 Found 151 hits with Last Name = 'moon' and Initial = 'mj'
Found 151 hits with Last Name = 'moon' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50084948
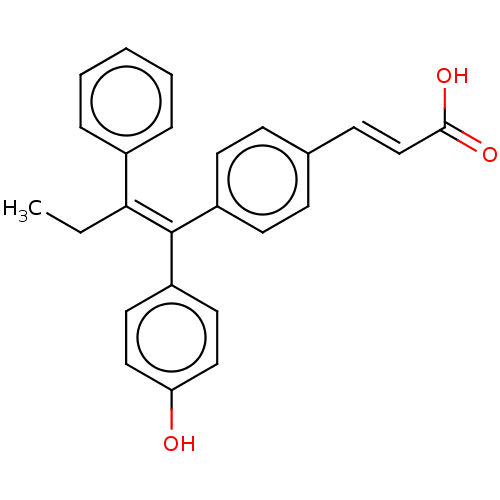
(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-beta (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50423773
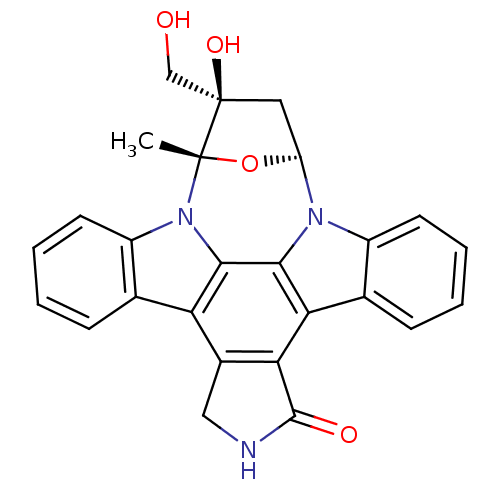
(A-1544750 | CEP-701 | KT-5555 | LESTAURTINIB | SP9...)Show SMILES C[C@]12O[C@@H](C[C@@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18?,25-,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50084948

(CHEMBL195515 | GW7604)Show SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(\C=C\C(O)=O)cc1)c1ccccc1 Show InChI InChI=1S/C25H22O3/c1-2-23(19-6-4-3-5-7-19)25(21-13-15-22(26)16-14-21)20-11-8-18(9-12-20)10-17-24(27)28/h3-17,26H,2H2,1H3,(H,27,28)/b17-10+,25-23+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-alpha (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50090462
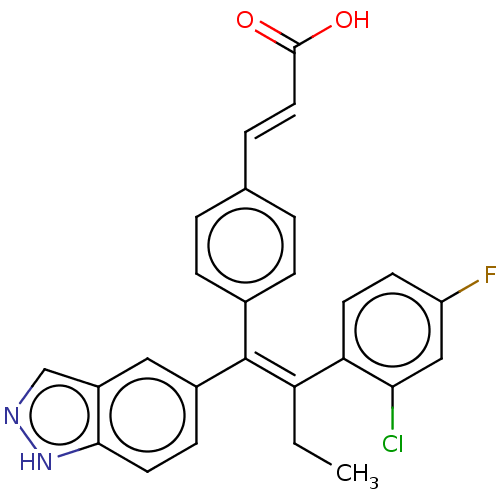
(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-alpha (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-beta (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313606
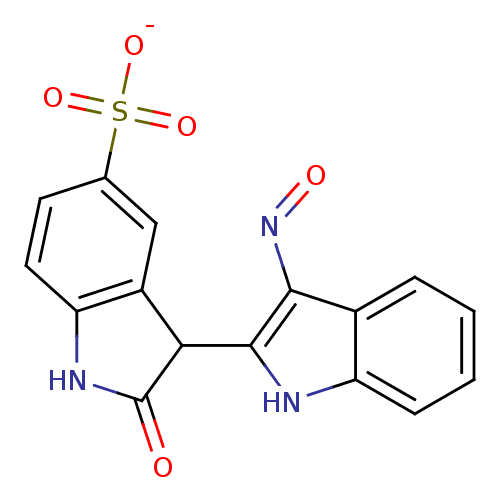
(CHEMBL1095687 | Sodium-3-[hydroxyimino]-2'-oxo-1,3...)Show SMILES [O-]S(=O)(=O)c1ccc2NC(=O)C(c2c1)c1[nH]c2ccccc2c1N=O Show InChI InChI=1S/C16H11N3O5S/c20-16-13(10-7-8(25(22,23)24)5-6-12(10)18-16)15-14(19-21)9-3-1-2-4-11(9)17-15/h1-7,13,17H,(H,18,20)(H,22,23,24)/p-1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313604
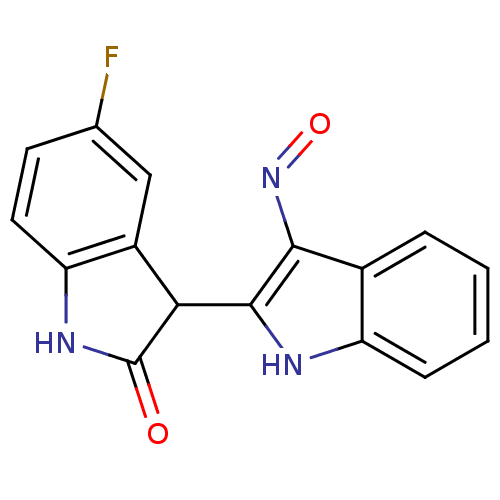
(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-beta (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-alpha (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313610

