

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Rattus norvegicus (rat)) | BDBM50122294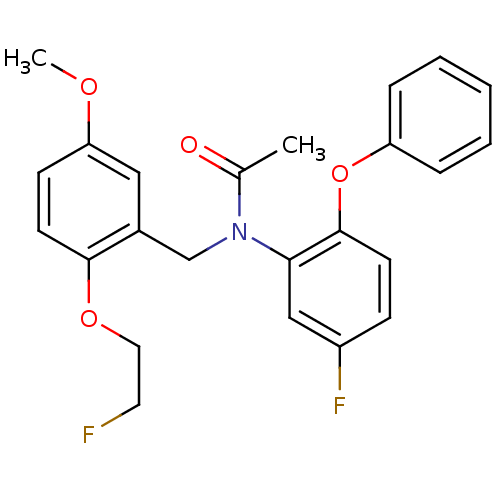 (CHEMBL292092 | N-(5-fluoro-2-phenoxyphenyl)-N-(2-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]DAA1106 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266889 (CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50312840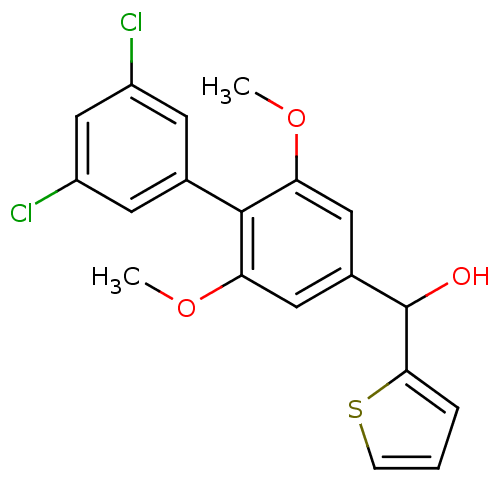 (CHEMBL1076680 | US9139546, 16 | [11C](3',5'-dichlo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to rat brain CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM22032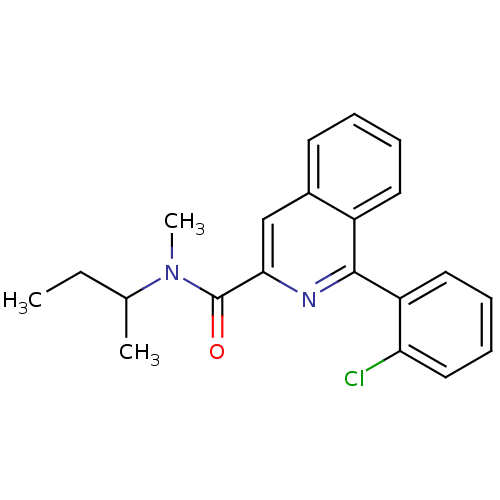 (1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50163592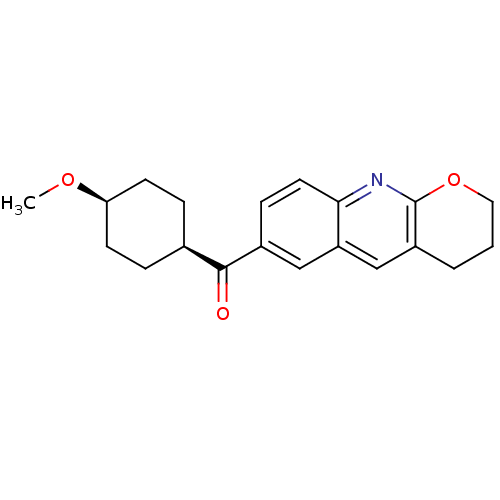 ((3,4-Dihydro-2H-1-oxa-9-aza-anthracen-6-yl)-(4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50301822 (5-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266861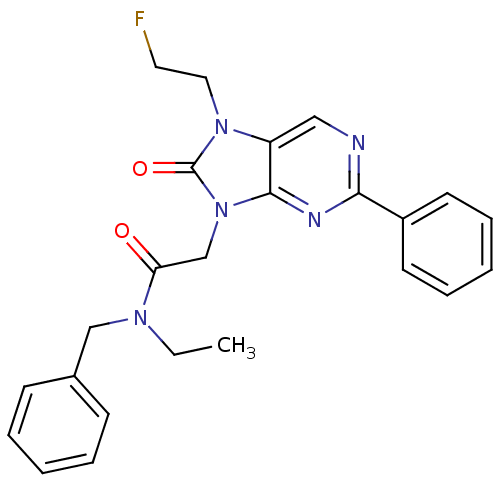 (CHEMBL515622 | N-benzyl-N-ethyl-2-(7-(2-fluoroethy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266863 (CHEMBL476811 | N-benzyl-N-(2-fluoroethyl)-2-(7-met...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266889 (CHEMBL513922 | N-benzyl-N-ethyl-2-(7-methyl-8-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50054139 ((R)1-(2-Chloro-phenyl)-isoquinoline-3-carboxylic a...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333368 (CHEMBL1645348 | [11C]-cis-(3-ethyl-2-methylquinoli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50266862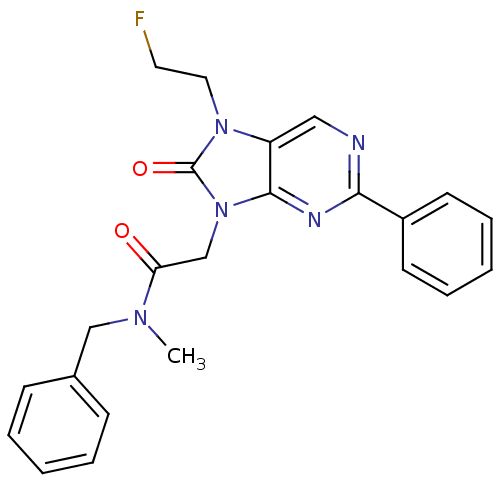 (CHEMBL476607 | N-benzyl-2-(7-(2-fluoroethyl)-8-oxo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]PK11195 from PBR receptor in rat brain membrane | Bioorg Med Chem Lett 19: 1707-10 (2009) Article DOI: 10.1016/j.bmcl.2009.01.093 BindingDB Entry DOI: 10.7270/Q2C82B6C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM50312841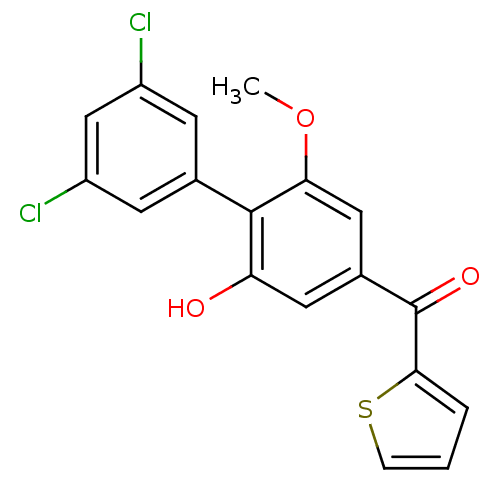 (CHEMBL1081609 | US9139546, 30 | [11C](3',5'-dichlo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to rat brain CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50273942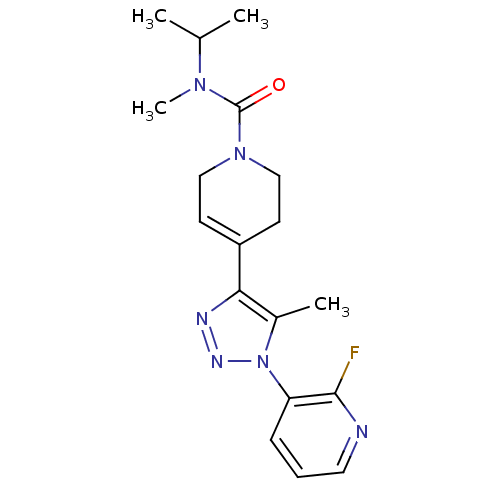 (4-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50312838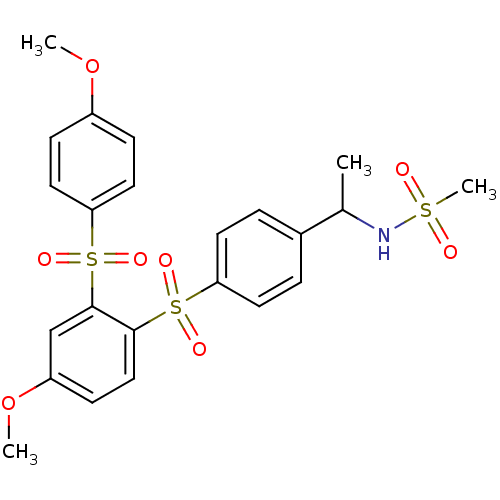 (CHEMBL1081623 | SCH-225336 | [11C]N-(1-(4-(4-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Homo sapiens (Human)) | BDBM50333371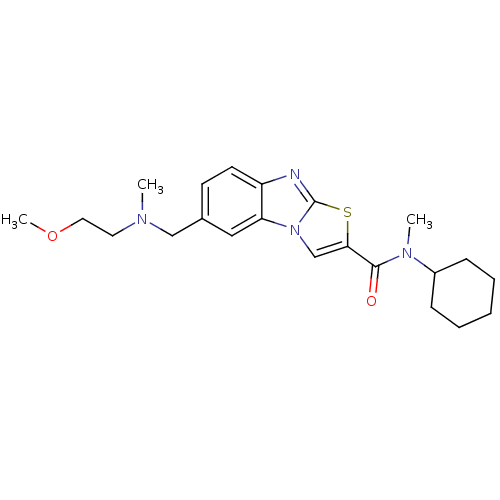 (CHEMBL1645351 | CHEMBL1771388 | [11C]-6-N-cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50401000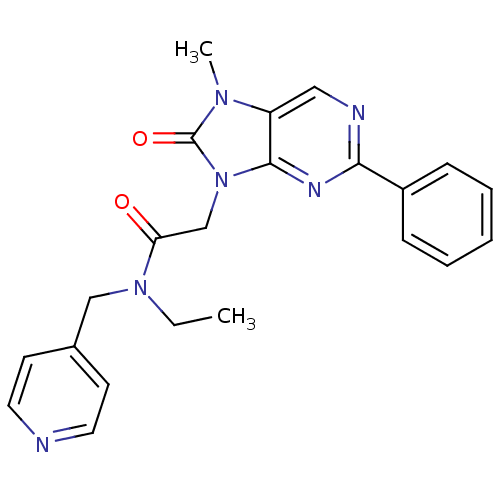 (CHEMBL2206282) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50312837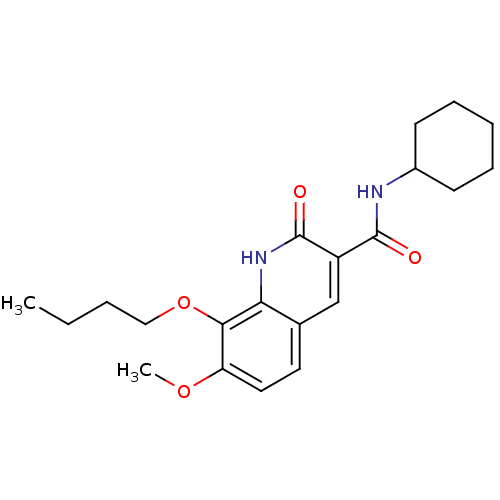 (CHEMBL1081610 | [11C]8-butoxy-N-cyclohexyl-7-metho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50400999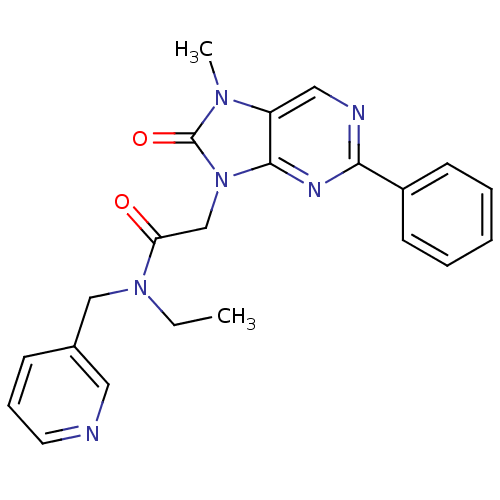 (CHEMBL2206283) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50312839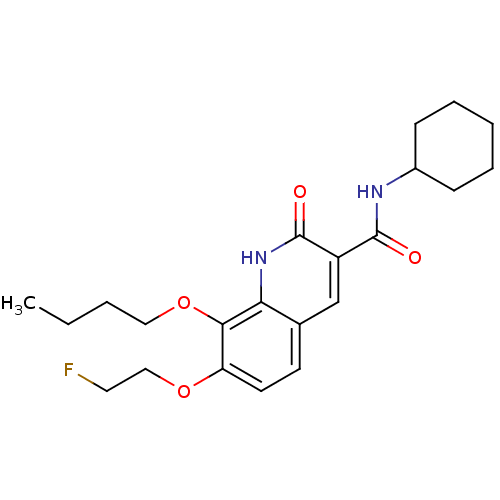 (CHEMBL1081622 | [18F]8-butoxy-N-cyclohexyl-7-(2-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to human CB2 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50400998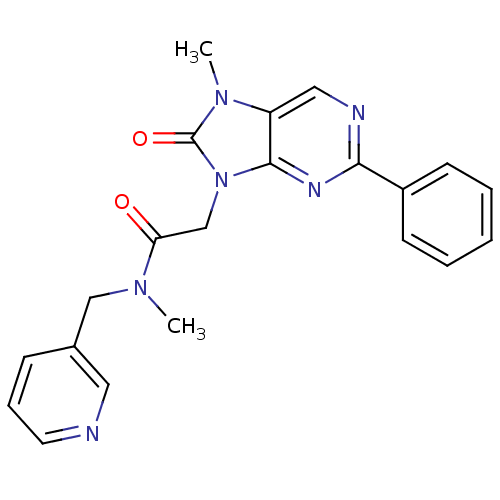 (CHEMBL2206284) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Displacement of [11C]-PK-11195 from TPSO in Sprague-Dawley rat brain homogenate after 30 mins by gamma counting | J Med Chem 54: 6040-9 (2011) Article DOI: 10.1021/jm200516a BindingDB Entry DOI: 10.7270/Q2C53N16 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50312841 (CHEMBL1081609 | US9139546, 30 | [11C](3',5'-dichlo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 503 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50312840 (CHEMBL1076680 | US9139546, 16 | [11C](3',5'-dichlo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor | Bioorg Med Chem Lett 20: 1565-8 (2010) Article