 Found 46 hits with Last Name = 'kirkpatrick' and Initial = 'j'
Found 46 hits with Last Name = 'kirkpatrick' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233679
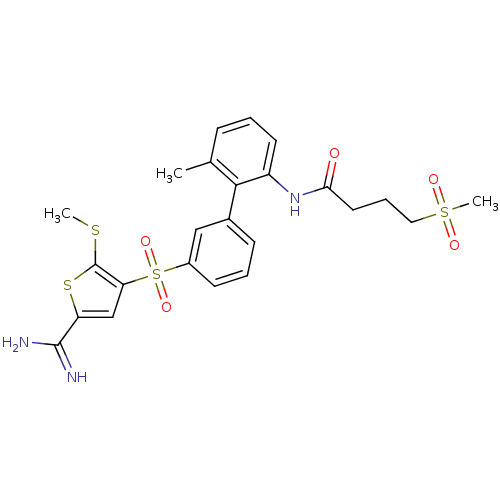
(CHEMBL399284 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C24H27N3O5S4/c1-15-7-4-10-18(27-21(28)11-6-12-35(3,29)30)22(15)16-8-5-9-17(13-16)36(31,32)20-14-19(23(25)26)34-24(20)33-2/h4-5,7-10,13-14H,6,11-12H2,1-3H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233691

(4-(2'-amino-6'-methyl-biphenyl-3-sulfonyl)-5-methy...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1N)C(N)=N Show InChI InChI=1S/C19H19N3O2S3/c1-11-5-3-8-14(20)17(11)12-6-4-7-13(9-12)27(23,24)16-10-15(18(21)22)26-19(16)25-2/h3-10H,20H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233674
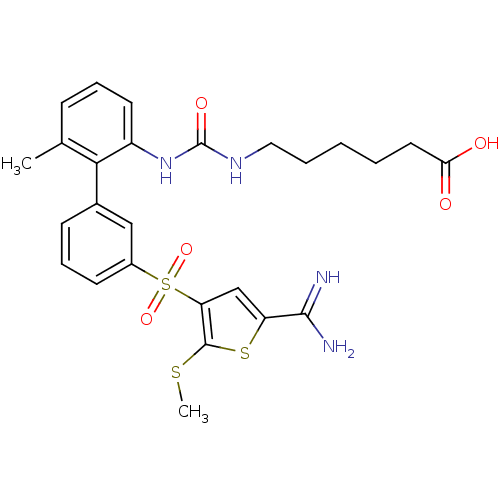
(6-{3-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophe...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCCCCC(O)=O)C(N)=N Show InChI InChI=1S/C26H30N4O5S3/c1-16-8-6-11-19(30-26(33)29-13-5-3-4-12-22(31)32)23(16)17-9-7-10-18(14-17)38(34,35)21-15-20(24(27)28)37-25(21)36-2/h6-11,14-15H,3-5,12-13H2,1-2H3,(H3,27,28)(H,31,32)(H2,29,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233686

(4-(2'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-3-4-9-15(12)13-7-5-8-14(10-13)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233692

(4-[3-(6-methyl-pyridin-2-yl)-benzenesulfonyl]-5-me...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)n1)C(N)=N Show InChI InChI=1S/C18H17N3O2S3/c1-11-5-3-8-14(21-11)12-6-4-7-13(9-12)26(22,23)16-10-15(17(19)20)25-18(16)24-2/h3-10H,1-2H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233688

(4-(2'-chloro-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1Cl)C(N)=N Show InChI InChI=1S/C18H15ClN2O2S3/c1-24-18-16(10-15(25-18)17(20)21)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)19/h2-10H,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233694

(5-[3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CCCCC(O)=O)C(N)=N Show InChI InChI=1S/C25H27N3O5S3/c1-15-7-5-10-18(28-21(29)11-3-4-12-22(30)31)23(15)16-8-6-9-17(13-16)36(32,33)20-14-19(24(26)27)35-25(20)34-2/h5-10,13-14H,3-4,11-12H2,1-2H3,(H3,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233677

