

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144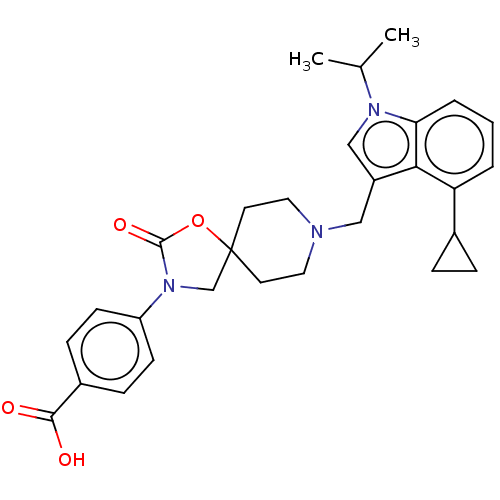 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123235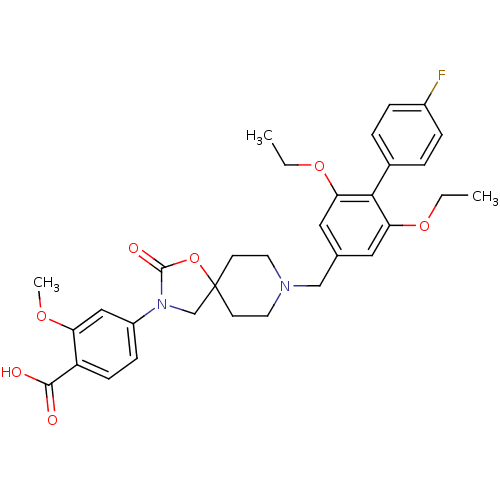 (US8742110, 3-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123247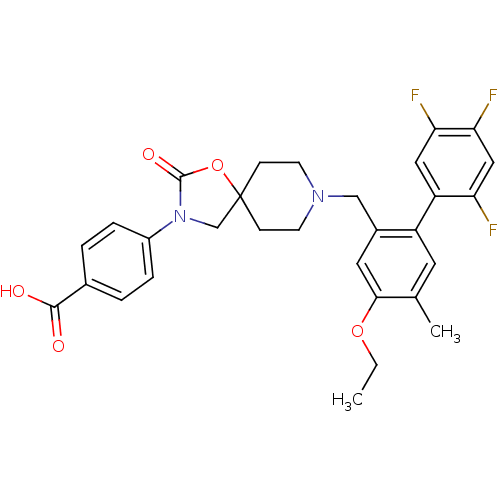 (US8742110, 4-11) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.152 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123253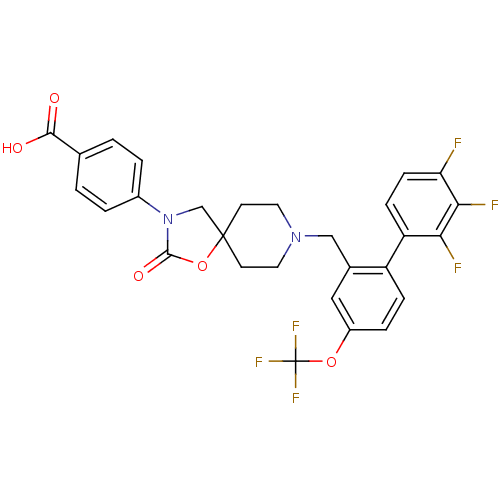 (US8742110, 4-17) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.165 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123270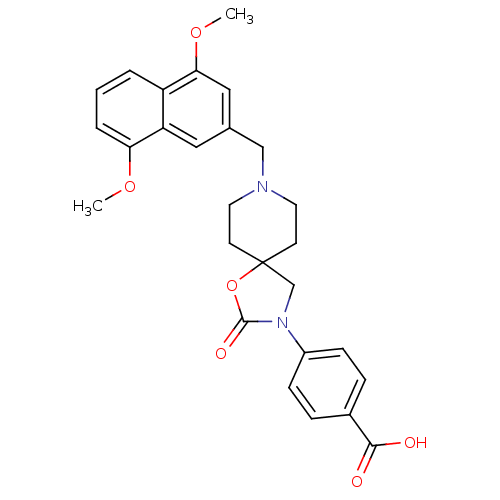 (US8742110, 5-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.172 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123248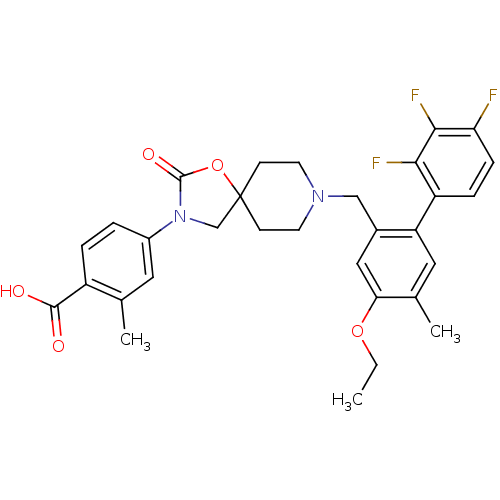 (US8742110, 4-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.188 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50081070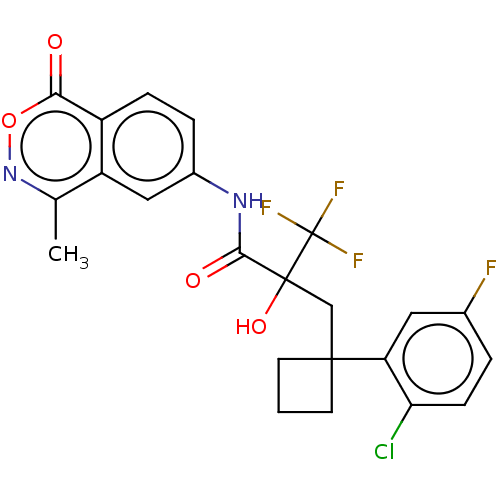 (CHEMBL3421892) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human PR | J Med Chem 58: 2658-77 (2015) Article