 Found 85 hits with Last Name = 'richon' and Initial = 'vm'
Found 85 hits with Last Name = 'richon' and Initial = 'vm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92649
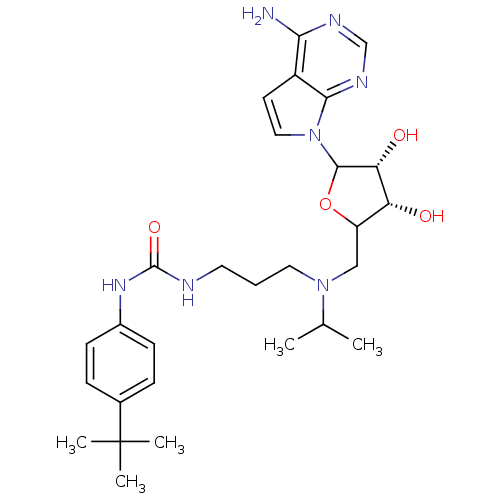
(EPZ004777)Show SMILES CC(C)N(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H41N7O4/c1-17(2)34(13-6-12-30-27(38)33-19-9-7-18(8-10-19)28(3,4)5)15-21-22(36)23(37)26(39-21)35-14-11-20-24(29)31-16-32-25(20)35/h7-11,14,16-17,21-23,26,36-37H,6,12-13,15H2,1-5H3,(H2,29,31,32)(H2,30,33,38)/t21?,22-,23-,26?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92648
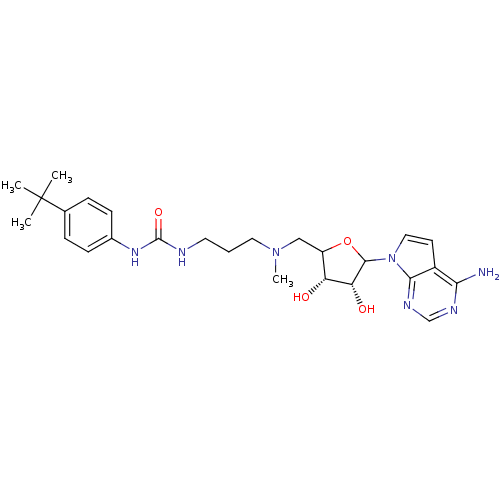
(EPZ004450)Show SMILES CN(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1ccc2c(N)ncnc12 |r| Show InChI InChI=1S/C26H37N7O4/c1-26(2,3)16-6-8-17(9-7-16)31-25(36)28-11-5-12-32(4)14-19-20(34)21(35)24(37-19)33-13-10-18-22(27)29-15-30-23(18)33/h6-10,13,15,19-21,24,34-35H,5,11-12,14H2,1-4H3,(H2,27,29,30)(H2,28,31,36)/t19?,20-,21-,24?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92647
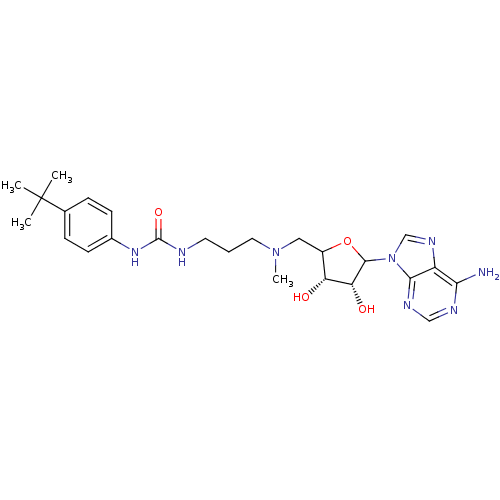
(EPZ003696)Show SMILES CN(CCCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C25H36N8O4/c1-25(2,3)15-6-8-16(9-7-15)31-24(36)27-10-5-11-32(4)12-17-19(34)20(35)23(37-17)33-14-30-18-21(26)28-13-29-22(18)33/h6-9,13-14,17,19-20,23,34-35H,5,10-12H2,1-4H3,(H2,26,28,29)(H2,27,31,36)/t17?,19-,20-,23?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92642
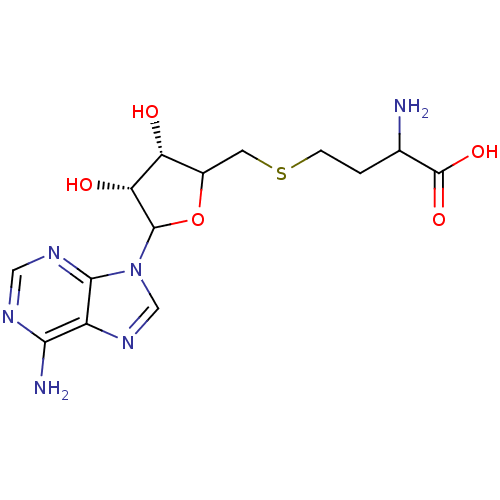
(SAH)Show SMILES NC(CCSCC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6?,7?,9-,10-,13?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92646
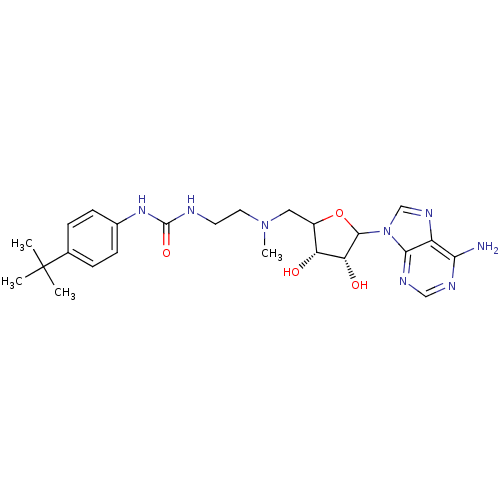
(EPZ003647)Show SMILES CN(CCNC(=O)Nc1ccc(cc1)C(C)(C)C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C24H34N8O4/c1-24(2,3)14-5-7-15(8-6-14)30-23(35)26-9-10-31(4)11-16-18(33)19(34)22(36-16)32-13-29-17-20(25)27-12-28-21(17)32/h5-8,12-13,16,18-19,22,33-34H,9-11H2,1-4H3,(H2,25,27,28)(H2,26,30,35)/t16?,18-,19-,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 845 | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92644
