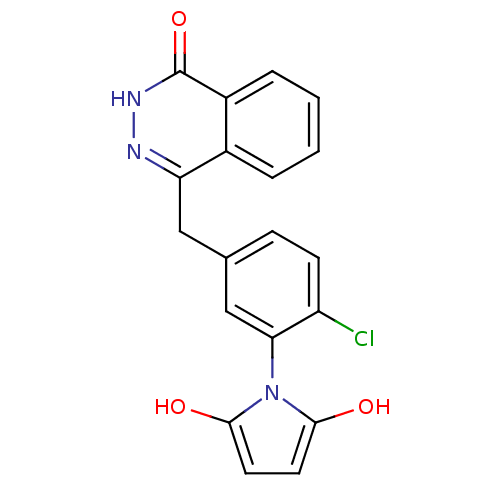 (1-(2-chloro-5-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165477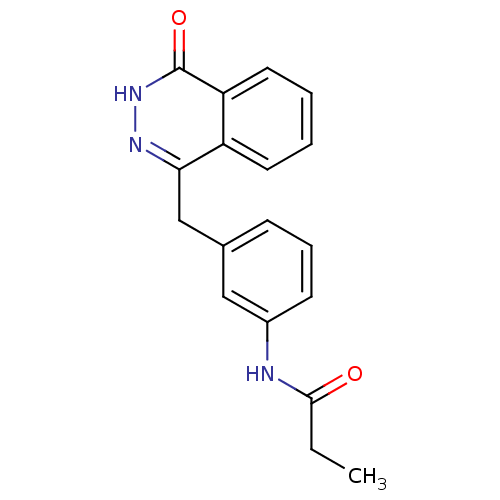 (CHEMBL371425 | N-(3-((4-oxo-3,4-dihydrophthalazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393861 (CHEMBL2158119) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27705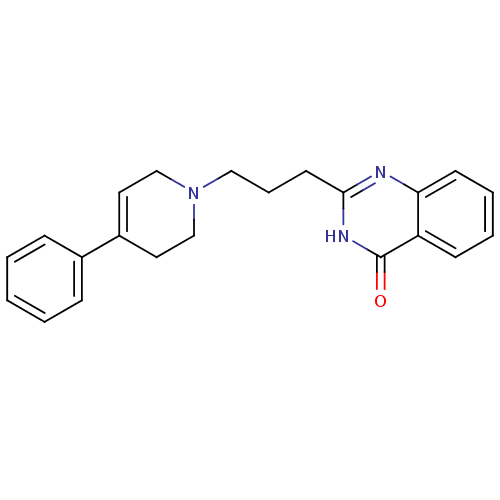 (2-[3-(4-phenyl-1,2,3,6-tetrahydropyridin-1-yl)prop...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50220868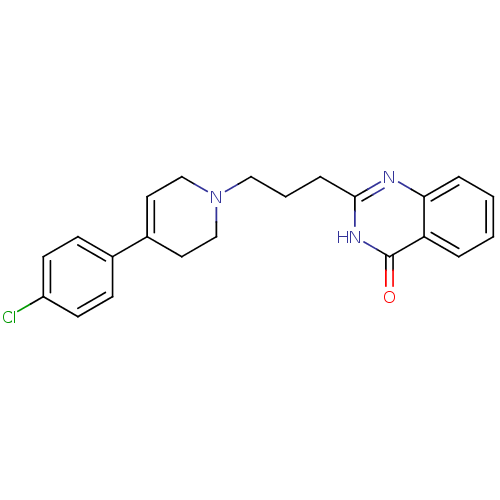 (2-(3-(4-(4-chlorophenyl)-5,6-dihydropyridin-1(2H)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50120265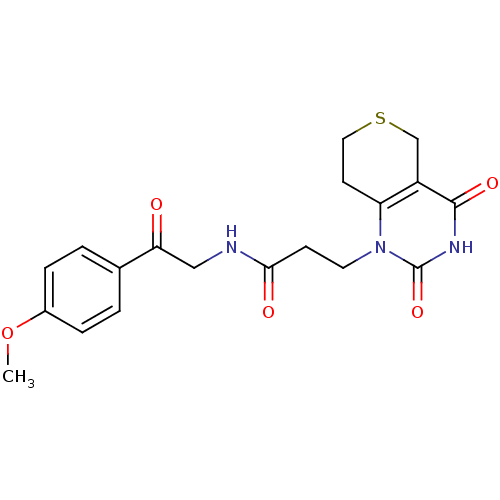 (3-(2,4-Dioxo-3,4,7,8-tetrahydro-2H,5H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255501 (3-(2,4-dioxo-2,3,4,5,7,8-hexahydro-1H-thiopyrano[4...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255360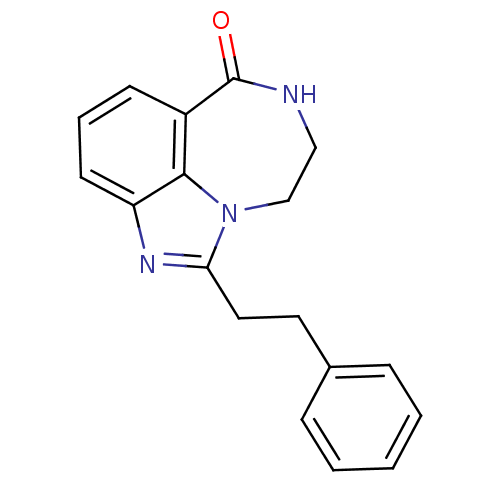 (1-Phenethyl-8,9-dihydro-7H-2,7,9a-triaza-benzo[cd]...