DOI: 10.1021/jm501601b BindingDB Entry DOI: 10.7270/Q2GM891C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160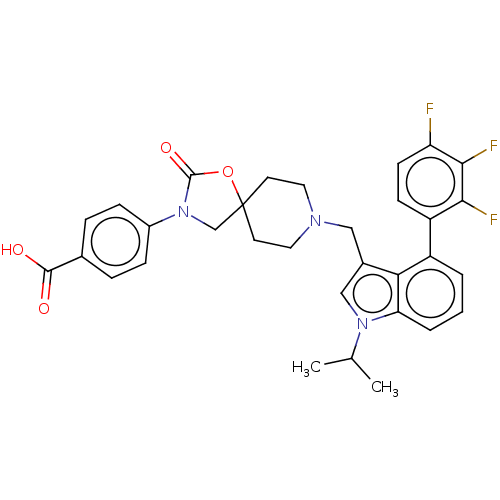 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468136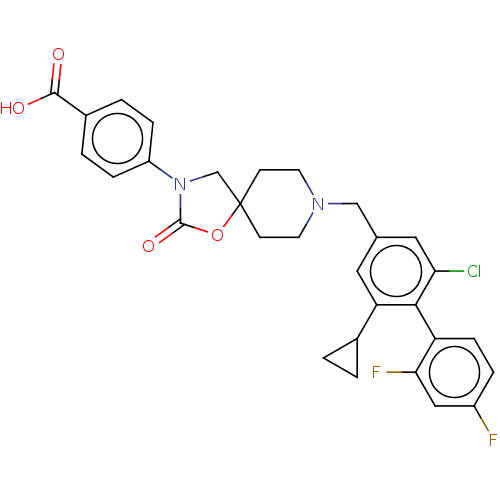 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123300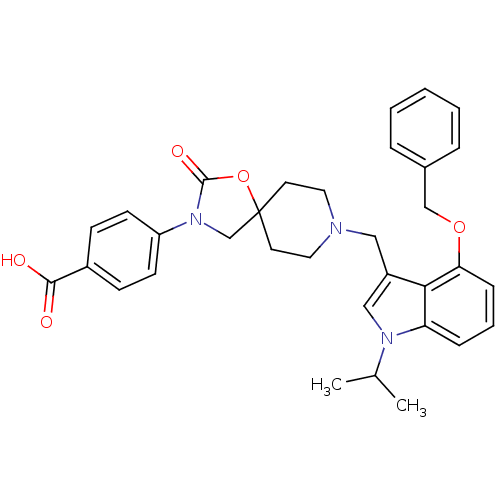 (US8742110, 6-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.225 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123254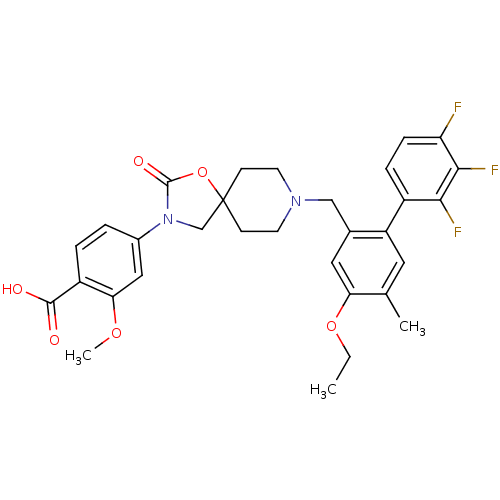 (US8742110, 4-18) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.228 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123271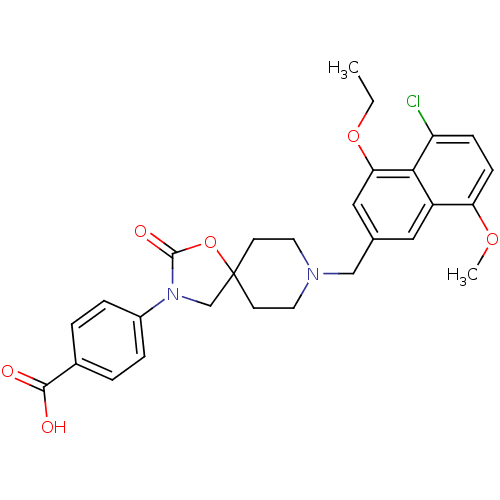 (US8742110, 5-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.233 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123255 (US8742110, 4-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.243 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123296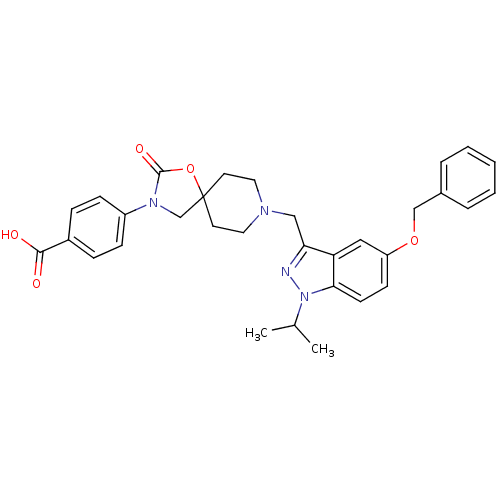 (US8742110, 6-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123252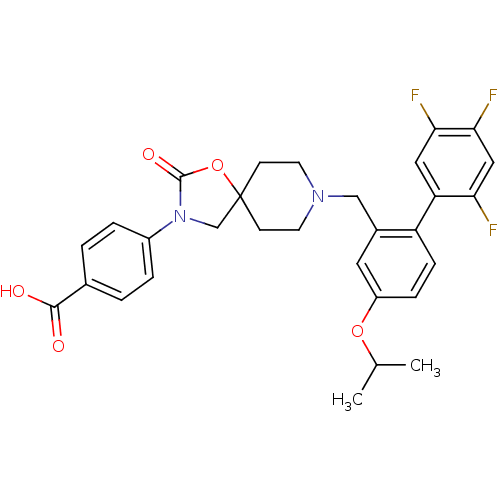 (US8742110, 4-16) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.272 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Mus musculus) | BDBM123216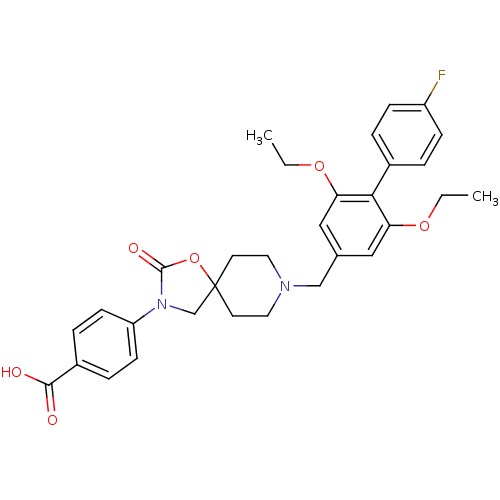 (US8742110, 3-1) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at mouse SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50081073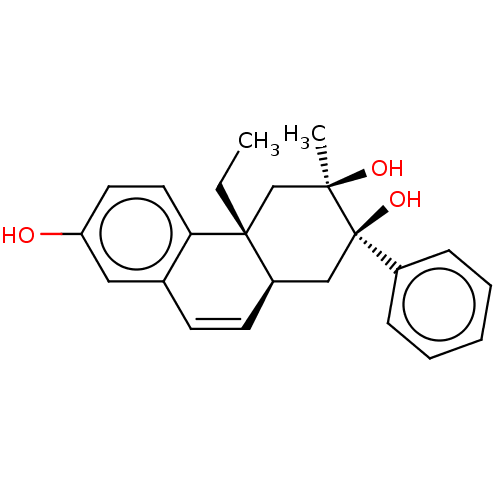 (CHEMBL3421889) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human GR | J Med Chem 58: 2658-77 (2015) Article DOI: 10.1021/jm501601b BindingDB Entry DOI: 10.7270/Q2GM891C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468144 (CHEMBL4286244) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123277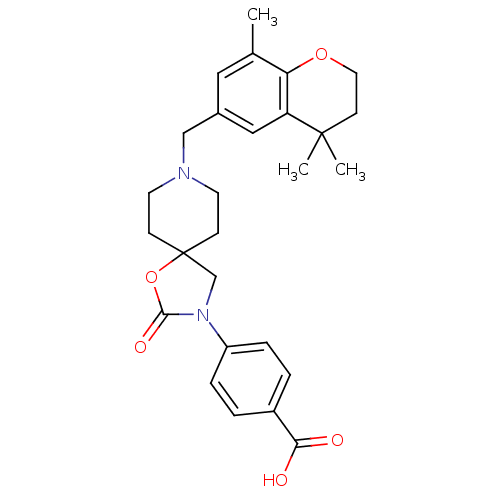 (US8742110, 5-13) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.317 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123227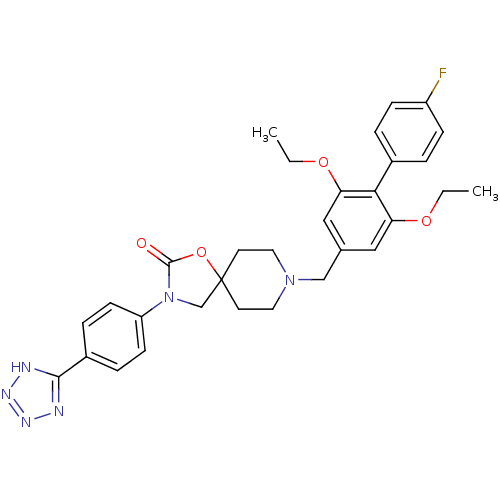 (US8742110, 3-12) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.323 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123306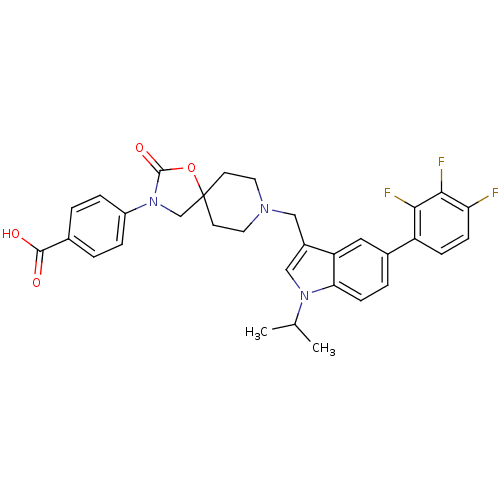 (US8742110, 6-24) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.342 