(5'-Methyl-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C17H13N3O2/c1-9-6-7-13-11(8-9)14(17(21)19-13)16-15(20-22)10-4-2-3-5-12(10)18-16/h2-8,14,18H,1H3,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM7393
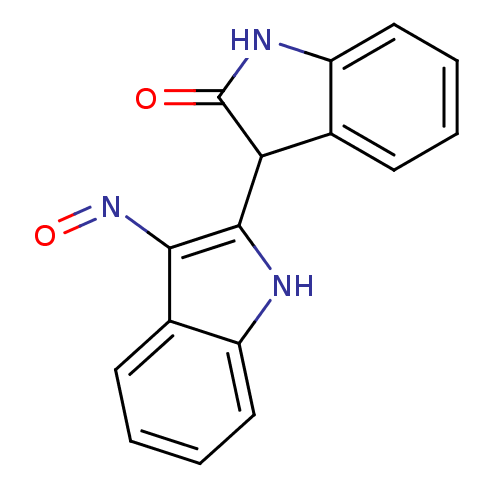
(3-[(2Z,3E)-3-(hydroxyimino)-2,3-dihydro-1H-indol-2...)Show InChI InChI=1S/C16H11N3O2/c20-16-13(9-5-1-3-7-11(9)18-16)15-14(19-21)10-6-2-4-8-12(10)17-15/h1-8,13,17H,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM19442
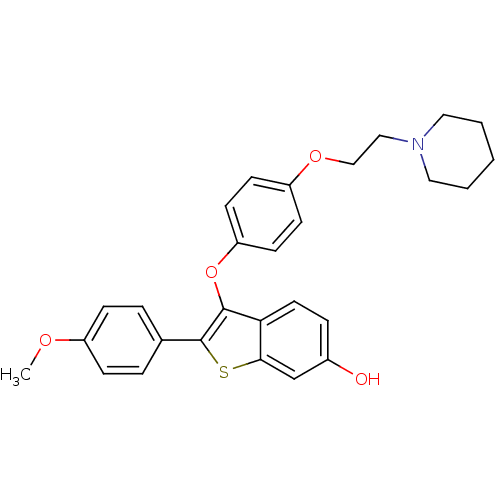
(2-(4-methoxyphenyl)-3-[4-(2-piperidin-1-ylethoxy)p...)Show SMILES COc1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H29NO4S/c1-31-22-8-5-20(6-9-22)28-27(25-14-7-21(30)19-26(25)34-28)33-24-12-10-23(11-13-24)32-18-17-29-15-3-2-4-16-29/h5-14,19,30H,2-4,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-alpha (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50304109