DOI: 10.1016/j.bmcl.2010.01.074 BindingDB Entry DOI: 10.7270/Q2N29X34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212110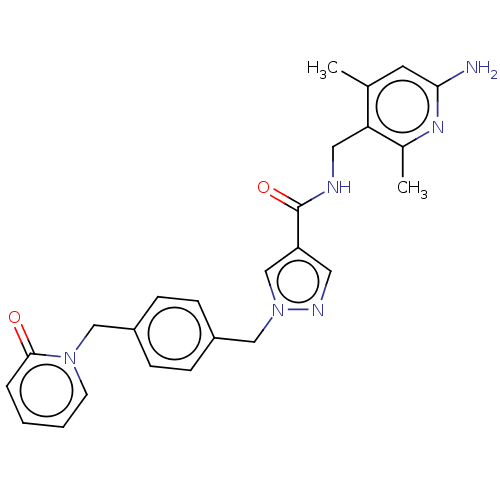 (US9290485, 142) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0224 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212116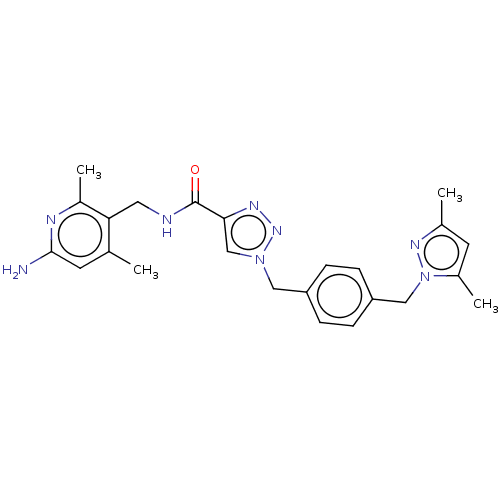 (US9290485, 148) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212114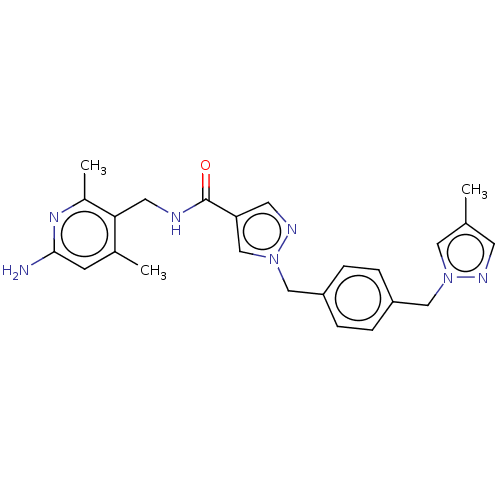 (US9290485, 146) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211977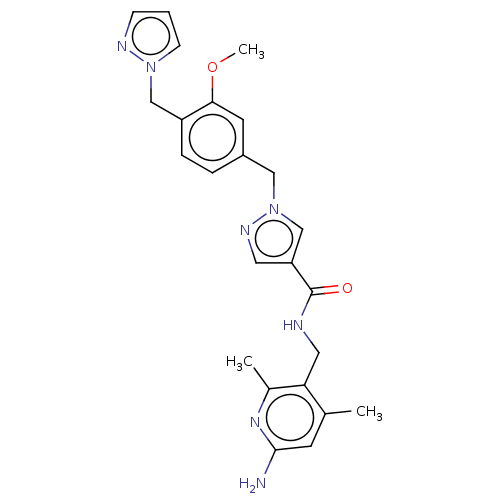 (US9290485, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0912 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212136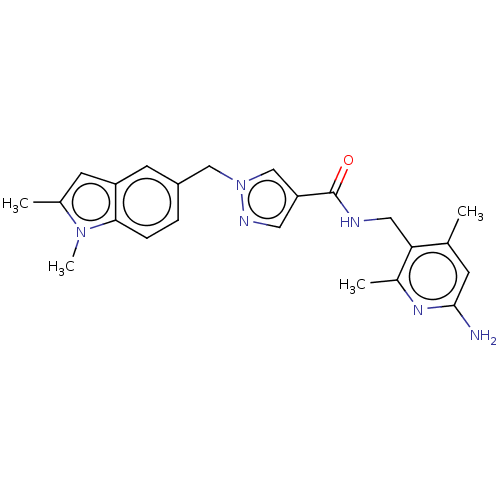 (US9290485, 168) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50228403 ((R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212115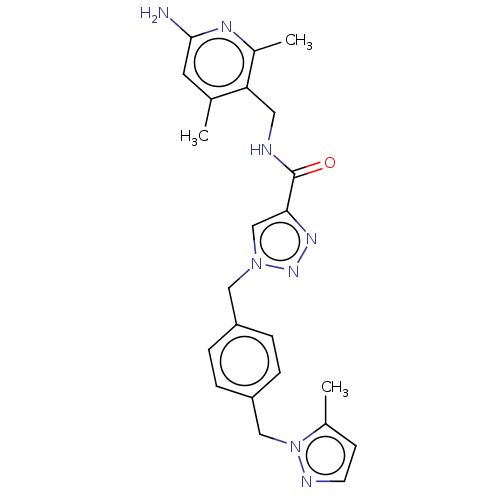 (US9290485, 147) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212004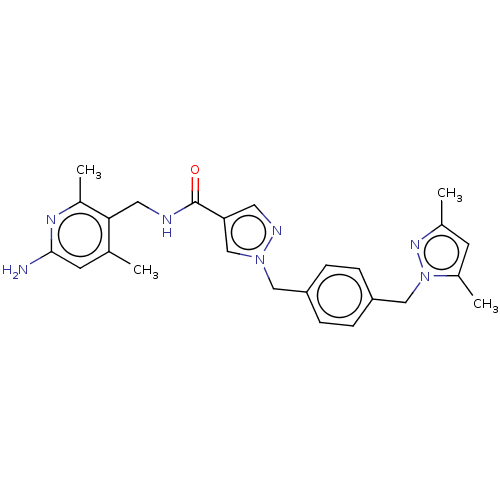 (US9290485, 36) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.134 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212134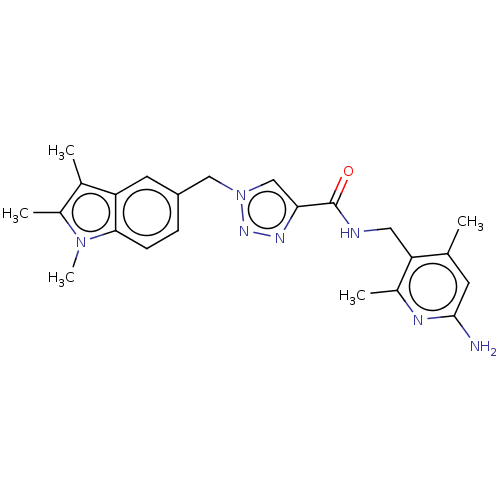 (US9290485, 166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM211974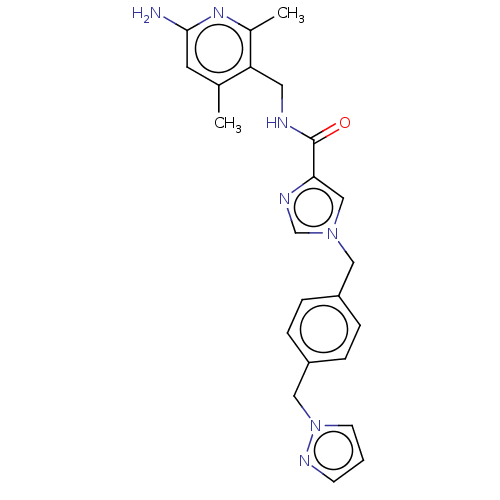 (US9290485, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212113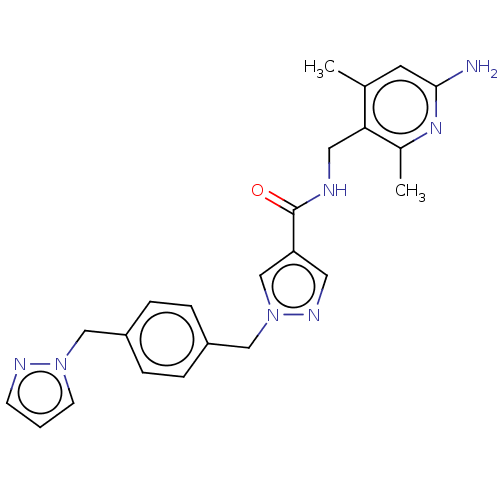 (US9290485, 145) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212135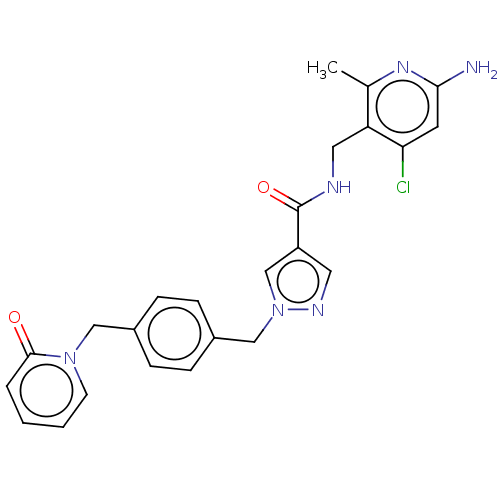 (US9290485, 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738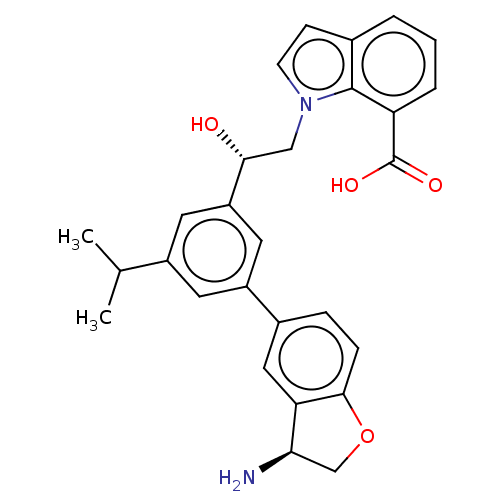 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212141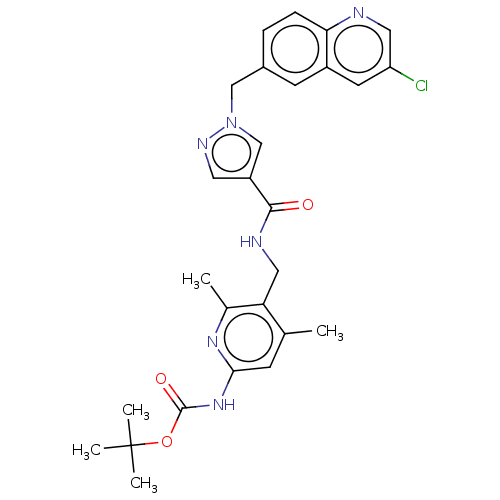 (US9290485, 173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364156 (CHEMBL1951432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364171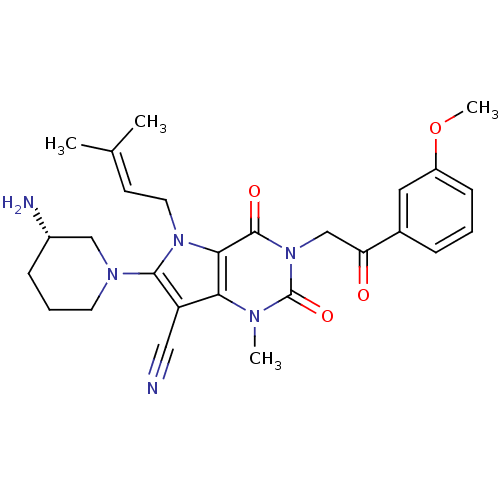 (CHEMBL1951598) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542741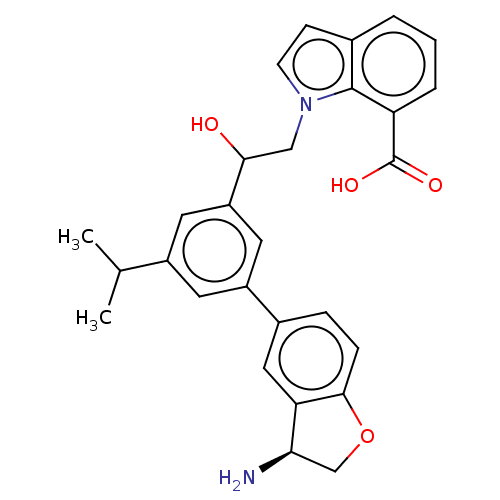 (CHEMBL4647950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212124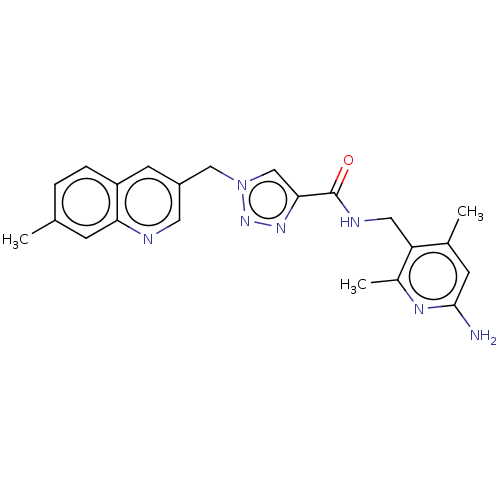 (US9290485, 156) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364158 (CHEMBL1951430) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364184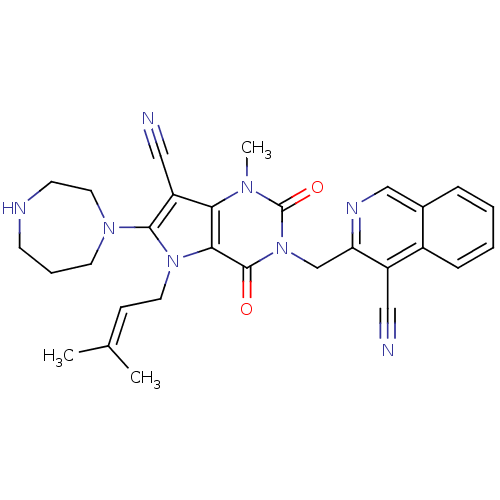 (CHEMBL1951611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364187 (CHEMBL1951416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50364173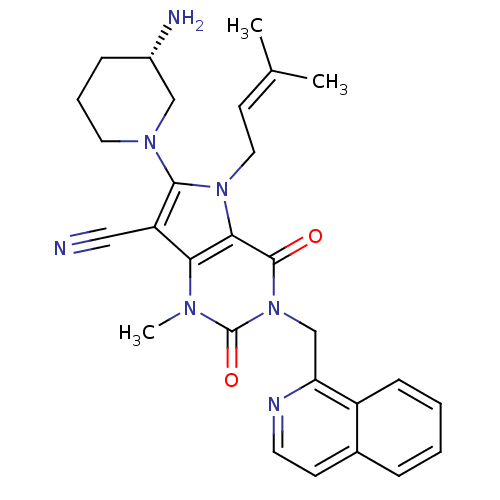 (CHEMBL1951599) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Argenta Discovery Ltd. Curated by ChEMBL | Assay Description Inhibition of human C-terminal step-tagged DPP4 expressed using baculovirus system | Bioorg Med Chem Lett 22: 1464-8 (2012) Article DOI: 10.1016/j.bmcl.2011.11.054 BindingDB Entry DOI: 