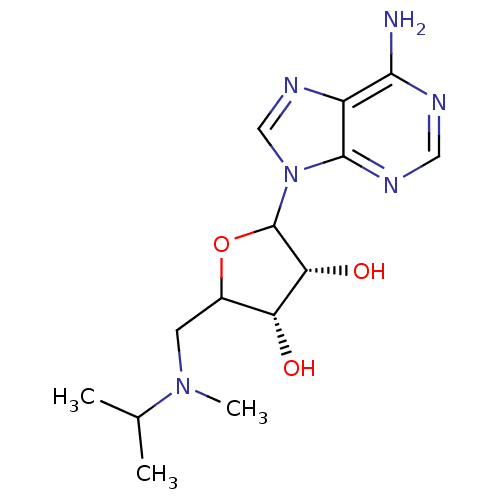
(EPZ002446)Show SMILES CC(C)N(C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H22N6O3/c1-7(2)19(3)4-8-10(21)11(22)14(23-8)20-6-18-9-12(15)16-5-17-13(9)20/h5-8,10-11,14,21-22H,4H2,1-3H3,(H2,15,16,17)/t8?,10-,11-,14?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92645
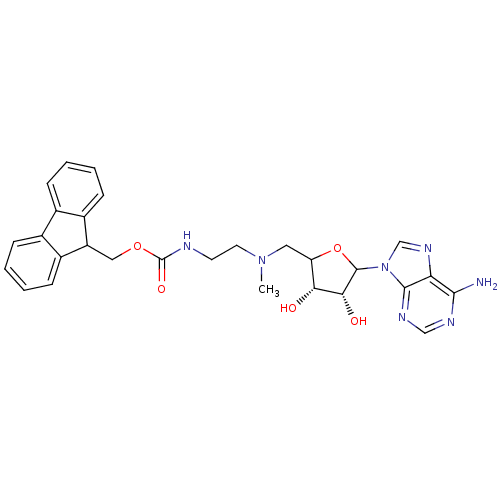
(EPZ003144)Show SMILES CN(CCNC(=O)OCC1c2ccccc2-c2ccccc12)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C28H31N7O5/c1-34(12-21-23(36)24(37)27(40-21)35-15-33-22-25(29)31-14-32-26(22)35)11-10-30-28(38)39-13-20-18-8-4-2-6-16(18)17-7-3-5-9-19(17)20/h2-9,14-15,20-21,23-24,27,36-37H,10-13H2,1H3,(H,30,38)(H2,29,31,32)/t21?,23-,24-,27?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase, H3 lysine-79 specific
(Homo sapiens (Human)) | BDBM92643
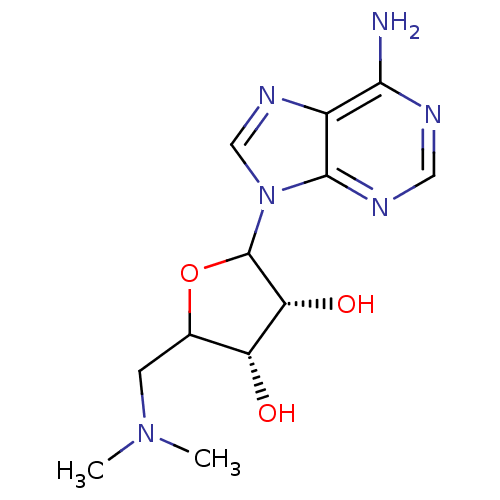
(EPZ000004)Show SMILES CN(C)CC1OC([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C12H18N6O3/c1-17(2)3-6-8(19)9(20)12(21-6)18-5-16-7-10(13)14-4-15-11(7)18/h4-6,8-9,12,19-20H,3H2,1-2H3,(H2,13,14,15)/t6?,8-,9-,12?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme Inc.
| Assay Description
Assay of DOT1L enzymatic activity were performed under balanced conditions using a radiometric assay. |
Chem Biol Drug Des 80: 971-80 (2012)
Article DOI: 10.1111/cbdd.12050
BindingDB Entry DOI: 10.7270/Q2Z89B12 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149902
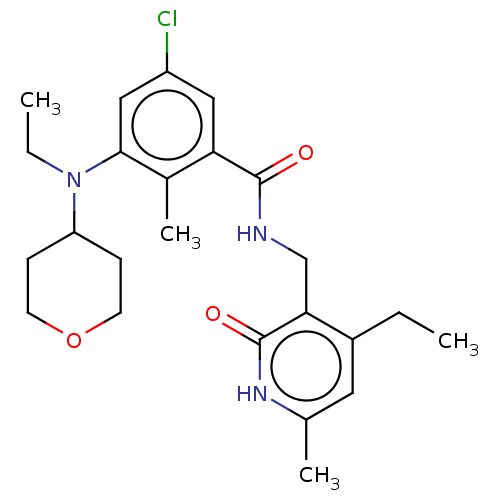
(CHEMBL3771372)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CC)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-5-17-11-15(3)27-24(30)21(17)14-26-23(29)20-12-18(25)13-22(16(20)4)28(6-2)19-7-9-31-10-8-19/h11-13,19H,5-10,14H2,1-4H3,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149895