((Z)-5'-Nitro-1H,1'H-[2,3']biindolylidene-3,2'-dion...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)C(c2c1)c1[nH]c2ccccc2c1N=O Show InChI InChI=1S/C16H10N4O4/c21-16-13(10-7-8(20(23)24)5-6-12(10)18-16)15-14(19-22)9-3-1-2-4-11(9)17-15/h1-7,13,17H,(H,18,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313609
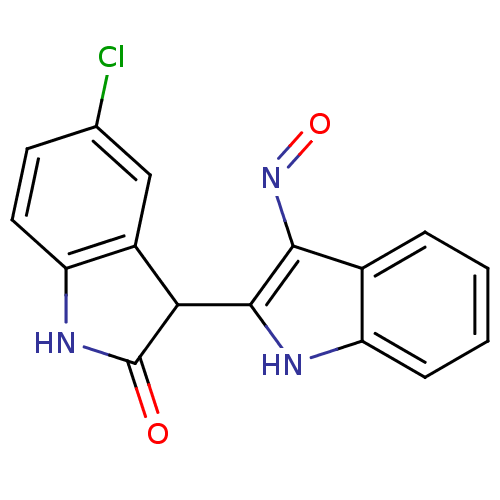
(5'-Chloro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10ClN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50304112

((Z)-5'-Amino-1H,1'H-[2,3']biindolylidene-3,2'-dion...)Show InChI InChI=1S/C16H12N4O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,17H2,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313614

(5'-Methyl-1H,1'H-[2,3']biindolylidene-3,2'-dione |...)Show InChI InChI=1S/C17H12N2O2/c1-9-6-7-13-11(8-9)14(17(21)19-13)15-16(20)10-4-2-3-5-12(10)18-15/h2-8,18H,1H3,(H,19,21)/b15-14- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 209 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313613
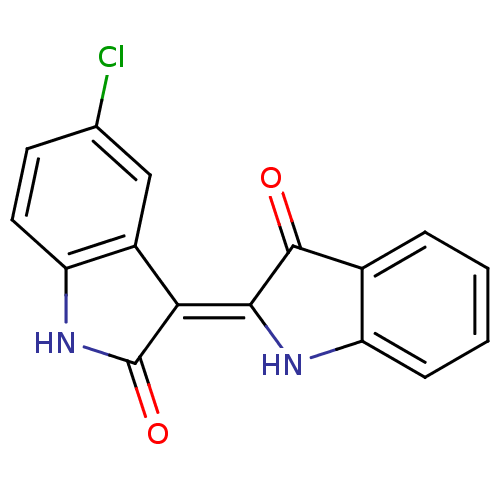
(5'-Chloro-1H,1'H-[2,3']biindolylidene-3,2'-dione |...)Show InChI InChI=1S/C16H9ClN2O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)14-15(20)9-3-1-2-4-11(9)18-14/h1-7,18H,(H,19,21)/b14-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 212 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM19442

(2-(4-methoxyphenyl)-3-[4-(2-piperidin-1-ylethoxy)p...)Show SMILES COc1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1 Show InChI InChI=1S/C28H29NO4S/c1-31-22-8-5-20(6-9-22)28-27(25-14-7-21(30)19-26(25)34-28)33-24-12-10-23(11-13-24)32-18-17-29-15-3-2-4-16-29/h5-14,19,30H,2-4,15-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-E2 from estrogen receptor-beta (unknown origin) by scintillation counting analysis |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313615