(4-(2'-hydroxymethyl-6'-methyl-biphenyl-3-sulfonyl)...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1CO)C(N)=N Show InChI InChI=1S/C20H20N2O3S3/c1-12-5-3-7-14(11-23)18(12)13-6-4-8-15(9-13)28(24,25)17-10-16(19(21)22)27-20(17)26-2/h3-10,23H,11H2,1-2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233689
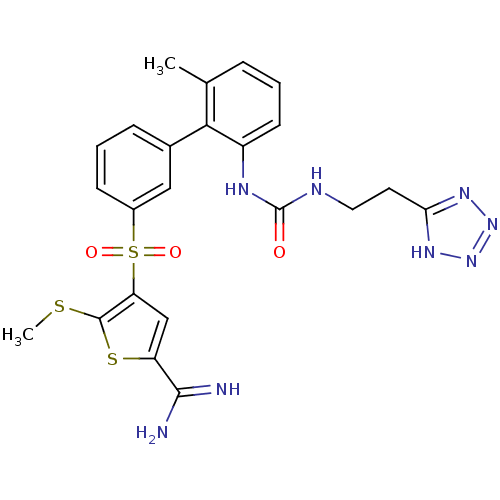
(5-methylsulfanyl-4-(6'-methyl-2'-{3-[2-(2H-tetrazo...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)NCCc1nnn[nH]1)C(N)=N Show InChI InChI=1S/C23H24N8O3S3/c1-13-5-3-8-16(27-23(32)26-10-9-19-28-30-31-29-19)20(13)14-6-4-7-15(11-14)37(33,34)18-12-17(21(24)25)36-22(18)35-2/h3-8,11-12H,9-10H2,1-2H3,(H3,24,25)(H2,26,27,32)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233680

(4-(2'-hydroxymethyl-biphenyl-3-sulfonyl)-5-methyls...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1CO)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-25-19-17(10-16(26-19)18(20)21)27(23,24)14-7-4-6-12(9-14)15-8-3-2-5-13(15)11-22/h2-10,22H,11H2,1H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233693

(CHEMBL252619 | N-[3'-(5-carbamimidoyl-2-methylsulf...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1NC(=O)CS(C)(=O)=O)C(N)=N Show InChI InChI=1S/C22H23N3O5S4/c1-13-6-4-9-16(25-19(26)12-33(3,27)28)20(13)14-7-5-8-15(10-14)34(29,30)18-11-17(21(23)24)32-22(18)31-2/h4-11H,12H2,1-3H3,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233681
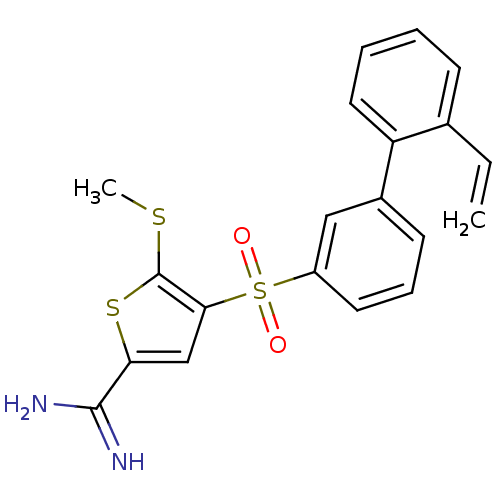
(5-methylsulfanyl-4-(2'-vinyl-biphenyl-3-sulfonyl)-...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1C=C)C(N)=N Show InChI InChI=1S/C20H18N2O2S3/c1-3-13-7-4-5-10-16(13)14-8-6-9-15(11-14)27(23,24)18-12-17(19(21)22)26-20(18)25-2/h3-12H,1H2,2H3,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233676