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123234 (US8742110, 3-19) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.359 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123264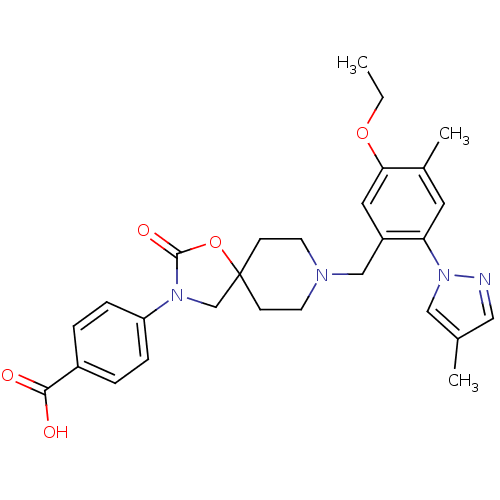 (US8742110, 4-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.395 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123257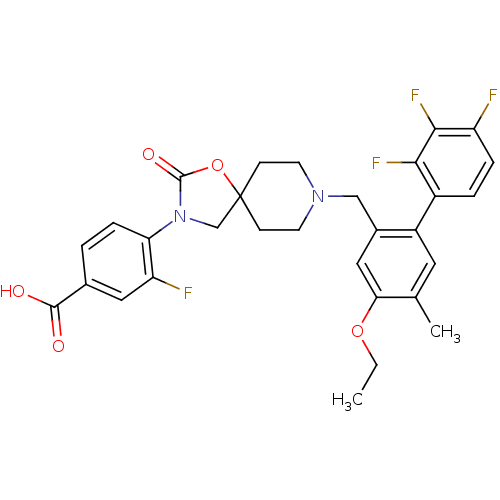 (US8742110, 4-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.397 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468157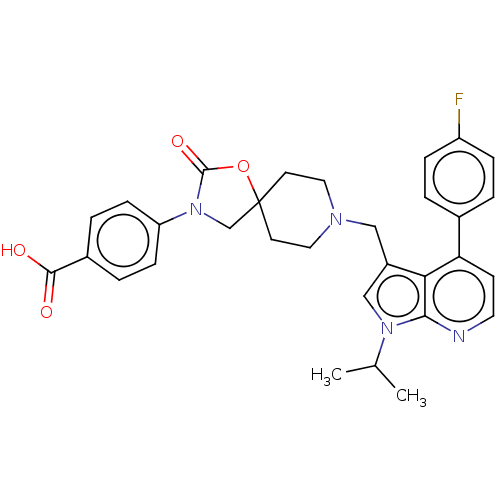 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468146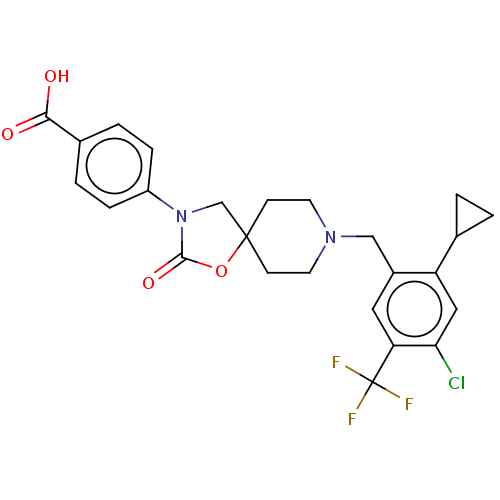 (CHEMBL4285917) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468157 (CHEMBL4289661) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468136 (CHEMBL4287248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50081070 (CHEMBL3421892) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human GR | J Med Chem 58: 2658-77 (2015) Article DOI: 10.1021/jm501601b BindingDB Entry DOI: 