(CHEMBL1085922 | Sodium-3,2'-dioxo-1,3,1',2'-tetrah...)Show SMILES [O-]S(=O)(=O)c1ccc2NC(=O)\C(=C3/Nc4ccccc4C3=O)c2c1 Show InChI InChI=1S/C16H10N2O5S/c19-15-9-3-1-2-4-11(9)17-14(15)13-10-7-8(24(21,22)23)5-6-12(10)18-16(13)20/h1-7,17H,(H,18,20)(H,21,22,23)/p-1/b14-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50304110
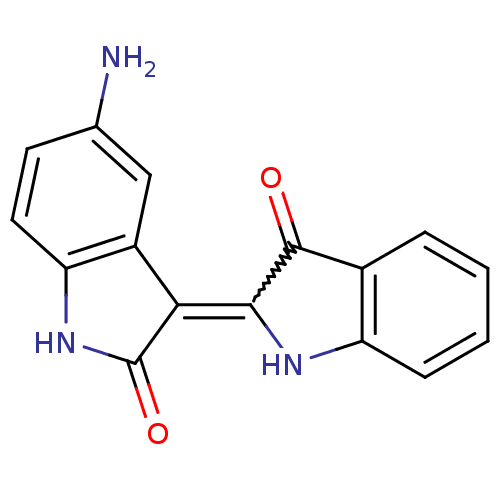
((Z)-5'-Amino-1H,1'H-[2,3']biindolylidene-3,2'-dion...)Show SMILES Nc1ccc2NC(=O)C(=C3Nc4ccccc4C3=O)c2c1 |w:9.18| Show InChI InChI=1S/C16H11N3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)14-15(20)9-3-1-2-4-11(9)18-14/h1-7,18H,17H2,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM7492
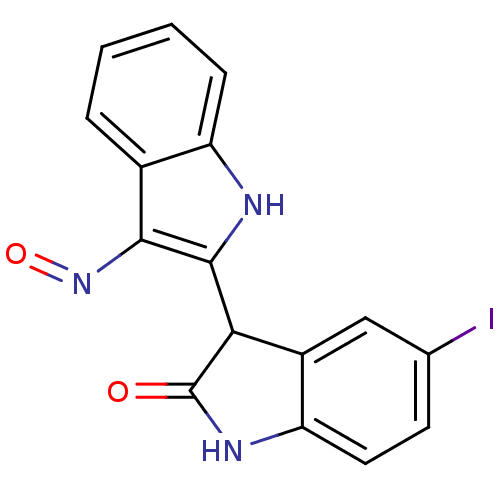
(3-[(2Z,3E)-3-(hydroxyimino)-2,3-dihydro-1H-indol-2...)Show InChI InChI=1S/C16H10IN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 418 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313608

(5',7'-Dimethyl-1H,1'H-[2,3']biindolylidene-3,2'-di...)Show InChI InChI=1S/C18H15N3O2/c1-9-7-10(2)15-12(8-9)14(18(22)20-15)17-16(21-23)11-5-3-4-6-13(11)19-17/h3-8,14,19H,1-2H3,(H,20,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 952 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to glucocorticoid receptor (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone-A receptor (unknown origin) by transcriptional reporter assay |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone-B receptor (unknown origin) by transcriptional reporter assay |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor (unknown origin) by transcriptional reporter assay |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor (unknown origin) by transcriptional reporter assay |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of VEGFR2 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of dopamine transporter (unknown origin) by cell-based assay |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to androgen receptor (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313612