(3'-(5-carbamimidoyl-2-methylsulfanyl-thiophene-3-s...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1c(C)cccc1C(O)=O)C(N)=N Show InChI InChI=1S/C20H18N2O4S3/c1-11-5-3-8-14(19(23)24)17(11)12-6-4-7-13(9-12)29(25,26)16-10-15(18(21)22)28-20(16)27-2/h3-10H,1-2H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233683
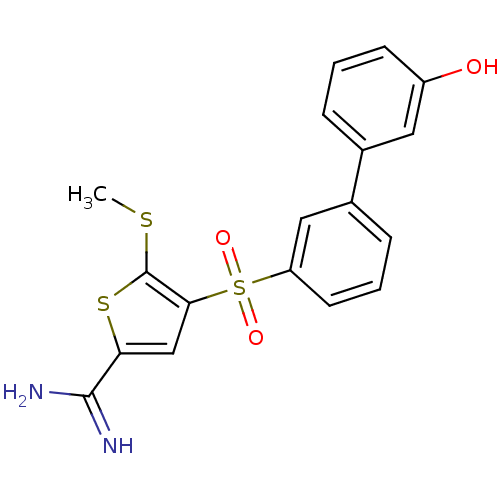
(4-(3'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(O)c1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-7-3-5-12(9-14)11-4-2-6-13(21)8-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233678

(4-(4'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccc(O)cc1)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)14-4-2-3-12(9-14)11-5-7-13(21)8-6-11/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233687

(4-(4'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccc(C)cc1)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-6-8-13(9-7-12)14-4-3-5-15(10-14)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233675
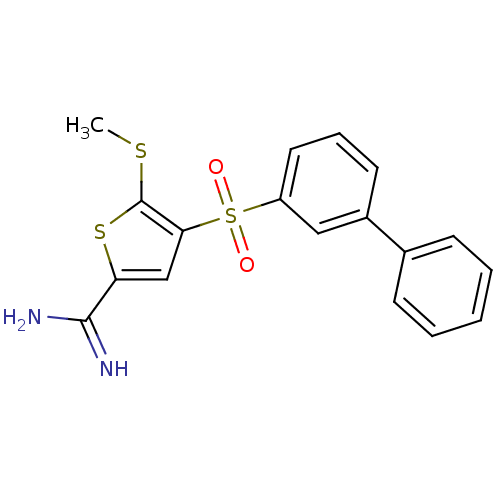
(4-(biphenyl-3-sulfonyl)-5-methylsulfanyl-thiophene...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1)C(N)=N Show InChI InChI=1S/C18H16N2O2S3/c1-23-18-16(11-15(24-18)17(19)20)25(21,22)14-9-5-8-13(10-14)12-6-3-2-4-7-12/h2-11H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233695

(4-(4-methoxy-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES COc1ccc(cc1)-c1cccc(c1)S(=O)(=O)c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-24-14-8-6-12(7-9-14)13-4-3-5-15(10-13)27(22,23)17-11-16(18(20)21)26-19(17)25-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233690
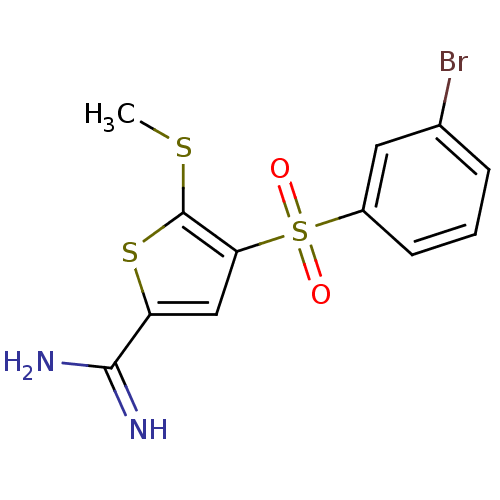
(4-(3-bromo-benzenesulfonyl)-5-methylsulfanyl-thiop...)Show InChI InChI=1S/C12H11BrN2O2S3/c1-18-12-10(6-9(19-12)11(14)15)20(16,17)8-4-2-3-7(13)5-8/h2-6H,1H3,(H3,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233685