10.7270/Q2GM891C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123250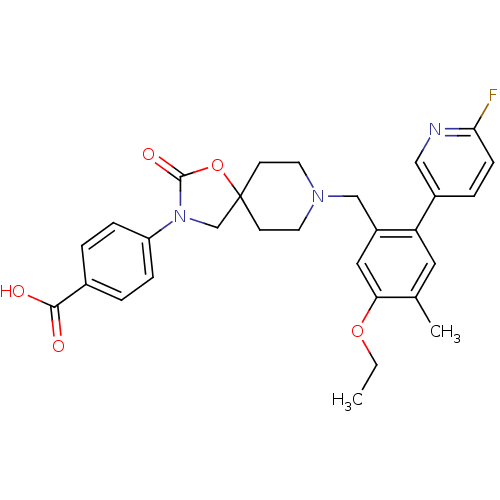 (US8742110, 4-14) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123243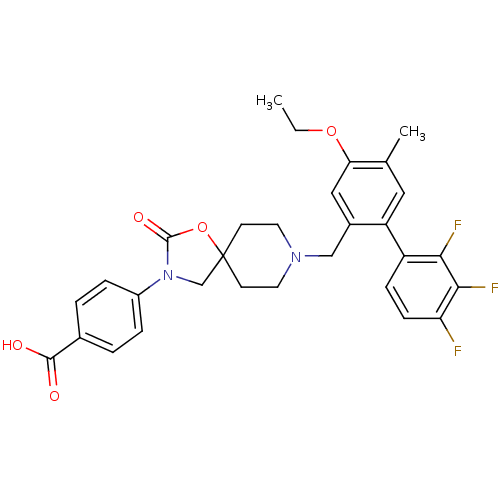 (US8742110, 4-7) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.409 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123179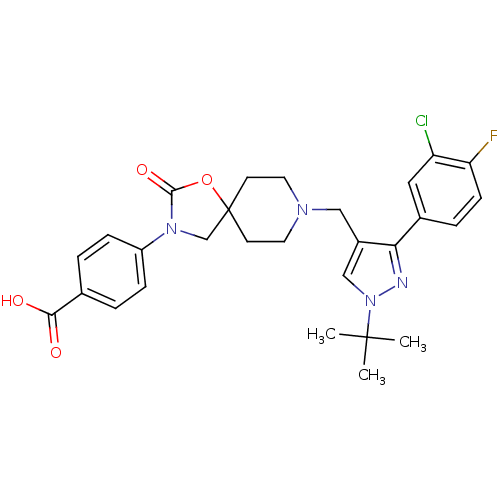 (US8742110, 1-28) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.421 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123272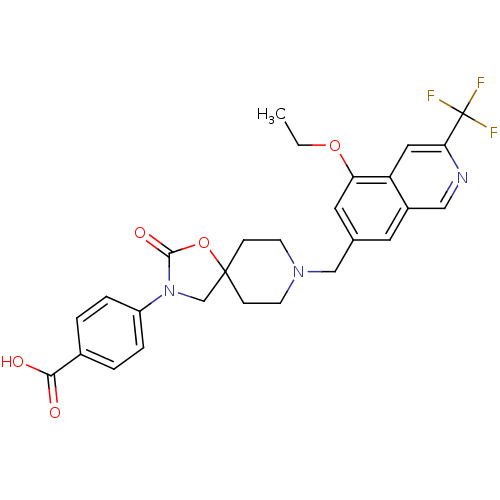 (US8742110, 5-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.424 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123236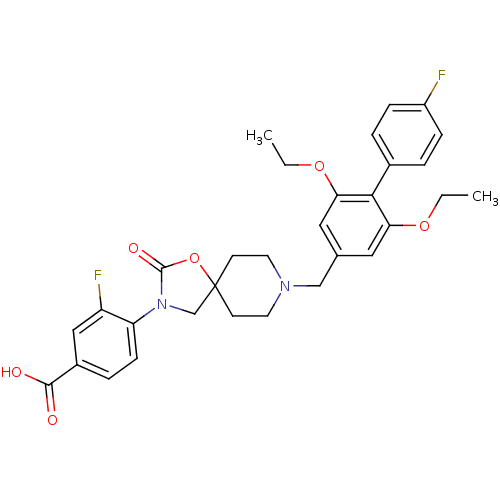 (US8742110, 3-21) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.437 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123245 (US8742110, 4-9) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.439 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123258 (US8742110, 4-22) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.468 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123244 (US8742110, 4-8) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.474 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123239 (US8742110, 4-3) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468160 (CHEMBL4293458) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Antagonist activity at human SSR5 expressed in CHOK1 cells assessed as inhibition of forskolin-induced cAMP accumulation preincubated for 15 mins fol... | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123240 (US8742110, 4-4) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.502 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123242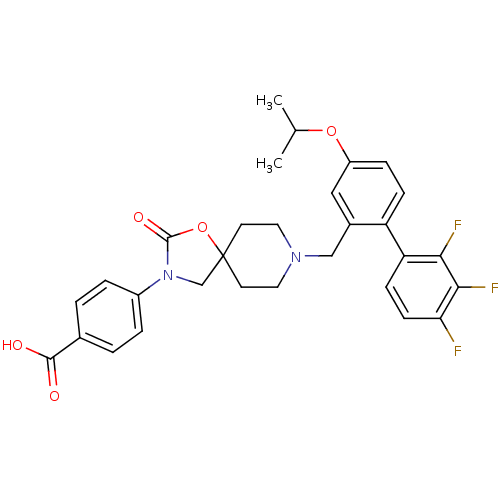 (US8742110, 4-6) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.546 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123307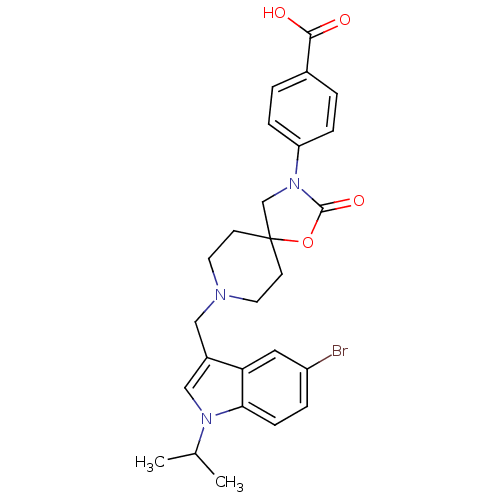 (US8742110, 6-25) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123241 (US8742110, 4-5) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.556 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123180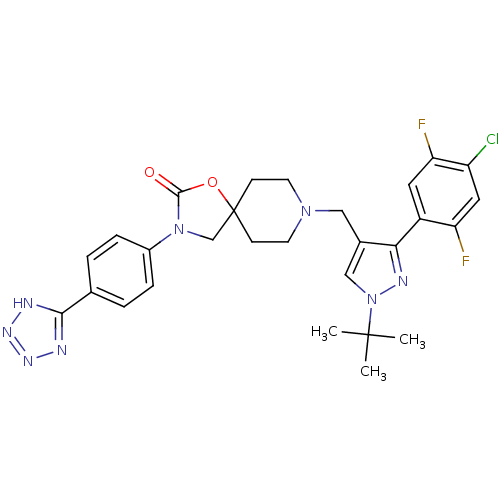 (US8742110, 