(CHEMBL3770000)Show SMILES CCN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30BrN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149889

(CHEMBL3769571)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30ClN3O3/c1-5-27(18-6-8-30-9-7-18)21-12-17(24)11-19(16(21)4)22(28)25-13-20-14(2)10-15(3)26-23(20)29/h10-12,18H,5-9,13H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190198
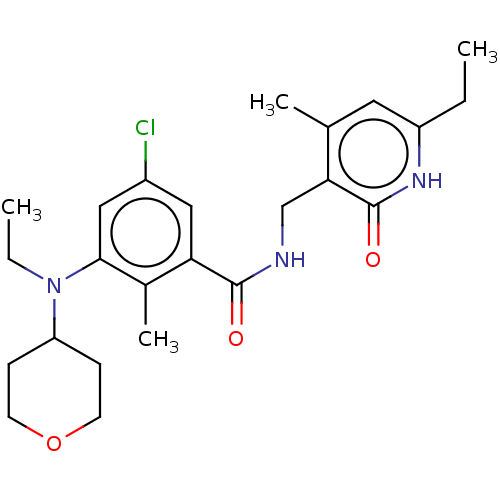
(EPZ008279 | US9175331, 27)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(CC)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-5-18-11-15(3)21(24(30)27-18)14-26-23(29)20-12-17(25)13-22(16(20)4)28(6-2)19-7-9-31-10-8-19/h11-13,19H,5-10,14H2,1-4H3,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149901
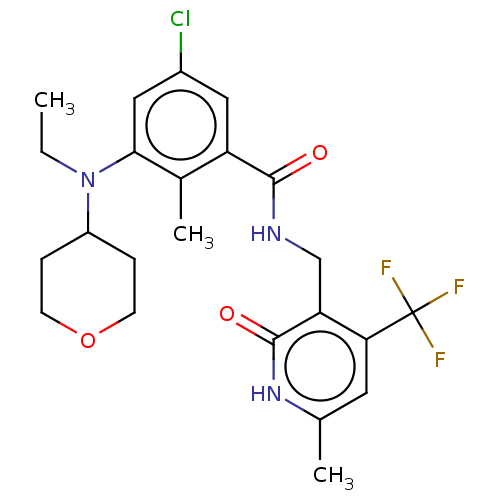
(CHEMBL3770820)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(cc(C)[nH]c2=O)C(F)(F)F)c1C Show InChI InChI=1S/C23H27ClF3N3O3/c1-4-30(16-5-7-33-8-6-16)20-11-15(24)10-17(14(20)3)21(31)28-12-18-19(23(25,26)27)9-13(2)29-22(18)32/h9-11,16H,4-8,12H2,1-3H3,(H,28,31)(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149896