(4-(2'-hydroxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1ccccc1O)C(N)=N Show InChI InChI=1S/C18H16N2O3S3/c1-24-18-16(10-15(25-18)17(19)20)26(22,23)12-6-4-5-11(9-12)13-7-2-3-8-14(13)21/h2-10,21H,1H3,(H3,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233682
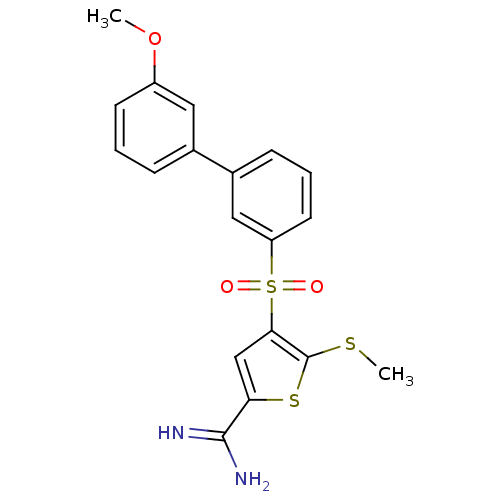
(4-(3-methoxy-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES COc1cccc(c1)-c1cccc(c1)S(=O)(=O)c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-24-14-7-3-5-12(9-14)13-6-4-8-15(10-13)27(22,23)17-11-16(18(20)21)26-19(17)25-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233684

(4-(2'-methoxy-biphenyl-3-sulfonyl)-5-methylsulfany...)Show SMILES COc1ccccc1-c1cccc(c1)S(=O)(=O)c1cc(sc1SC)C(N)=N Show InChI InChI=1S/C19H18N2O3S3/c1-24-15-9-4-3-8-14(15)12-6-5-7-13(10-12)27(22,23)17-11-16(18(20)21)26-19(17)25-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Complement C1s subcomponent
(Homo sapiens (Human)) | BDBM50233696
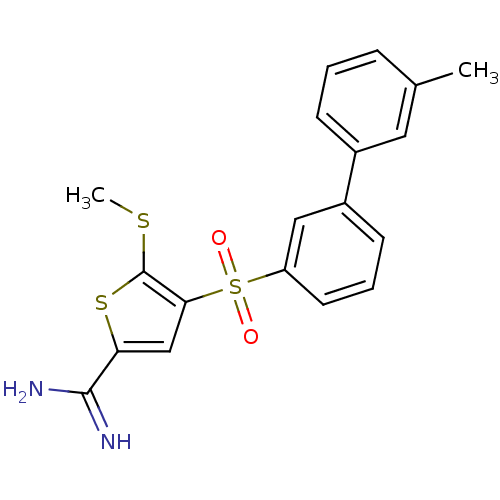
(4-(3'-methyl-biphenyl-3-sulfonyl)-5-methylsulfanyl...)Show SMILES CSc1sc(cc1S(=O)(=O)c1cccc(c1)-c1cccc(C)c1)C(N)=N Show InChI InChI=1S/C19H18N2O2S3/c1-12-5-3-6-13(9-12)14-7-4-8-15(10-14)26(22,23)17-11-16(18(20)21)25-19(17)24-2/h3-11H,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Complement C1s subcomponent |
Bioorg Med Chem Lett 18: 1603-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.064
BindingDB Entry DOI: 10.7270/Q2K35TD4 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217358