1-29) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.582 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50081072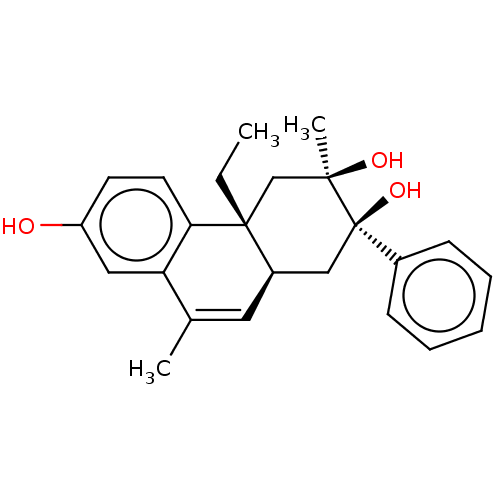 (CHEMBL3421890) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor in human SW1353 cells assessed as repression of IL-1-induced MMP-13 production after 24 hrs by EL... | J Med Chem 58: 2658-77 (2015) Article DOI: 10.1021/jm501601b BindingDB Entry DOI: 10.7270/Q2GM891C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM50468153 (CHEMBL4279392) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Curated by ChEMBL | Assay Description Displacement of [3-125I-Tyr11]-SRIF-14 or [3-125I-Tyr11]-SRIF-28 from human SSR5 expressed in CHOK1 cell membranes | ACS Med Chem Lett 9: 1088-1093 (2018) Article DOI: 10.1021/acsmedchemlett.8b00306 BindingDB Entry DOI: 10.7270/Q2V127H3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50258579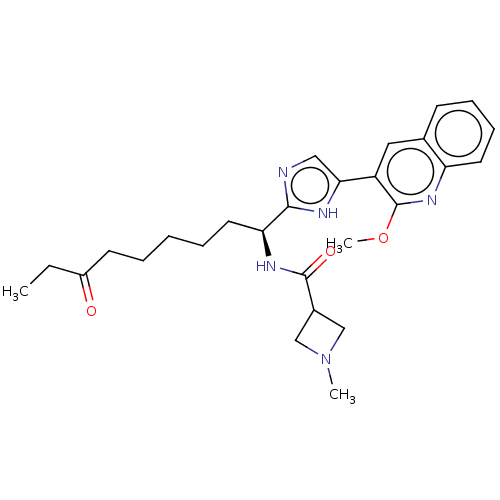 ((S)-N-(1-(5-(2-methoxyquinolin-3-yl)-1H-imidazol-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human FLAG-tagged HDAC3 expressed in human HEK293F cells co-expressing His6-tagged SMRT (1 to 899 