(5'-Iodo-1H,1'H-[2,3']biindolylidene-3,2'-dione | C...)Show InChI InChI=1S/C16H9IN2O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)14-15(20)9-3-1-2-4-11(9)18-14/h1-7,18H,(H,19,21)/b14-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313611
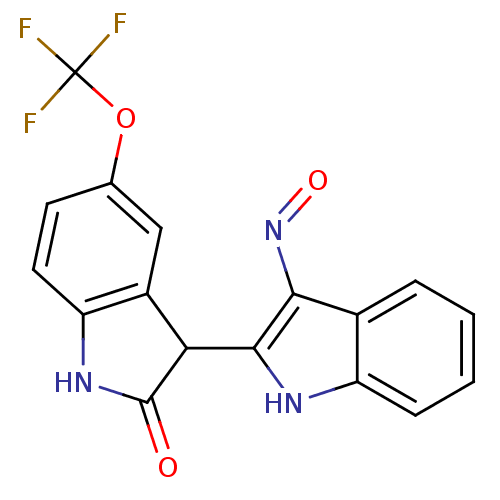
(5'-Trifluoromethoxy-1H,1'H-[2,3']biindolylidene-3,...)Show SMILES FC(F)(F)Oc1ccc2NC(=O)C(c2c1)c1[nH]c2ccccc2c1N=O Show InChI InChI=1S/C17H10F3N3O3/c18-17(19,20)26-8-5-6-12-10(7-8)13(16(24)22-12)15-14(23-25)9-3-1-2-4-11(9)21-15/h1-7,13,21H,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313607
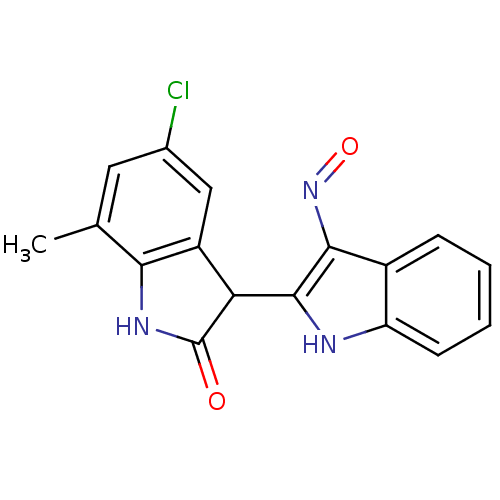
(5'-Chloro-7'-methyl-1H,1'H-[2,3']biindolylidene-3,...)Show SMILES Cc1cc(Cl)cc2C(C(=O)Nc12)c1[nH]c2ccccc2c1N=O Show InChI InChI=1S/C17H12ClN3O2/c1-8-6-9(18)7-11-13(17(22)20-14(8)11)16-15(21-23)10-4-2-3-5-12(10)19-16/h2-7,13,19H,1H3,(H,20,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50304121
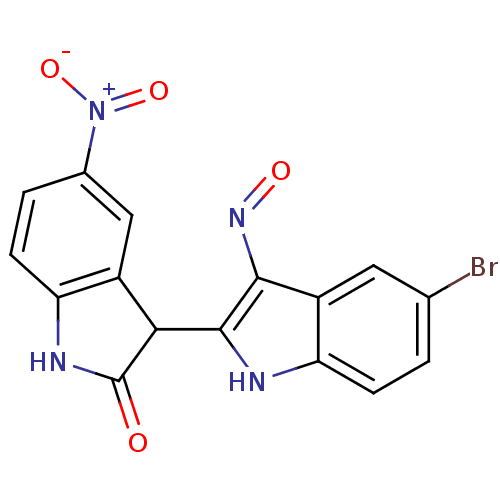
((Z)-5-Bromo-5'-nitro-1H,1'H-[2,3']biindolylidene-3...)Show SMILES [O-][N+](=O)c1ccc2NC(=O)C(c2c1)c1[nH]c2ccc(Br)cc2c1N=O Show InChI InChI=1S/C16H9BrN4O4/c17-7-1-3-12-10(5-7)14(20-23)15(18-12)13-9-6-8(21(24)25)2-4-11(9)19-16(13)22/h1-6,13,18H,(H,19,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of Met by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50349806

(INDIRUBIN)Show InChI InChI=1S/C16H10N2O2/c19-15-10-6-2-4-8-12(10)17-14(15)13-9-5-1-3-7-11(9)18-16(13)20/h1-8,17H,(H,18,20)/b14-13+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of EGFR by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of insulin receptor by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Macrophage-stimulating protein receptor
(Homo sapiens (Human)) | BDBM50313604

