 Found 672 hits with Last Name = 'smit' and Initial = 'mj'
Found 672 hits with Last Name = 'smit' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50118719
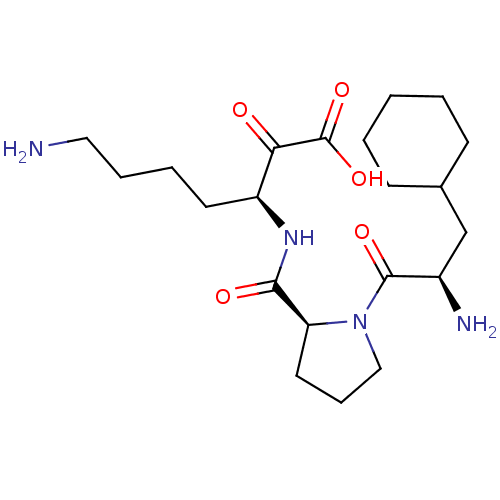
(7-Amino-3-{[1-(2-amino-3-cyclohexyl-propionyl)-pyr...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)C(O)=O Show InChI InChI=1S/C21H36N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h14-17H,1-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118730
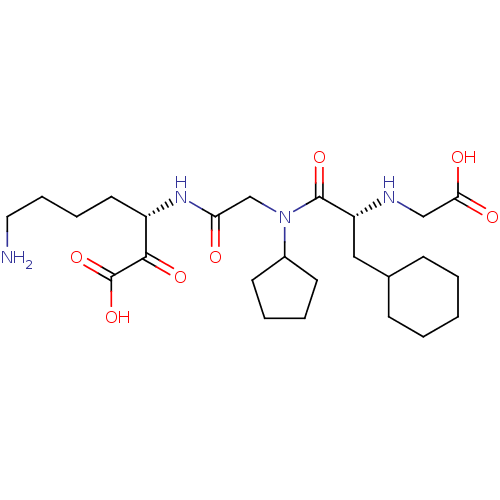
(7-Amino-3-(2-{[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CCCC1)C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C25H42N4O7/c26-13-7-6-12-19(23(33)25(35)36)28-21(30)16-29(18-10-4-5-11-18)24(34)20(27-15-22(31)32)14-17-8-2-1-3-9-17/h17-20,27H,1-16,26H2,(H,28,30)(H,31,32)(H,35,36)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118739
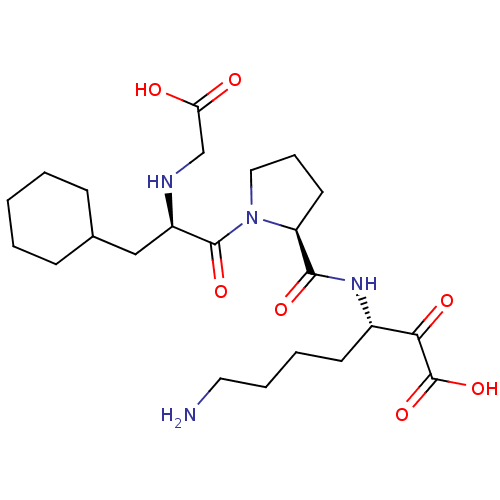
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C23H38N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h15-18,25H,1-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118728
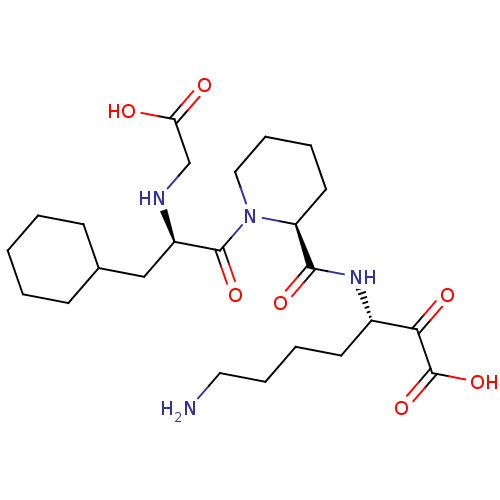