(CHEMBL3771013)Show SMILES CN(C1CCNCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H29BrN4O2/c1-13-9-14(2)26-22(29)19(13)12-25-21(28)18-10-16(23)11-20(15(18)3)27(4)17-5-7-24-8-6-17/h9-11,17,24H,5-8,12H2,1-4H3,(H,25,28)(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149884
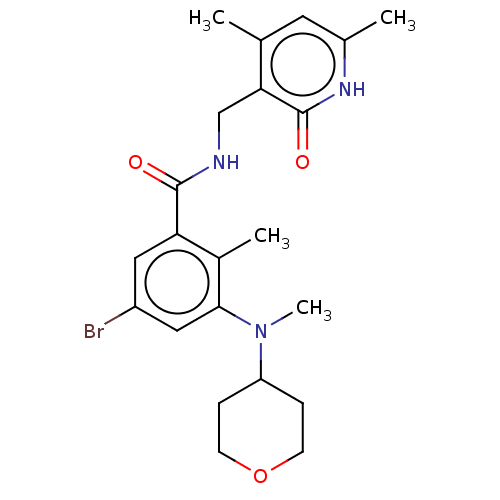
(CHEMBL3770973)Show SMILES CN(C1CCOCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O3/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-5-7-29-8-6-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149882
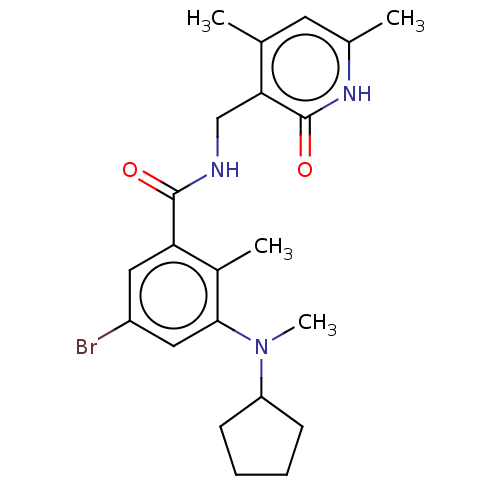
(CHEMBL3771083)Show SMILES CN(C1CCCC1)c1cc(Br)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28BrN3O2/c1-13-9-14(2)25-22(28)19(13)12-24-21(27)18-10-16(23)11-20(15(18)3)26(4)17-7-5-6-8-17/h9-11,17H,5-8,12H2,1-4H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149705

(CHEMBL3771343)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCN(CC2)C(=O)C(C)(C)C)c(=O)[nH]1 Show InChI InChI=1S/C25H31BrN6O3/c1-14-10-15(2)29-23(34)18(14)12-27-22(33)17-11-20(26)30-21-19(17)13-28-32(21)16-6-8-31(9-7-16)24(35)25(3,4)5/h10-11,13,16H,6-9,12H2,1-5H3,(H,27,33)(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149706
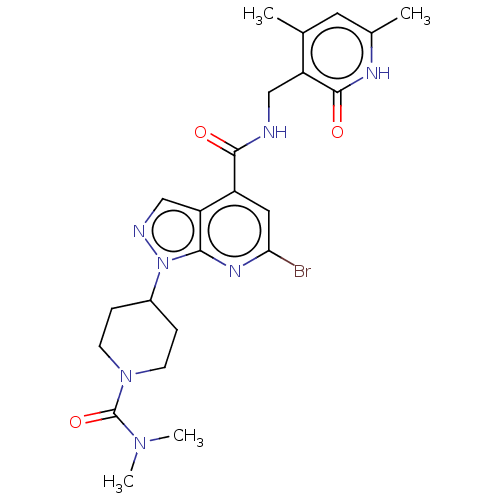
(CHEMBL3769652)Show SMILES CN(C)C(=O)N1CCC(CC1)n1ncc2c(cc(Br)nc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C23H28BrN7O3/c1-13-9-14(2)27-22(33)17(13)11-25-21(32)16-10-19(24)28-20-18(16)12-26-31(20)15-5-7-30(8-6-15)23(34)29(3)4/h9-10,12,15H,5-8,11H2,1-4H3,(H,25,32)(H,27,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149703
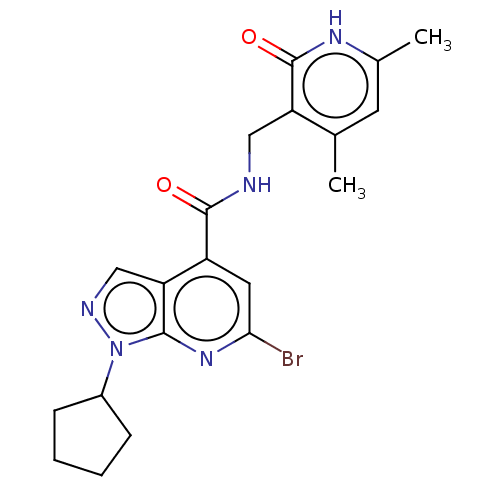
(CHEMBL3770438)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H22BrN5O2/c1-11-7-12(2)24-20(28)15(11)9-22-19(27)14-8-17(21)25-18-16(14)10-23-26(18)13-5-3-4-6-13/h7-8,10,13H,3-6,9H2,1-2H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149909

(CHEMBL3770154)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)n(C)c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(19-7-9-31-10-8-19)22-13-18(25)12-20(17(22)4)23(29)26-14-21-15(2)11-16(3)27(5)24(21)30/h11-13,19H,6-10,14H2,1-5H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149898