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C23H25N7O3/c1-32-27-15-20-21(24)25-16-26-22(20)29-11-13-30(14-12-29)23(31)28-17-7-9-19(10-8-17)33-18-5-3-2-4-6-18/h2-10,15-16H,11-14H2,1H3,(H,28,31)(H2,24,25,26)/b27-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217367
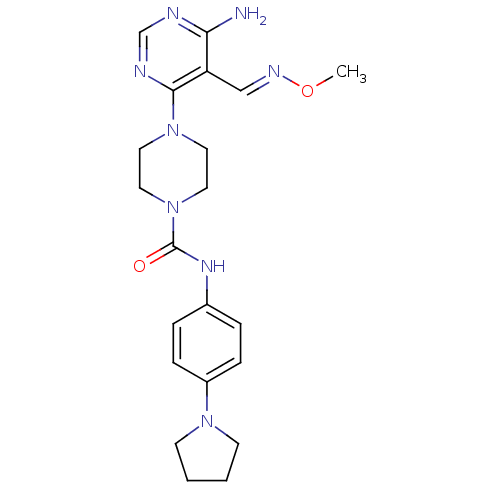
(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C21H28N8O2/c1-31-25-14-18-19(22)23-15-24-20(18)28-10-12-29(13-11-28)21(30)26-16-4-6-17(7-5-16)27-8-2-3-9-27/h4-7,14-15H,2-3,8-13H2,1H3,(H,26,30)(H2,22,23,24)/b25-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217357
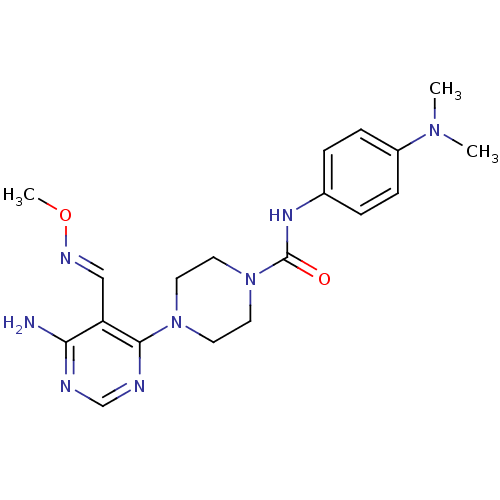
(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N(C)C Show InChI InChI=1S/C19H26N8O2/c1-25(2)15-6-4-14(5-7-15)24-19(28)27-10-8-26(9-11-27)18-16(12-23-29-3)17(20)21-13-22-18/h4-7,12-13H,8-11H2,1-3H3,(H,24,28)(H2,20,21,22)/b23-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217366

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)C(C)C Show InChI InChI=1S/C20H27N7O2/c1-14(2)15-4-6-16(7-5-15)25-20(28)27-10-8-26(9-11-27)19-17(12-24-29-3)18(21)22-13-23-19/h4-7,12-14H,8-11H2,1-3H3,(H,25,28)(H2,21,22,23)/b24-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217350

(4-(6-amino-5-((ethoxyimino)methyl)pyrimidin-4-yl)-...)Show SMILES CCO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C21H29N7O3/c1-4-30-25-13-18-19(22)23-14-24-20(18)27-9-11-28(12-10-27)21(29)26-16-5-7-17(8-6-16)31-15(2)3/h5-8,13-15H,4,9-12H2,1-3H3,(H,26,29)(H2,22,23,24)/b25-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217362
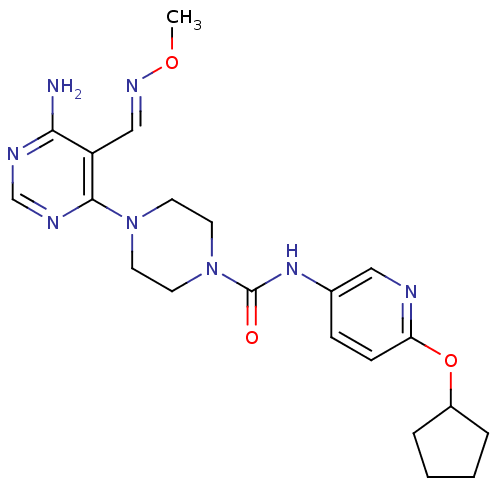
(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC2CCCC2)nc1 Show InChI InChI=1S/C21H28N8O3/c1-31-26-13-17-19(22)24-14-25-20(17)28-8-10-29(11-9-28)21(30)27-15-6-7-18(23-12-15)32-16-4-2-3-5-16/h6-7,12-14,16H,2-5,8-11H2,1H3,(H,27,30)(H2,22,24,25)/b26-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217348