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255630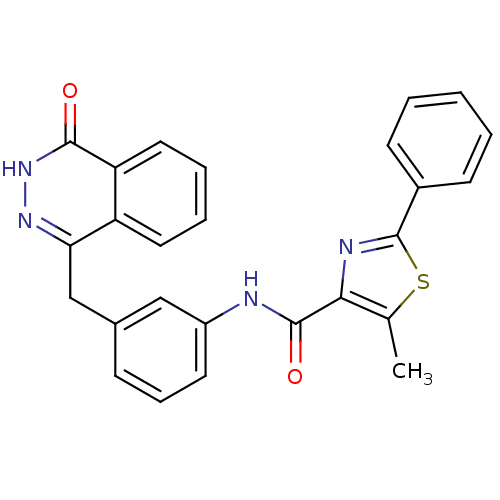 (5-methyl-N-(3-((4-oxo-3,4-dihydrophthalazin-1-yl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165484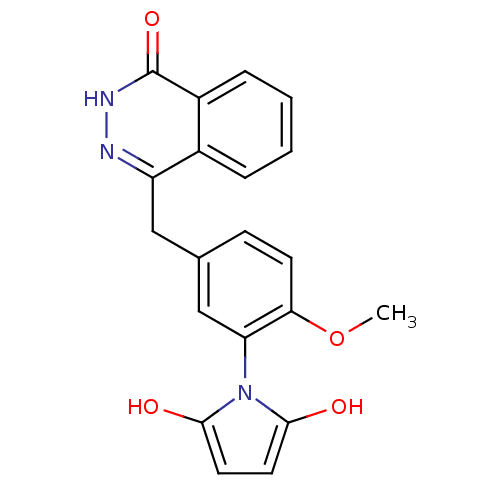 (1-(2-methoxy-5-((4-oxo-3,4-dihydrophthalazin-1-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165474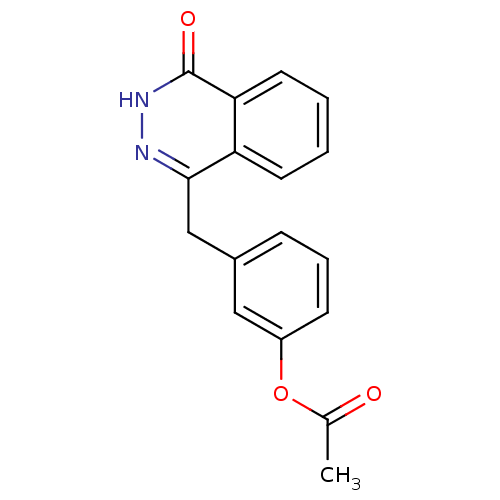 (3-((4-oxo-3,4-dihydrophthalazin-1-yl)methyl)phenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50384894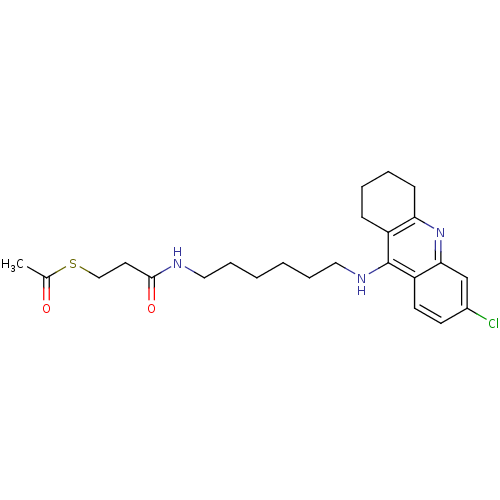 (CHEMBL2036262) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase using acetylcholine chloride as substrate incubated for 5 mins prior to substrate addition measured... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255572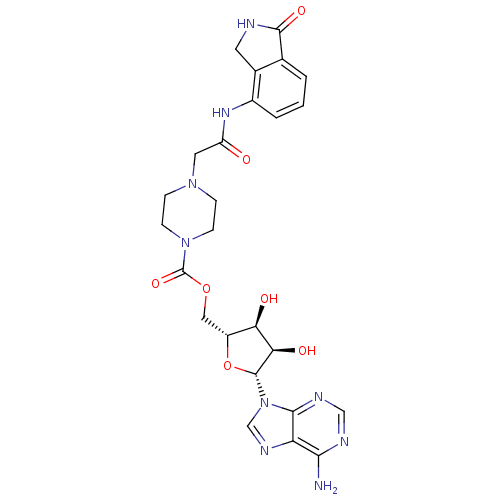 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393854 (CHEMBL2158126) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255502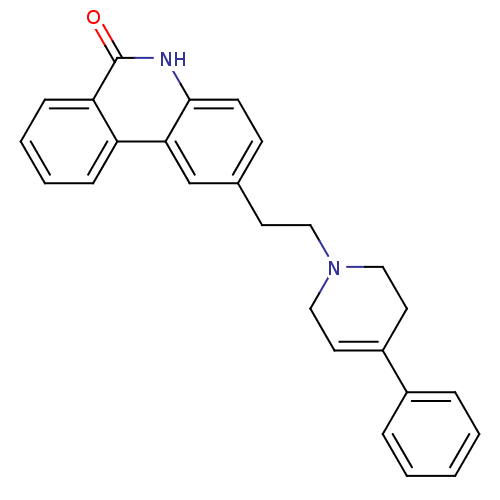 (2-(2-(4-phenyl-5,6-dihydropyridin-1(2H)-yl)ethyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165483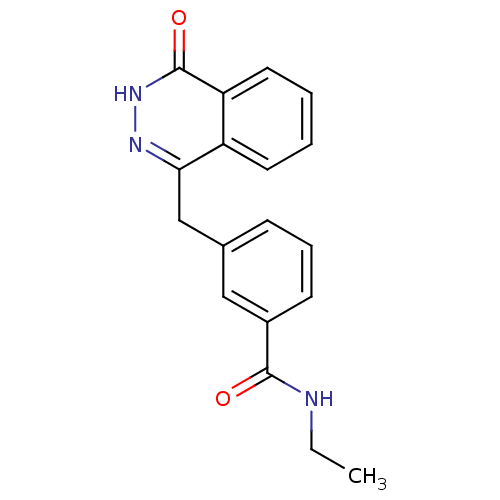 (CHEMBL381208 | N-Ethyl-3-(4-oxo-3,4-dihydro-phthal...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165471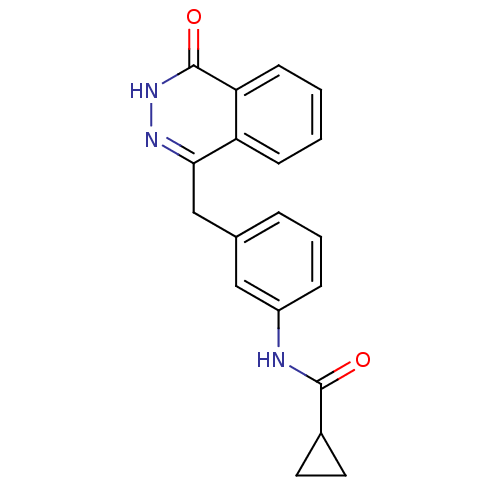 (CHEMBL196507 | Cyclopropanecarboxylic acid [3-(4-o...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50240980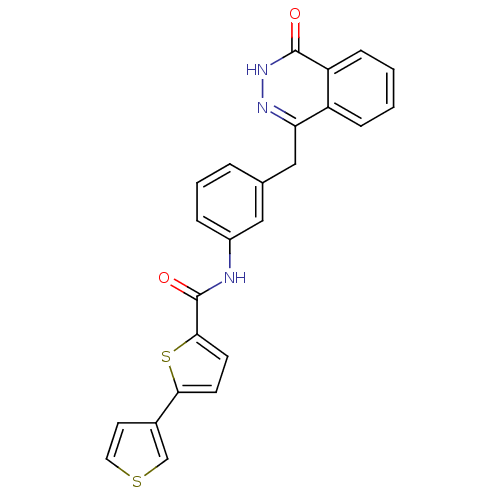 (CHEMBL372450 | N-(3-((4-oxo-3,4-dihydrophthalazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255411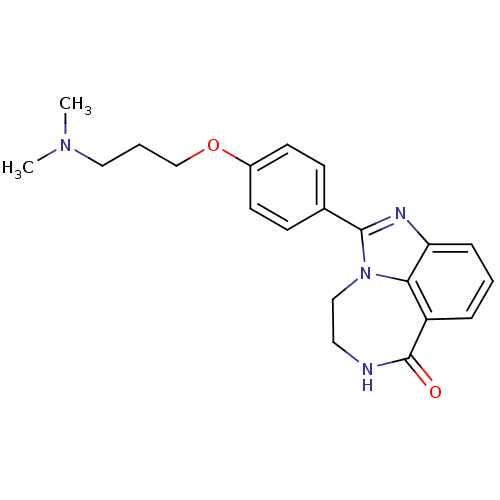 (1-[4-(3-Dimethylamino-propoxy)-phenyl]-8,9-dihydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27716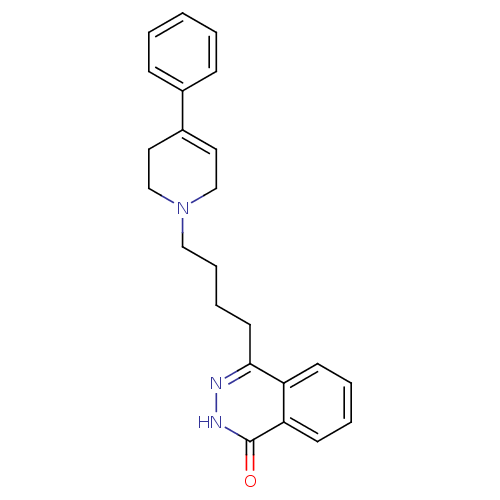 (4-(4-(4-phenyl-5,6-dihydropyridin-1(2H)-yl)butyl)p...