(CHEMBL3771269)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(C)cc[nH]c2=O)c1C Show InChI InChI=1S/C22H28ClN3O3/c1-4-26(17-6-9-29-10-7-17)20-12-16(23)11-18(15(20)3)21(27)25-13-19-14(2)5-8-24-22(19)28/h5,8,11-12,17H,4,6-7,9-10,13H2,1-3H3,(H,24,28)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149602
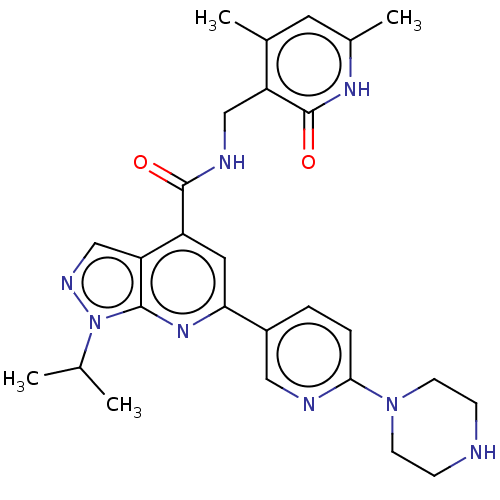
(CHEMBL3770239 | US10273223, Compound A-10 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(nc1)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C27H32N8O2/c1-16(2)35-25-22(15-31-35)20(26(36)30-14-21-17(3)11-18(4)32-27(21)37)12-23(33-25)19-5-6-24(29-13-19)34-9-7-28-8-10-34/h5-6,11-13,15-16,28H,7-10,14H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149907

(CHEMBL3770109)Show SMILES CCN(C1CCOCC1)c1ccc(Cl)c(C(=O)N(C)Cc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C24H32ClN3O3/c1-6-28(18-9-11-31-12-10-18)21-8-7-20(25)22(17(21)4)24(30)27(5)14-19-15(2)13-16(3)26-23(19)29/h7-8,13,18H,6,9-12,14H2,1-5H3,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149899

(CHEMBL3770788)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2ccc(C)[nH]c2=O)c1C Show InChI InChI=1S/C22H28ClN3O3/c1-4-26(18-7-9-29-10-8-18)20-12-17(23)11-19(15(20)3)22(28)24-13-16-6-5-14(2)25-21(16)27/h5-6,11-12,18H,4,7-10,13H2,1-3H3,(H,24,28)(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149900
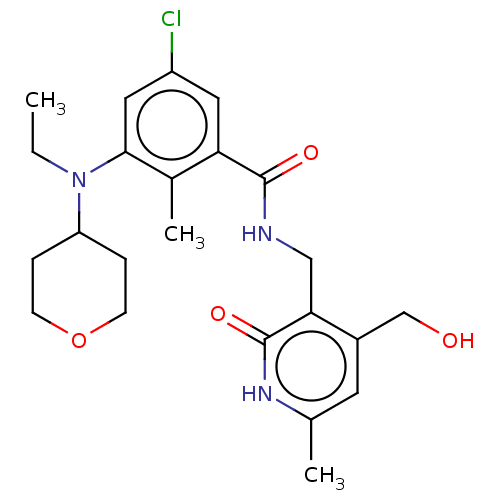
(CHEMBL3769624)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CO)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C23H30ClN3O4/c1-4-27(18-5-7-31-8-6-18)21-11-17(24)10-19(15(21)3)22(29)25-12-20-16(13-28)9-14(2)26-23(20)30/h9-11,18,28H,4-8,12-13H2,1-3H3,(H,25,29)(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149704

(CHEMBL3771135)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCN(CC2)C(=O)OC(C)(C)C)c(=O)[nH]1 Show InChI InChI=1S/C25H31BrN6O4/c1-14-10-15(2)29-23(34)18(14)12-27-22(33)17-11-20(26)30-21-19(17)13-28-32(21)16-6-8-31(9-7-16)24(35)36-25(3,4)5/h10-11,13,16H,6-9,12H2,1-5H3,(H,27,33)(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 263 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 283 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149616
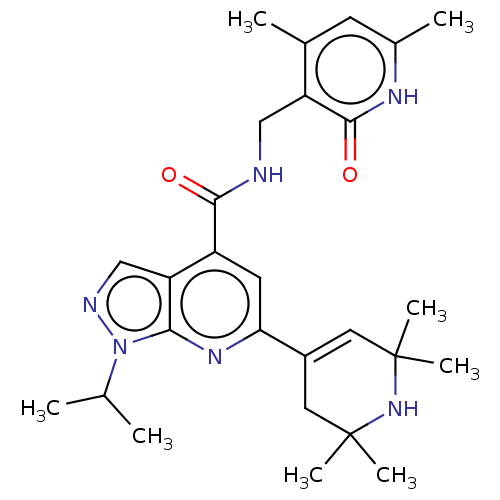
(CHEMBL3770922 | US10273223, Compound B-36 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)C1=CC(C)(C)NC(C)(C)C1)C(=O)NCc1c(C)cc(C)[nH]c1=O |t:14| Show InChI InChI=1S/C27H36N6O2/c1-15(2)33-23-21(14-29-33)19(24(34)28-13-20-16(3)9-17(4)30-25(20)35)10-22(31-23)18-11-26(5,6)32-27(7,8)12-18/h9-11,14-15,32H,12-13H2,1-8H3,(H,28,34)(H,30,35) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM190179