(4-(6-amino-5-((2-morpholinoethoxyimino)methyl)pyri...)Show SMILES CC(C)Oc1ccc(NC(=O)N2CCN(CC2)c2ncnc(N)c2\C=N\OCCN2CCOCC2)cc1 Show InChI InChI=1S/C25H36N8O4/c1-19(2)37-21-5-3-20(4-6-21)30-25(34)33-9-7-32(8-10-33)24-22(23(26)27-18-28-24)17-29-36-16-13-31-11-14-35-15-12-31/h3-6,17-19H,7-16H2,1-2H3,(H,30,34)(H2,26,27,28)/b29-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217349

(4-(6-amino-5-((2-morpholino-2-oxoethoxyimino)methy...)Show SMILES Nc1ncnc(N2CCN(CC2)C(=O)Nc2ccc(cc2)N2CCCC2)c1\C=N\OCC(=O)N1CCOCC1 Show InChI InChI=1S/C26H35N9O4/c27-24-22(17-30-39-18-23(36)33-13-15-38-16-14-33)25(29-19-28-24)34-9-11-35(12-10-34)26(37)31-20-3-5-21(6-4-20)32-7-1-2-8-32/h3-6,17,19H,1-2,7-16,18H2,(H,31,37)(H2,27,28,29)/b30-17+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217361

(4-(6-amino-5-((ethoxyimino)methyl)pyrimidin-4-yl)-...)Show SMILES CCO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C23H32N8O2/c1-2-33-27-16-20-21(24)25-17-26-22(20)30-12-14-31(15-13-30)23(32)28-18-6-8-19(9-7-18)29-10-4-3-5-11-29/h6-9,16-17H,2-5,10-15H2,1H3,(H,28,32)(H2,24,25,26)/b27-16+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217360
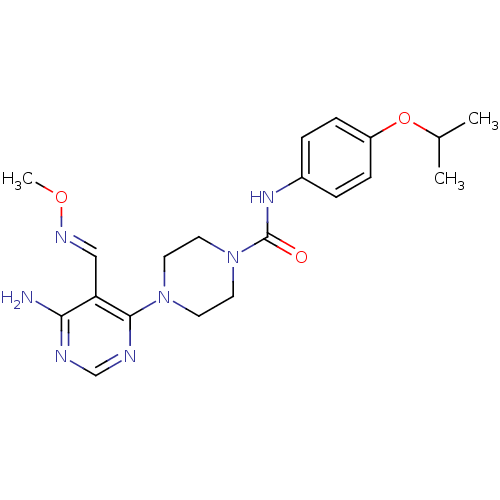
(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C20H27N7O3/c1-14(2)30-16-6-4-15(5-7-16)25-20(28)27-10-8-26(9-11-27)19-17(12-24-29-3)18(21)22-13-23-19/h4-7,12-14H,8-11H2,1-3H3,(H,25,28)(H2,21,22,23)/b24-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217359

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCCCC1 Show InChI InChI=1S/C22H30N8O2/c1-32-26-15-19-20(23)24-16-25-21(19)29-11-13-30(14-12-29)22(31)27-17-5-7-18(8-6-17)28-9-3-2-4-10-28/h5-8,15-16H,2-4,9-14H2,1H3,(H,27,31)(H2,23,24,25)/b26-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217352