residues) using Fluor-de-... | Citation and Details Article DOI: 10.1021/acsmedchemlett.1c00074 BindingDB Entry DOI: 10.7270/Q2V41004 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50081069 (CHEMBL3421871) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human GR | J Med Chem 58: 2658-77 (2015) Article DOI: 10.1021/jm501601b BindingDB Entry DOI: 10.7270/Q2GM891C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123256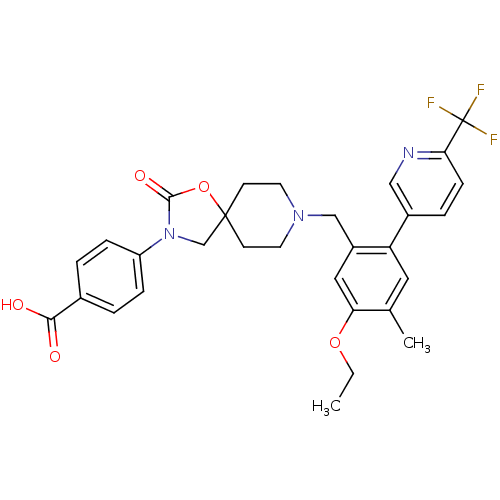 (US8742110, 4-20) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.632 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 5 (Homo sapiens (Human)) | BDBM123269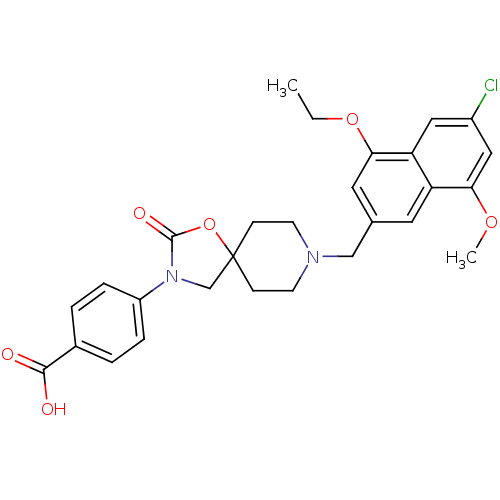 (US8742110, 5-5) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | 7.8 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description SSTR5 binding assays can be performed by labeling somatostatin and determining the ability of a compound to inhibit somatostatin binding. (Poitout et... | US Patent US8742110 (2014) BindingDB Entry DOI: 10.7270/Q26H4G39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 841 total ) | Next | Last >> |