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase using acetylcholine chloride as substrate incubated for 5 mins prior to substrate addition measured... | J Med Chem 55: 3588-92 (2012) Article DOI: 10.1021/jm300124p BindingDB Entry DOI: 10.7270/Q2QJ7JBD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 64.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27723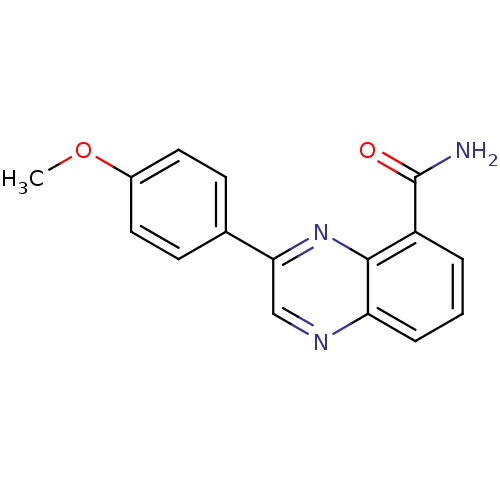 (3-(4-methoxyphenyl)quinoxaline-5-carboxamide | CHE...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393868 (CHEMBL2158112) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50393860 (CHEMBL2158120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50393853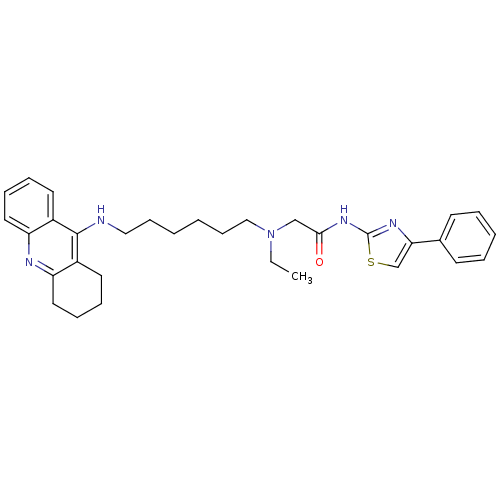 (CHEMBL2158127) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine chloride substrate incubated for 5 mins before substrate addition measured at 3 min interval ... | Bioorg Med Chem 20: 6513-22 (2012) Article DOI: 10.1016/j.bmc.2012.08.040 BindingDB Entry DOI: 10.7270/Q20Z74DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM27724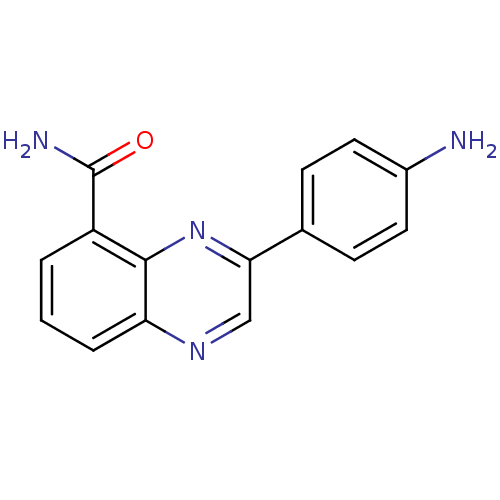 (3-(4-aminophenyl)quinoxaline-5-carboxamide | CHEMB...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50255795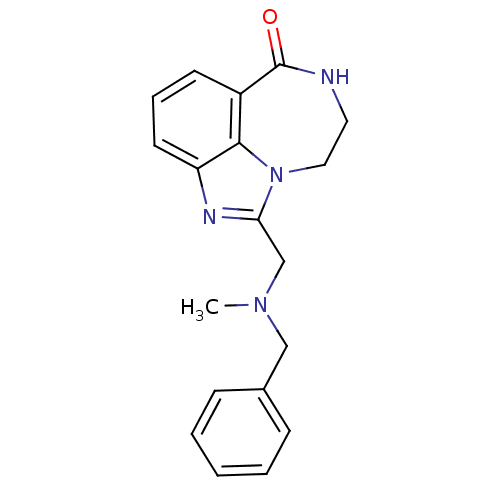 (1-[(Benzyl-methyl-amino)-methyl]-8,9-dihydro-7H-2,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase 1 (Homo sapiens (Human)) | BDBM50165481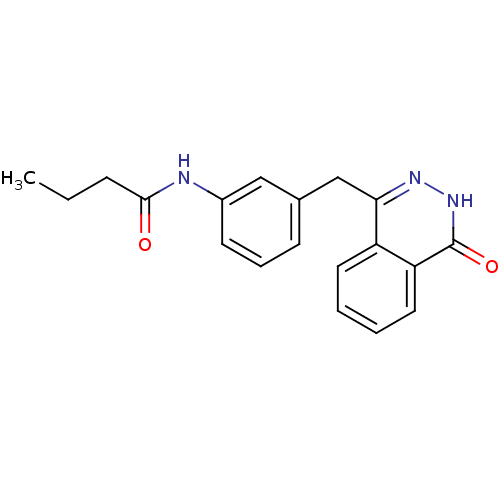 (CHEMBL425560 | N-(3-((4-oxo-3,4-dihydrophthalazin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Huazhong University of Science and Technology Curated by ChEMBL | Assay Description Inhibition of human recombinant PARP1 | J Med Chem 52: 718-25 (2009) Article DOI: 10.1021/jm800902t BindingDB Entry DOI: 10.7270/Q2C53KPW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 112 total ) | Next | Last >> |