(EPZ005030 | US10273223, Compound A-2 | US9175331, ...)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc(CN2CCOCC2)cc1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H34N6O3/c1-18(2)35-27-25(16-31-35)23(28(36)30-15-24-19(3)13-20(4)32-29(24)37)14-26(33-27)22-7-5-21(6-8-22)17-34-9-11-38-12-10-34/h5-8,13-14,16,18H,9-12,15,17H2,1-4H3,(H,30,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50009672

(AdoHcy | CHEMBL418052 | S-(5'-adenosyl)-L-homocyst...)Show SMILES N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O Show InChI InChI=1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
US Patent
| n/a | n/a | 467 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
S-Adenosyl-L-homocysteine (SAH) was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive c... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
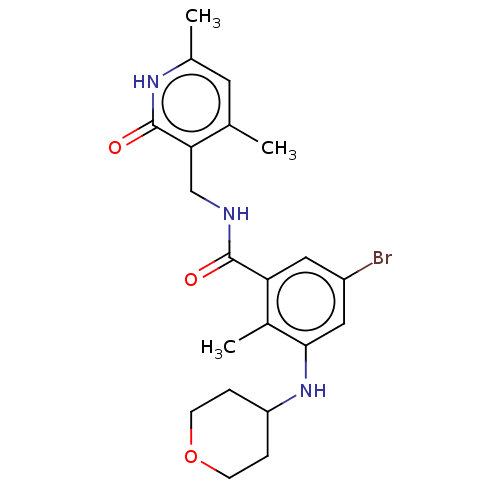
(Homo sapiens (Human)) | BDBM50149883
(CHEMBL3770619)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)cc(NC3CCOCC3)c2C)c(=O)[nH]1 Show InChI InChI=1S/C21H26BrN3O3/c1-12-8-13(2)24-21(27)18(12)11-23-20(26)17-9-15(22)10-19(14(17)3)25-16-4-6-28-7-5-16/h8-10,16,25H,4-7,11H2,1-3H3,(H,23,26)(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
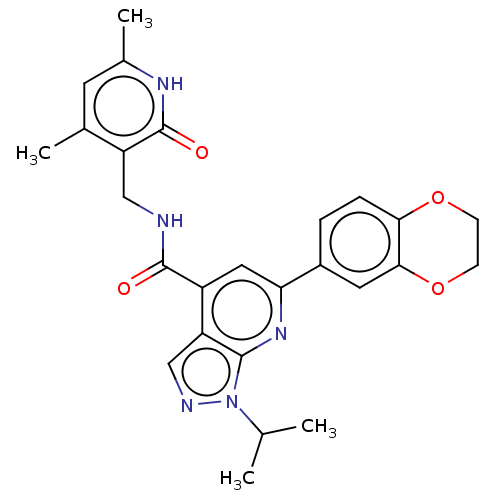
(Homo sapiens (Human)) | BDBM50075053
(CHEMBL3414618)Show SMILES CC(C)n1ncc2c(cc(nc12)-c1ccc2OCCOc2c1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C26H27N5O4/c1-14(2)31-24-20(13-28-31)18(25(32)27-12-19-15(3)9-16(4)29-26(19)33)11-21(30-24)17-5-6-22-23(10-17)35-8-7-34-22/h5-6,9-11,13-14H,7-8,12H2,1-4H3,(H,27,32)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149879

(CHEMBL3770626)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCOCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H22BrN5O3/c1-11-7-12(2)24-20(28)15(11)9-22-19(27)14-8-17(21)25-18-16(14)10-23-26(18)13-3-5-29-6-4-13/h7-8,10,13H,3-6,9H2,1-2H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149638
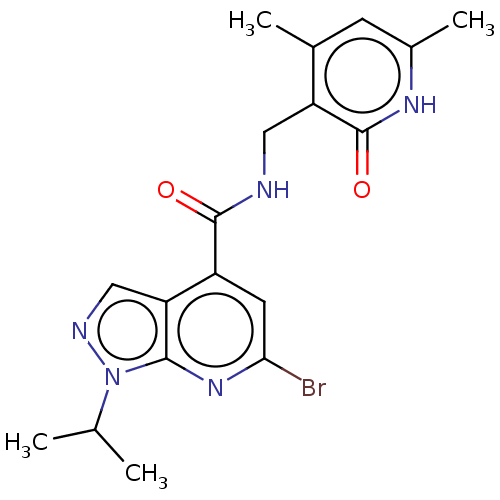
(CHEMBL3770418 | US10273223, Compound B-88 | US9637...)Show SMILES CC(C)n1ncc2c(cc(Br)nc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C18H20BrN5O2/c1-9(2)24-16-14(8-21-24)12(6-15(19)23-16)17(25)20-7-13-10(3)5-11(4)22-18(13)26/h5-6,8-9H,7H2,1-4H3,(H,20,25)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149903