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione 3...)Show InChI InChI=1S/C16H10FN3O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)15-14(20-22)9-3-1-2-4-11(9)18-15/h1-7,13,18H,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of Ron by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50304116
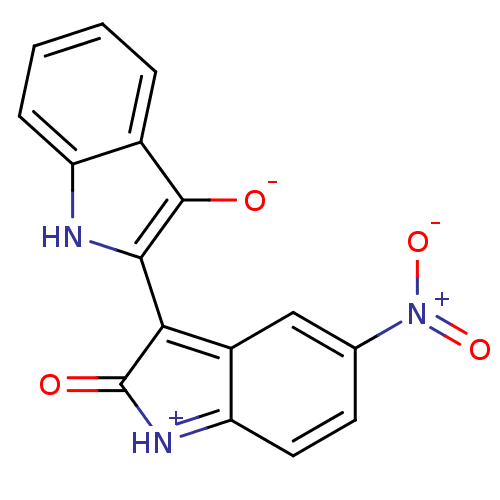
((Z)-5'-Nitro-1H,1'H-[2,3']biindolylidene-3,2'-dion...)Show SMILES [O-]c1c([nH]c2ccccc12)C1=c2cc(ccc2=[NH+]C1=O)[N+]([O-])=O |c:12,19| Show InChI InChI=1S/C16H9N3O4/c20-15-9-3-1-2-4-11(9)17-14(15)13-10-7-8(19(22)23)5-6-12(10)18-16(13)21/h1-7,17,20H | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313617

(1-Acetyl-1H,1'H-[2,3']biindolylidene-3,2'-dione | ...)Show SMILES CC(=O)N1\C(C(=O)c2ccccc12)=C1/C(=O)Nc2ccccc12 Show InChI InChI=1S/C18H12N2O3/c1-10(21)20-14-9-5-3-7-12(14)17(22)16(20)15-11-6-2-4-8-13(11)19-18(15)23/h2-9H,1H3,(H,19,23)/b16-15- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50313616

(5'-Fluoro-1H,1'H-[2,3']biindolylidene-3,2'-dione |...)Show InChI InChI=1S/C16H9FN2O2/c17-8-5-6-12-10(7-8)13(16(21)19-12)14-15(20)9-3-1-2-4-11(9)18-14/h1-7,18H,(H,19,21)/b14-13- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 by HTRF assay |
Bioorg Med Chem Lett 20: 2033-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.039
BindingDB Entry DOI: 10.7270/Q29W0GF0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50090462

(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50508065
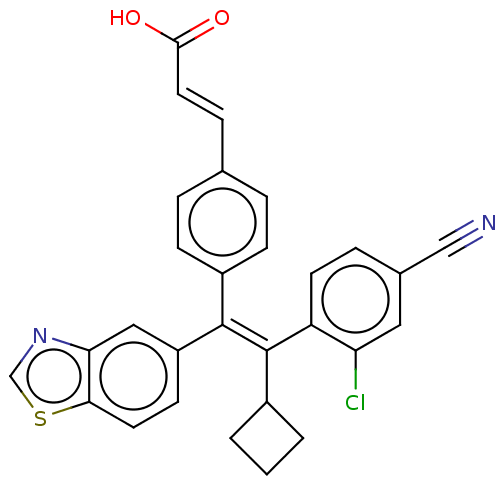
(CHEMBL4563585)Show SMILES OC(=O)\C=C\c1ccc(cc1)C(=C(\C1CCC1)c1ccc(cc1Cl)C#N)\c1ccc2scnc2c1 Show InChI InChI=1S/C29H21ClN2O2S/c30-24-14-19(16-31)6-11-23(24)29(20-2-1-3-20)28(22-10-12-26-25(15-22)32-17-35-26)21-8-4-18(5-9-21)7-13-27(33)34/h4-15,17,20H,1-3H2,(H,33,34)/b13-7+,29-28+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Induction of ERalpha degradation in human MCF7 cells in phenol red free RPMI medium containing 5% charcoal-stripped FBS incubated for 4 hrs by IRDye ... |
Bioorg Med Chem Lett 29: 367-372 (2019)
Article DOI: 10.1016/j.bmcl.2018.12.042
BindingDB Entry DOI: 10.7270/Q2639T19 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data