10.7270/Q2SF2WN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM212109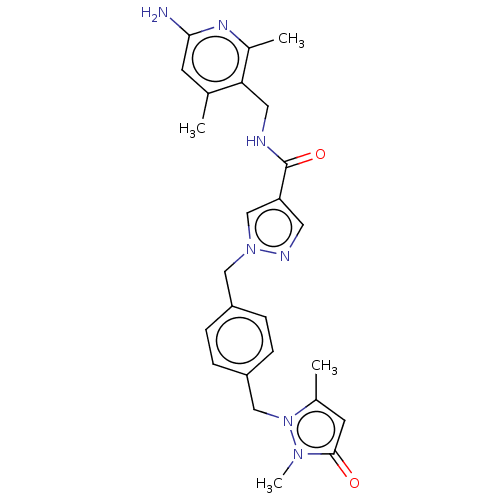 (US9290485, 141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.8 | 25 |
NOVARTIS AG US Patent | Assay Description Enzymatic reactions were conducted in ⿿assay buffer⿿, comprising 50 mM Hepes/NaOH at pH 7.8, 150 mM NaCl, 1 mM EDTA and 0.05% (w/v) CHAPS. For th... | US Patent US9290485 (2016) BindingDB Entry DOI: 10.7270/Q26H4G73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 1 (Mus musculus) | BDBM50333367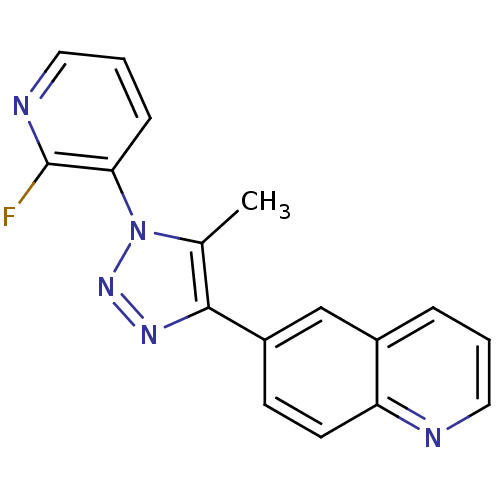 (6-(1-(2-fluoropyridin-3-yl)-5-methyl-1H-1,2,3-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Radiological Sciences Curated by ChEMBL | Assay Description Binding affinity to mouse mGluR1 | Bioorg Med Chem 19: 102-10 (2011) Article DOI: 10.1016/j.bmc.2010.11.048 BindingDB Entry DOI: 10.7270/Q21R6QSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 647 total ) | Next | Last >> |