(CHEMBL3769599)Show SMILES CCN(C1CCOCC1)c1cc(Cl)cc(C(=O)NCc2c(CN(C)C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C25H35ClN4O3/c1-6-30(20-7-9-33-10-8-20)23-13-19(26)12-21(17(23)3)24(31)27-14-22-18(15-29(4)5)11-16(2)28-25(22)32/h11-13,20H,6-10,14-15H2,1-5H3,(H,27,31)(H,28,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149647
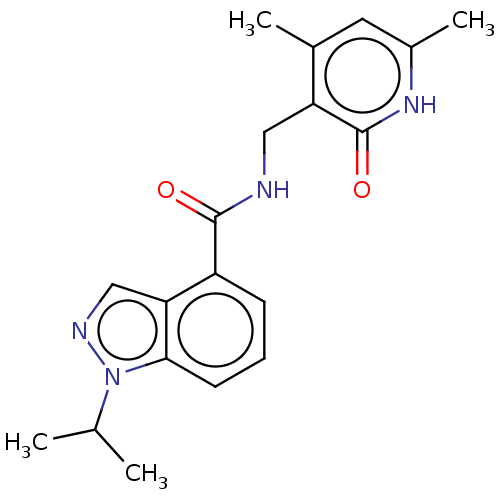
(CHEMBL3771291 | US10273223, Compound D-71 | US9637...)Show SMILES CC(C)n1ncc2c(cccc12)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C19H22N4O2/c1-11(2)23-17-7-5-6-14(16(17)10-21-23)18(24)20-9-15-12(3)8-13(4)22-19(15)25/h5-8,10-11H,9H2,1-4H3,(H,20,24)(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149624
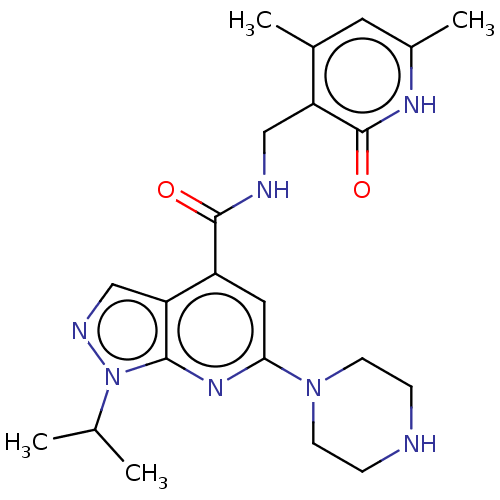
(CHEMBL3769631 | US10273223, Compound B-92 | US9637...)Show SMILES CC(C)n1ncc2c(cc(nc12)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C22H29N7O2/c1-13(2)29-20-18(12-25-29)16(10-19(27-20)28-7-5-23-6-8-28)21(30)24-11-17-14(3)9-15(4)26-22(17)31/h9-10,12-13,23H,5-8,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149887
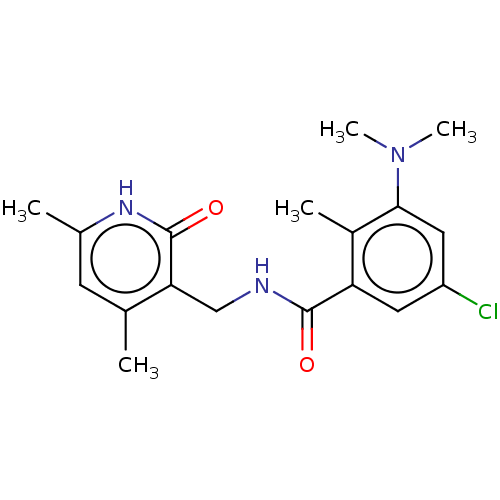
(CHEMBL3771140)Show SMILES CN(C)c1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C18H22ClN3O2/c1-10-6-11(2)21-18(24)15(10)9-20-17(23)14-7-13(19)8-16(12(14)3)22(4)5/h6-8H,9H2,1-5H3,(H,20,23)(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149878

(CHEMBL3770059)Show SMILES Cc1cc(C)c(CNC(=O)c2cc(Br)nc3n(ncc23)C2CCNCC2)c(=O)[nH]1 Show InChI InChI=1S/C20H23BrN6O2/c1-11-7-12(2)25-20(29)15(11)9-23-19(28)14-8-17(21)26-18-16(14)10-24-27(18)13-3-5-22-6-4-13/h7-8,10,13,22H,3-6,9H2,1-2H3,(H,23,28)(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149634