(4-(6-amino-5-((ethoxyimino)methyl)pyrimidin-4-yl)-...)Show SMILES CCO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC2CCC2)nc1 Show InChI InChI=1S/C21H28N8O3/c1-2-31-26-13-17-19(22)24-14-25-20(17)28-8-10-29(11-9-28)21(30)27-15-6-7-18(23-12-15)32-16-4-3-5-16/h6-7,12-14,16H,2-5,8-11H2,1H3,(H,27,30)(H2,22,24,25)/b26-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217353
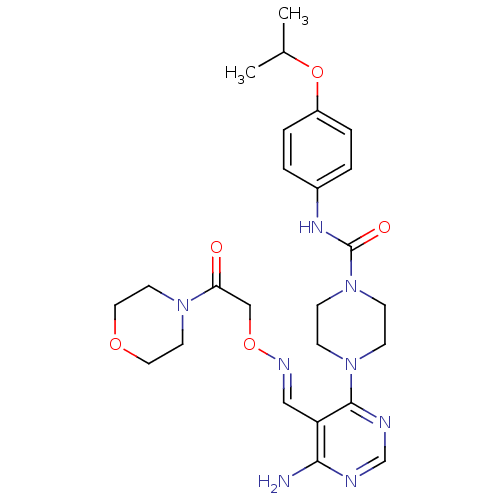
((E)-4-(6-amino-5-((2-morpholino-2-oxoethoxyimino)m...)Show SMILES CC(C)Oc1ccc(NC(=O)N2CCN(CC2)c2ncnc(N)c2\C=N\OCC(=O)N2CCOCC2)cc1 Show InChI InChI=1S/C25H34N8O5/c1-18(2)38-20-5-3-19(4-6-20)30-25(35)33-9-7-32(8-10-33)24-21(23(26)27-17-28-24)15-29-37-16-22(34)31-11-13-36-14-12-31/h3-6,15,17-18H,7-14,16H2,1-2H3,(H,30,35)(H2,26,27,28)/b29-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217347
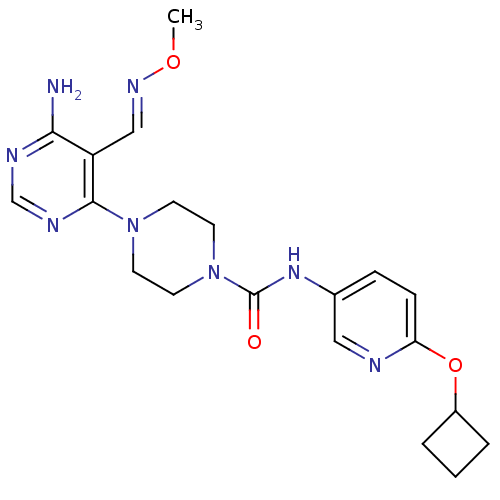
(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC2CCC2)nc1 Show InChI InChI=1S/C20H26N8O3/c1-30-25-12-16-18(21)23-13-24-19(16)27-7-9-28(10-8-27)20(29)26-14-5-6-17(22-11-14)31-15-3-2-4-15/h5-6,11-13,15H,2-4,7-10H2,1H3,(H,26,29)(H2,21,23,24)/b25-12+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217365

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)C1CCCCC1 Show InChI InChI=1S/C23H31N7O2/c1-32-27-15-20-21(24)25-16-26-22(20)29-11-13-30(14-12-29)23(31)28-19-9-7-18(8-10-19)17-5-3-2-4-6-17/h7-10,15-17H,2-6,11-14H2,1H3,(H,28,31)(H2,24,25,26)/b27-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217356

