

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM155343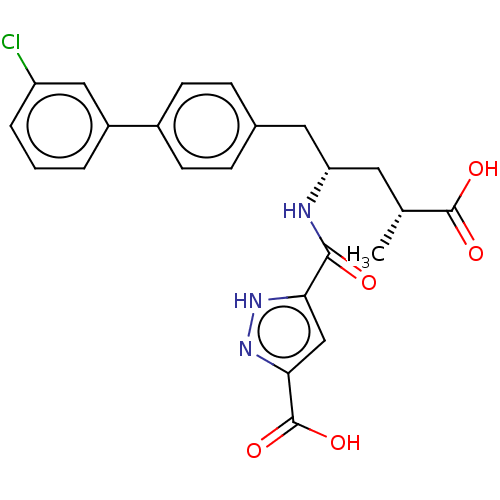 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155343 (US9006249, Example 49-2 | US9603819, Example 49-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153121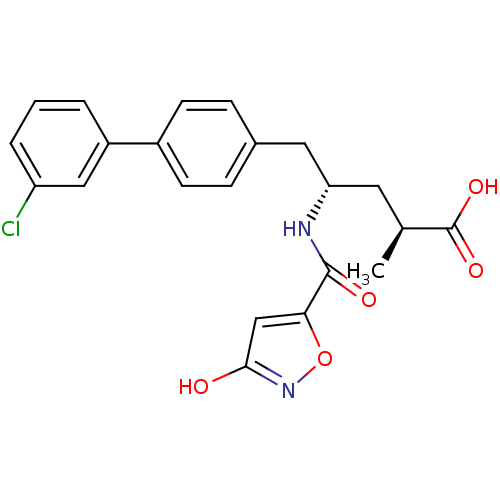 (US8993631, 29-2 | US9006249, Example 49-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM309469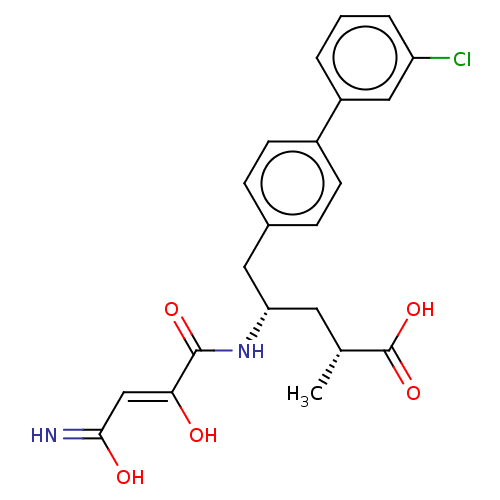 ((2R,4S)-5-(3′-Chloro-biphenyl-4-yl)-4-[(3-hy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130367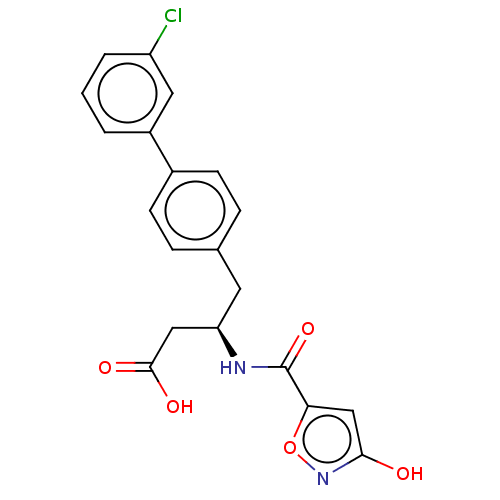 (US8822534, Example 11-14) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM138390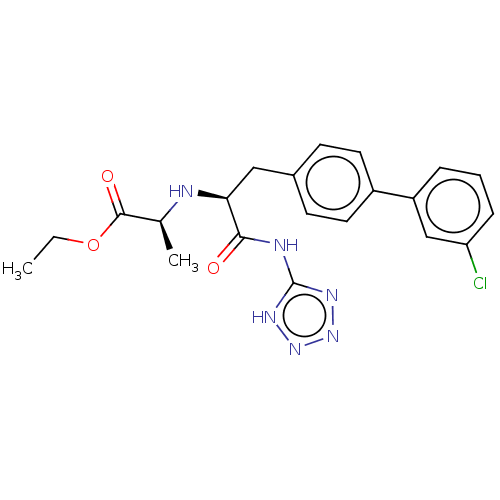 (US8877786, Example 3-1) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description The in vitro inhibition of recombinant human neutral endopeptidase (NEP, EC 3.424.11) can be determined as follows:Recombinant human neutral endopept... | US Patent US8877786 (2014) BindingDB Entry DOI: 10.7270/Q28914KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130365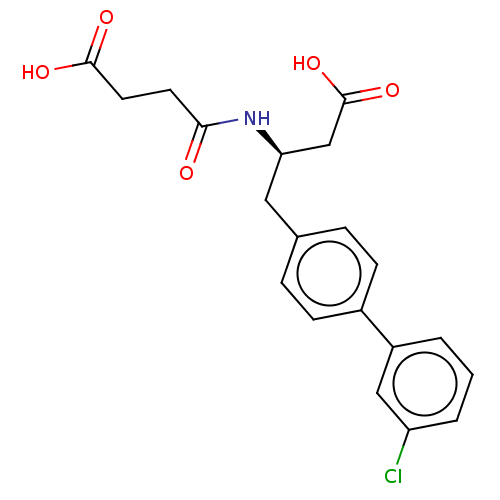 (US8822534, Example 11-1 | US8822534, Example 12-1 ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM138396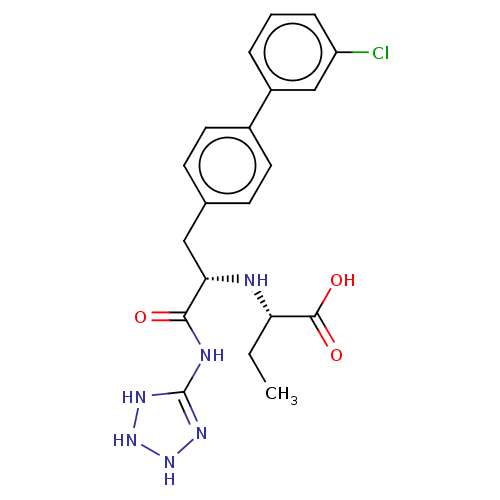 (US8877786, Example 3-13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description The in vitro inhibition of recombinant human neutral endopeptidase (NEP, EC 3.424.11) can be determined as follows:Recombinant human neutral endopept... | US Patent US8877786 (2014) BindingDB Entry DOI: 10.7270/Q28914KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM138395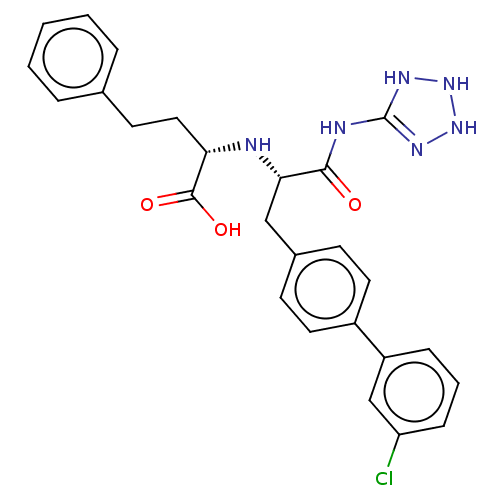 (US8877786, Example 3-12) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description The in vitro inhibition of recombinant human neutral endopeptidase (NEP, EC 3.424.11) can be determined as follows:Recombinant human neutral endopept... | US Patent US8877786 (2014) BindingDB Entry DOI: 10.7270/Q28914KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344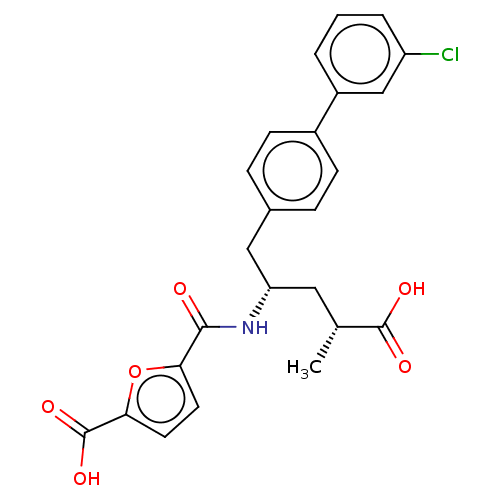 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM138391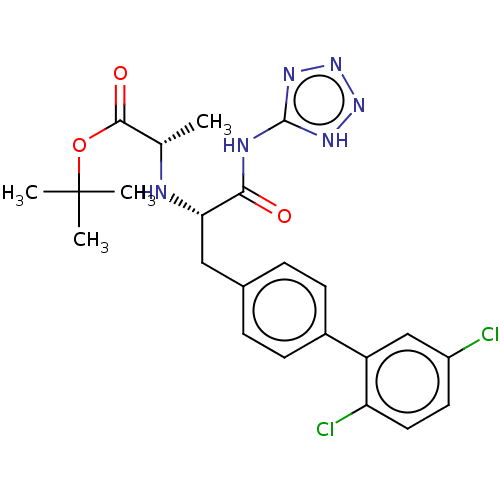 (US8877786, Example 3-2) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description The in vitro inhibition of recombinant human neutral endopeptidase (NEP, EC 3.424.11) can be determined as follows:Recombinant human neutral endopept... | US Patent US8877786 (2014) BindingDB Entry DOI: 10.7270/Q28914KV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323359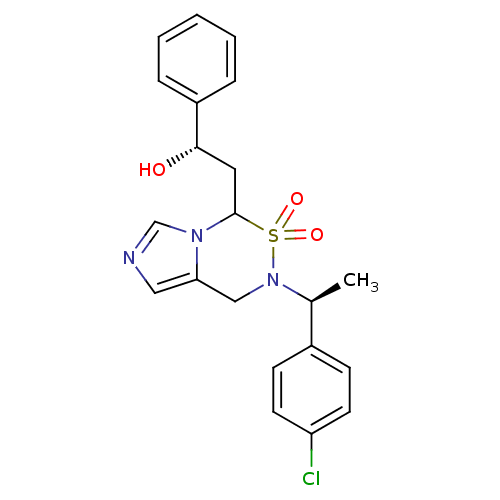 ((S)-2-{6-[(S)-1-(4-Chloro-phenyl)-ethyl]-5,5-dioxo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153128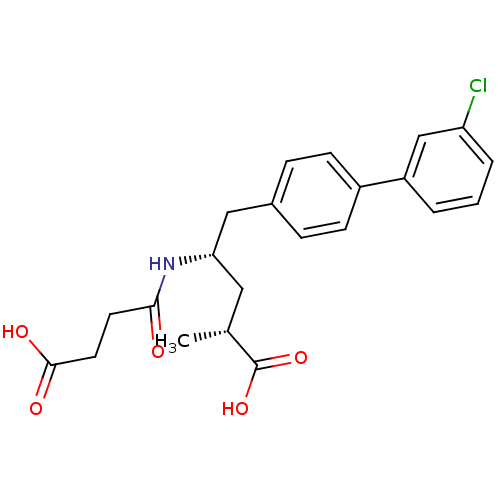 (US8993631, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM155344 (US9006249, Example 49-3 | US9603819, Example 49-3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM130359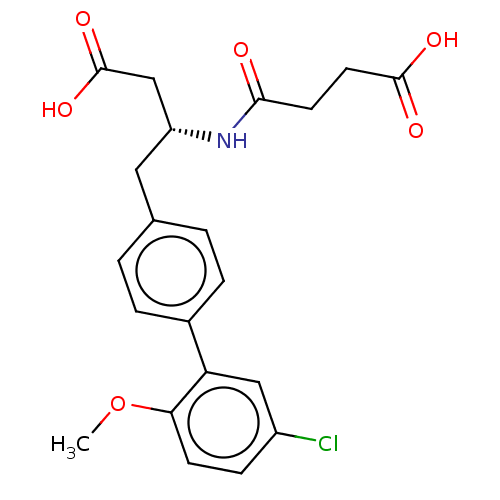 (US8822534, Example 5-39 | US8993631, 5-8) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in membranes prepared from rabbit | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130360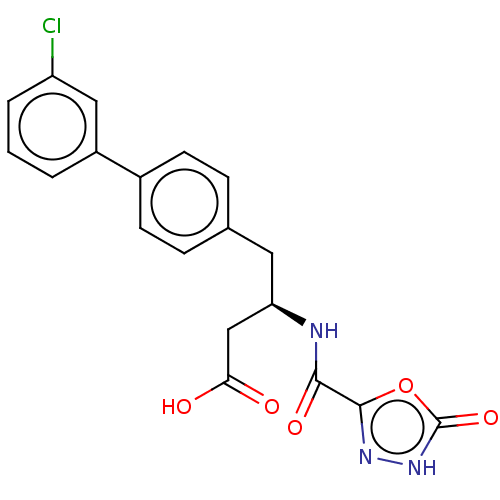 (US8822534, Example 5-46 | US8993631, 5-9) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130366 (US8822534, Example 11-11 | US8993631, 9-6) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibition of enkephalinase activity in synaptic membranes prepared from rat striatum | J Med Chem 32: 2519-26 (1989) BindingDB Entry DOI: 10.7270/Q2668DSV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034841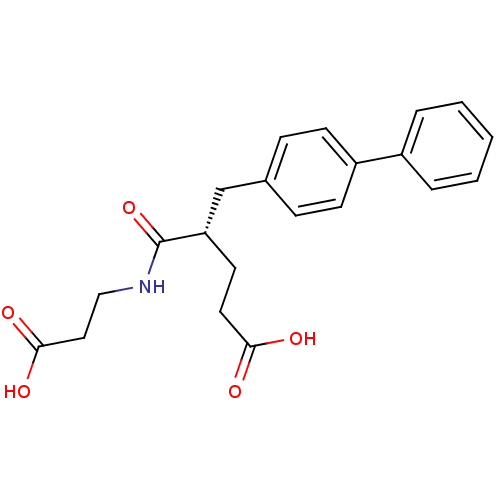 ((S)-5-Biphenyl-4-yl-4-(2-carboxy-ethylcarbamoyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130359 (US8822534, Example 5-39 | US8993631, 5-8) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130362 (US8822534, Example 5-55 | US8993631, 5-11) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107813 ((S)-methyl 5-(6-methyl-4'-(trifluoromethyl)bipheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034846 ((2R,4R)-5-Biphenyl-4-yl-4-(3-carboxy-propionylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323356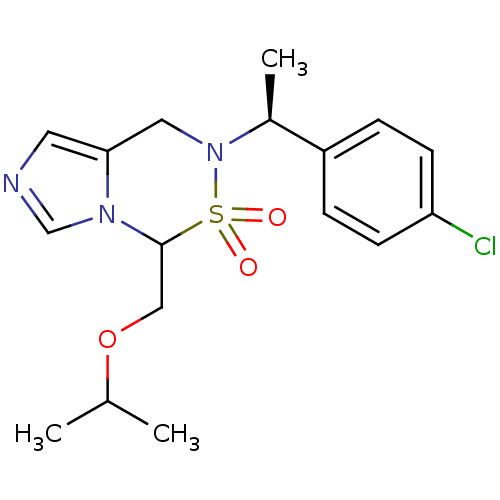 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-isopropoxymeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130369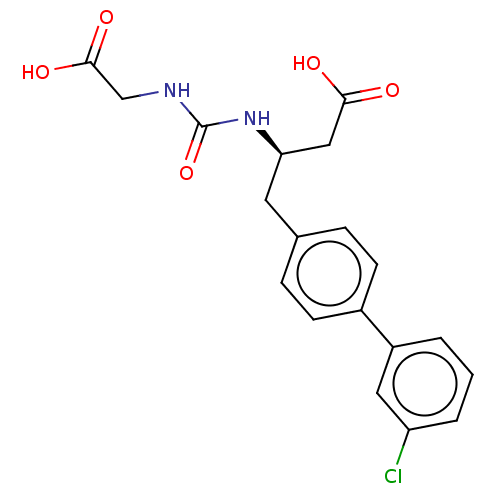 (US8822534, Example 14-1 | US8993631, 11-1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50509659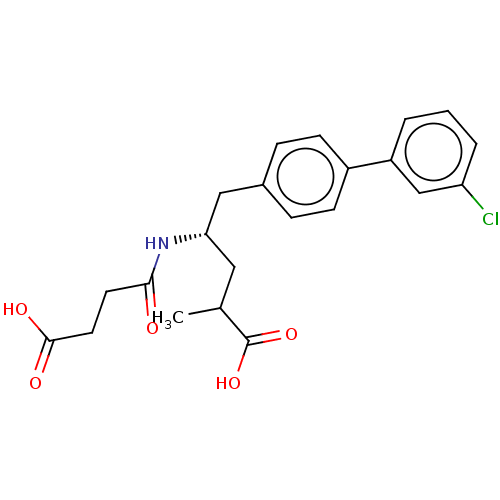 (CHEMBL4443138) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50034851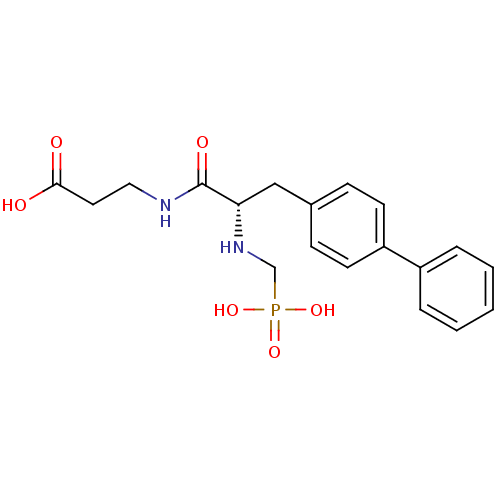 (3-[(S)-3-Biphenyl-4-yl-2-(phosphonomethyl-amino)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description The compound was tested in vitro for inhibition of Neutral endopeptidase by using Leu enkephalin as substrate | J Med Chem 38: 1689-700 (1995) BindingDB Entry DOI: 10.7270/Q2Z89BD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323355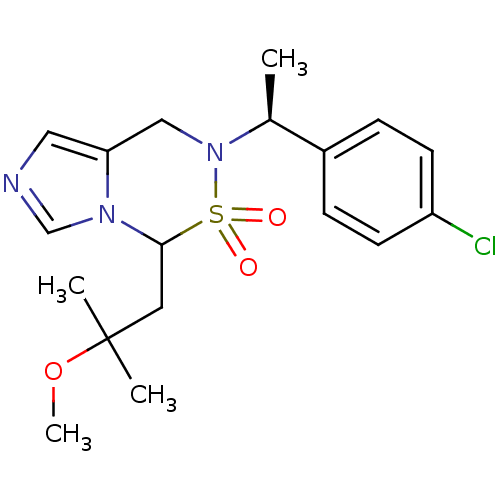 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-(2-methoxy-2-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107808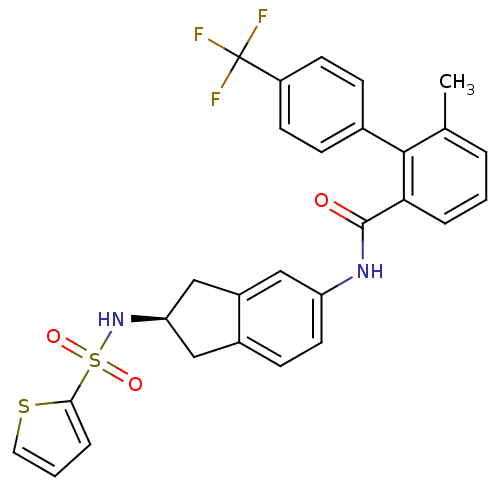 (6-Methyl-4'-trifluoromethyl-biphenyl-2-carboxylic ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50056252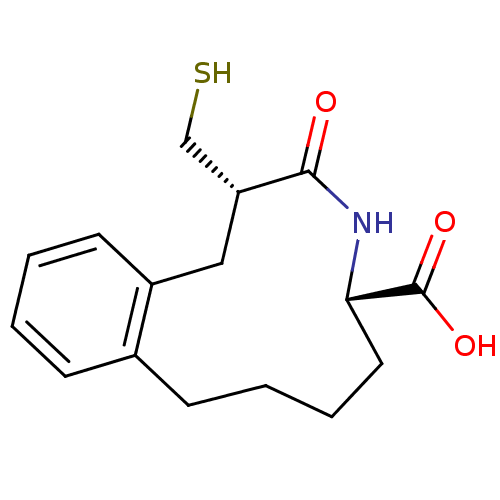 ((6S,9S)-6-Mercaptomethyl-7-oxo-6,7,8,9,10,11,12,13...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY Corporation Curated by ChEMBL | Assay Description Inhibitory concentration required to inhibit neutral endopeptidase (NEP) | J Med Chem 40: 506-14 (1997) Article DOI: 10.1021/jm960583g BindingDB Entry DOI: 10.7270/Q2TM797S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50056252 ((6S,9S)-6-Mercaptomethyl-7-oxo-6,7,8,9,10,11,12,13...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase | J Med Chem 40: 495-505 (1997) Article DOI: 10.1021/jm960582o BindingDB Entry DOI: 10.7270/Q2ZC81Z3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444548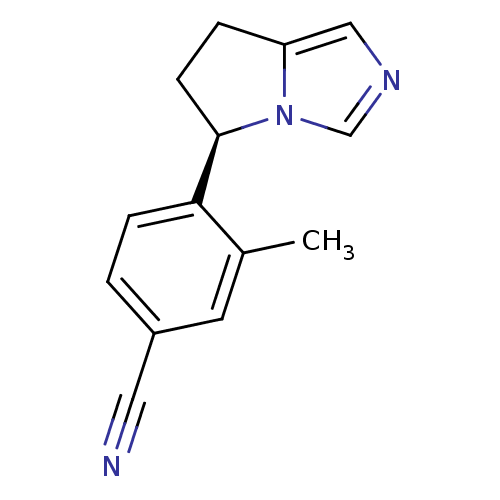 (CHEMBL3099696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description The activity of a compound according to the present invention can be assessed by the following in vitro & in vivo methods and/or by the following in ... | US Patent US9603819 (2017) BindingDB Entry DOI: 10.7270/Q20V8FVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107774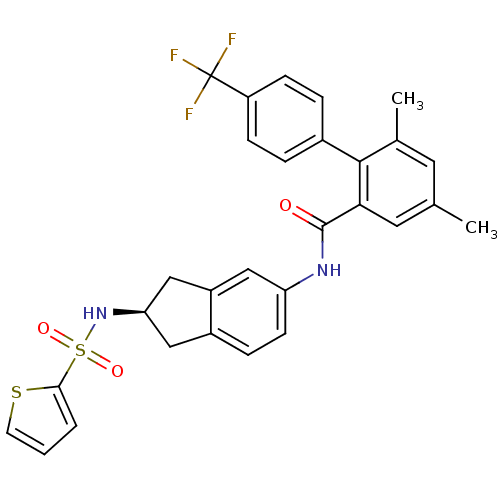 (4,6-Dimethyl-4'-trifluoromethyl-biphenyl-2-carboxy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153108 (US8993631, 16-5 | US9006249, Example 3-32 | US9603...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description See A fluorescence lifetime-based assay for protease inhibitor profiling on human kallikrein 7 Doering K, Meder G, Hinnenberger M, Woelcke J, Mayr L ... | US Patent US9006249 (2015) BindingDB Entry DOI: 10.7270/Q2NV9H0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50500190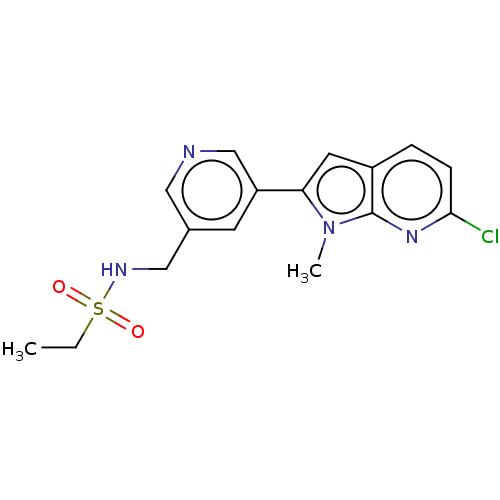 (CHEMBL3746080) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate after 2 hrs by scintillation proximity assay | J Med Chem 58: 9382-94 (2015) Article DOI: 10.1021/acs.jmedchem.5b01545 BindingDB Entry DOI: 10.7270/Q2CC13QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130365 (US8822534, Example 11-1 | US8822534, Example 12-1 ...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107809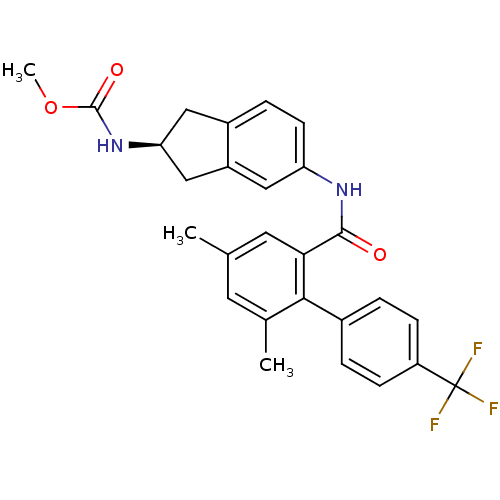 (CHEMBL143284 | {5-[(4,6-Dimethyl-4'-trifluoromethy...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Membrane metallo-endopeptidase-like 1 (Homo sapiens (Human)) | BDBM130370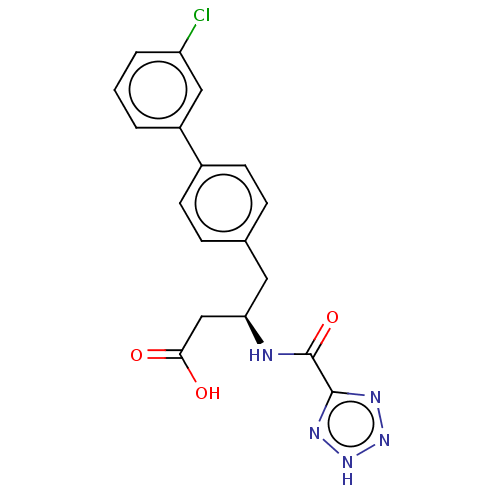 (US8822534, Example 15-1 | US8993631, 12-1) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Novartis AG US Patent | Assay Description Recombinant human neutral endopeptidase (expressed in insect cells and purified using standard methods, final concentration 7 μM) is pre-incubat... | US Patent US8822534 (2014) BindingDB Entry DOI: 10.7270/Q2513WW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107797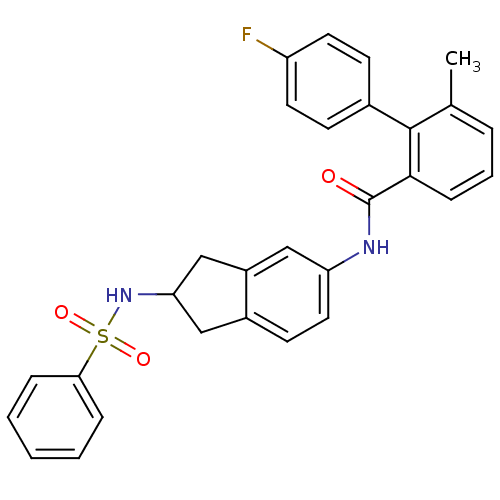 (4'-Fluoro-6-methyl-biphenyl-2-carboxylic acid (2-b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50323357 (6-[(S)-1-(4-Chloro-phenyl)-ethyl]-4-cyclopropylmet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 by cell-based assay | Bioorg Med Chem Lett 20: 4324-7 (2010) Article DOI: 10.1016/j.bmcl.2010.06.086 BindingDB Entry DOI: 10.7270/Q2QJ7HGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apolipoprotein B-100 (Homo sapiens (Human)) | BDBM50107790 (4'-Trifluoromethyl-biphenyl-2-carboxylic acid (2-b...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Reasearch Curated by ChEMBL | Assay Description Inhibition of apolipoprotein B (apoB) secreted by human hepatoma cells (Hep G2) at 100 nM | J Med Chem 44: 4677-87 (2001) BindingDB Entry DOI: 10.7270/Q23F4NXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 525 total ) | Next | Last >> |