(CHEMBL3771177 | US10273223, Compound B-109)Show SMILES CC(C)c1cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2cnn(C(C)C)c2n1 Show InChI InChI=1S/C21H27N5O2/c1-11(2)18-8-15(17-10-23-26(12(3)4)19(17)25-18)20(27)22-9-16-13(5)7-14(6)24-21(16)28/h7-8,10-12H,9H2,1-6H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149636
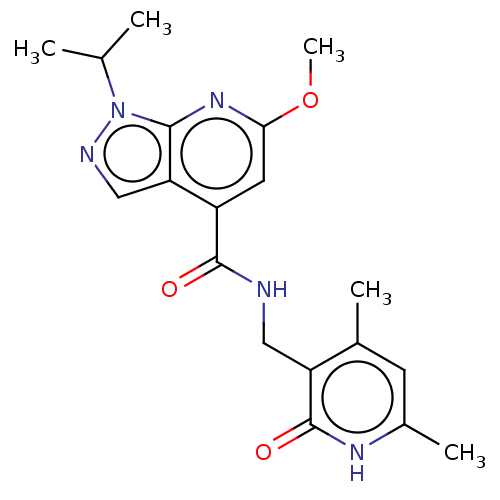
(CHEMBL3770249 | US10273223, Compound B-118 | US963...)Show SMILES COc1cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2cnn(C(C)C)c2n1 Show InChI InChI=1S/C19H23N5O3/c1-10(2)24-17-15(9-21-24)13(7-16(23-17)27-5)18(25)20-8-14-11(3)6-12(4)22-19(14)26/h6-7,9-10H,8H2,1-5H3,(H,20,25)(H,22,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM139690
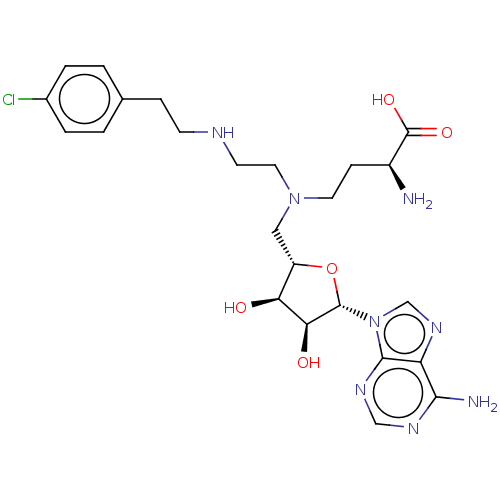
(US8895245, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)C[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17-,19-,20-,23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US8895245 (2014)
BindingDB Entry DOI: 10.7270/Q2WW7GBK |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM227458
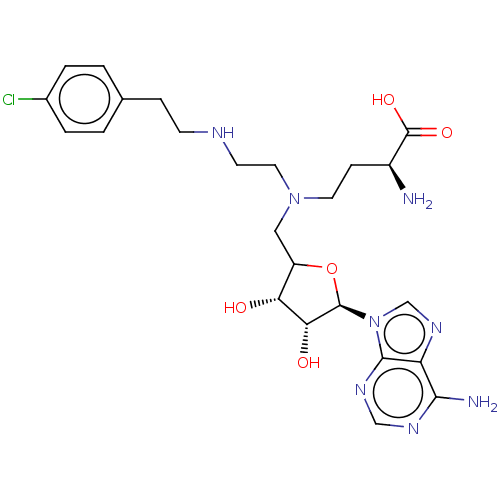
(US9333217, 75)Show SMILES N[C@@H](CCN(CCNCCc1ccc(Cl)cc1)CC1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12)C(O)=O |r| Show InChI InChI=1S/C24H33ClN8O5/c25-15-3-1-14(2-4-15)5-7-28-8-10-32(9-6-16(26)24(36)37)11-17-19(34)20(35)23(38-17)33-13-31-18-21(27)29-12-30-22(18)33/h1-4,12-13,16-17,19-20,23,28,34-35H,5-11,26H2,(H,36,37)(H2,27,29,30)/t16-,17?,19+,20+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 7.6 | n/a |
Epizyme, Inc.
US Patent
| Assay Description
Compound 75 was serially diluted 3 fold in DMSO for 10 points and 1 μL was plated in a 384 well microtiter plate. Positive control (100% inhibit... |
US Patent US9333217 (2016)
BindingDB Entry DOI: 10.7270/Q2XP73TJ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50149885
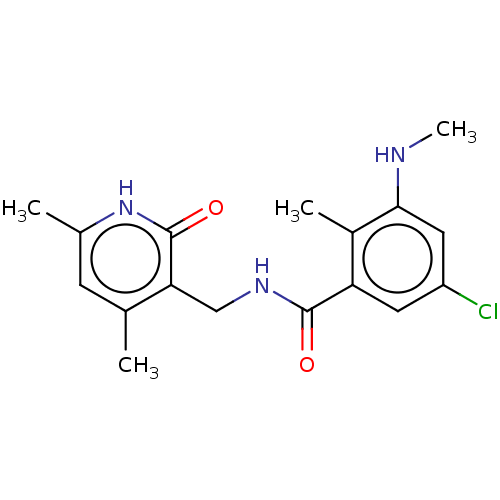
(CHEMBL3770845)Show SMILES CNc1cc(Cl)cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C Show InChI InChI=1S/C17H20ClN3O2/c1-9-5-10(2)21-17(23)14(9)8-20-16(22)13-6-12(18)7-15(19-4)11(13)3/h5-7,19H,8H2,1-4H3,(H,20,22)(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme
Curated by ChEMBL
| Assay Description
Inhibition of methyltransferase activity of human EZH2 using chicken oligonucleotide as substrate by pull down assay |
J Med Chem 59: 1556-64 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01501
BindingDB Entry DOI: 10.7270/Q2B56MM7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data