(4-(6-amino-5-((2-(methylsulfonamido)ethoxyimino)me...)Show SMILES CC(C)Oc1ccc(NC(=O)N2CCN(CC2)c2ncnc(N)c2\C=N\OCCNS(C)(=O)=O)cc1 Show InChI InChI=1S/C22H32N8O5S/c1-16(2)35-18-6-4-17(5-7-18)28-22(31)30-11-9-29(10-12-30)21-19(20(23)24-15-25-21)14-26-34-13-8-27-36(3,32)33/h4-7,14-16,27H,8-13H2,1-3H3,(H,28,31)(H2,23,24,25)/b26-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 146 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217355

((E)-4-(6-amino-5-((2-(methylsulfonamido)ethoxyimin...)Show SMILES CS(=O)(=O)NCCO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCCC1 Show InChI InChI=1S/C23H33N9O4S/c1-37(34,35)28-8-15-36-27-16-20-21(24)25-17-26-22(20)31-11-13-32(14-12-31)23(33)29-18-4-6-19(7-5-18)30-9-2-3-10-30/h4-7,16-17,28H,2-3,8-15H2,1H3,(H,29,33)(H2,24,25,26)/b27-16+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217363

((E)-4-(6-amino-5-((2-morpholinoethoxyimino)methyl)...)Show SMILES Nc1ncnc(N2CCN(CC2)C(=O)Nc2ccc(cc2)N2CCCC2)c1\C=N\OCCN1CCOCC1 Show InChI InChI=1S/C26H37N9O3/c27-24-23(19-30-38-18-15-32-13-16-37-17-14-32)25(29-20-28-24)34-9-11-35(12-10-34)26(36)31-21-3-5-22(6-4-21)33-7-1-2-8-33/h3-6,19-20H,1-2,7-18H2,(H,31,36)(H2,27,28,29)/b30-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217351

(4-(6-amino-5-((3-hydroxypropoxyimino)methyl)pyrimi...)Show SMILES CC(C)Oc1ccc(NC(=O)N2CCN(CC2)c2ncnc(N)c2\C=N\OCCCO)cc1 Show InChI InChI=1S/C22H31N7O4/c1-16(2)33-18-6-4-17(5-7-18)27-22(31)29-10-8-28(9-11-29)21-19(20(23)24-15-25-21)14-26-32-13-3-12-30/h4-7,14-16,30H,3,8-13H2,1-2H3,(H,27,31)(H2,23,24,25)/b26-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217354

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(cc1)N1CCOCC1 Show InChI InChI=1S/C21H28N8O3/c1-31-25-14-18-19(22)23-15-24-20(18)28-6-8-29(9-7-28)21(30)26-16-2-4-17(5-3-16)27-10-12-32-13-11-27/h2-5,14-15H,6-13H2,1H3,(H,26,30)(H2,22,23,24)/b25-14+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50217364

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C17H20ClN7O2/c1-27-22-10-14-15(19)20-11-21-16(14)24-6-8-25(9-7-24)17(26)23-13-4-2-12(18)3-5-13/h2-5,10-11H,6-9H2,1H3,(H,23,26)(H2,19,20,21)/b22-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of human FLT3 |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50217360

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C20H27N7O3/c1-14(2)30-16-6-4-15(5-7-16)25-20(28)27-10-8-26(9-11-27)19-17(12-24-29-3)18(21)22-13-23-19/h4-7,12-14H,8-11H2,1-3H3,(H,25,28)(H2,21,22,23)/b24-12+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of c-kit |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Homo sapiens (Human)) | BDBM50217360

(4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...)Show SMILES CO\N=C\c1c(N)ncnc1N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 Show InChI InChI=1S/C20H27N7O3/c1-14(2)30-16-6-4-15(5-7-16)25-20(28)27-10-8-26(9-11-27)19-17(12-24-29-3)18(21)22-13-23-19/h4-7,12-14H,8-11H2,1-3H3,(H,25,28)(H2,21,22,23)/b24-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRbeta |
Bioorg Med Chem Lett 17: 4861-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.06.046
BindingDB Entry DOI: 10.7270/Q2T72H47 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data