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C24H40N4O7/c25-12-6-4-10-17(21(31)24(34)35)27-22(32)19-11-5-7-13-28(19)23(33)18(26-15-20(29)30)14-16-8-2-1-3-9-16/h16-19,26H,1-15,25H2,(H,27,32)(H,29,30)(H,34,35)/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118732
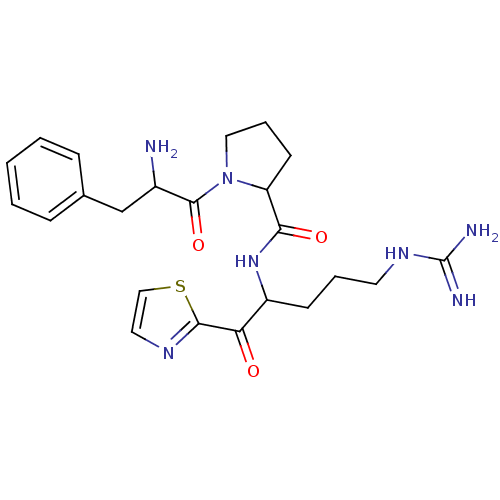
(1-(2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NC(Cc1ccccc1)C(=O)N1CCCC1C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118729
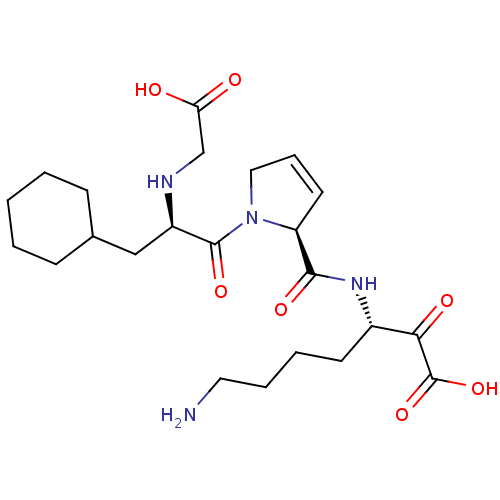
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1C=CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O |c:10| Show InChI InChI=1S/C23H36N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h6,10,15-18,25H,1-5,7-9,11-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118731
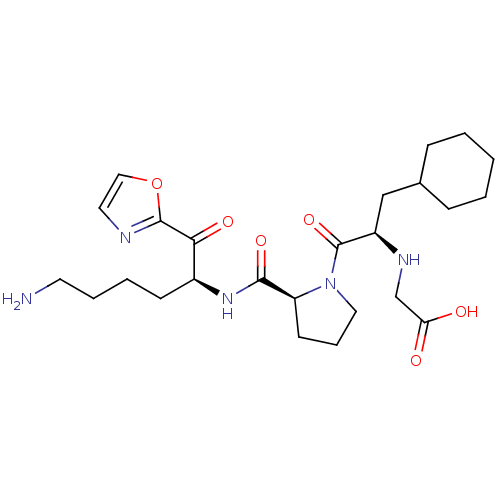
((2-{2-[5-Amino-1-(oxazole-2-carbonyl)-pentylcarbam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1ncco1 Show InChI InChI=1S/C25H39N5O6/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118735
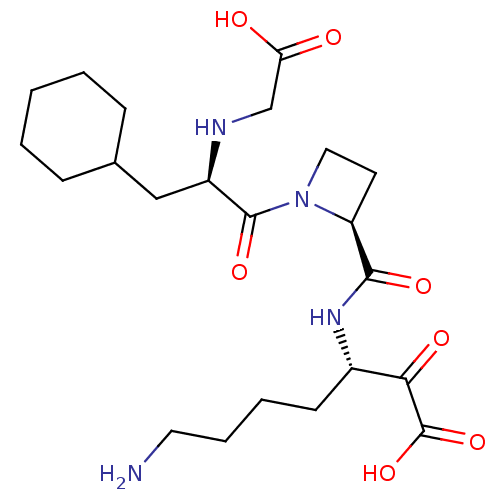
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C22H36N4O7/c23-10-5-4-8-15(19(29)22(32)33)25-20(30)17-9-11-26(17)21(31)16(24-13-18(27)28)12-14-6-2-1-3-7-14/h14-17,24H,1-13,23H2,(H,25,30)(H,27,28)(H,32,33)/t15-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118738
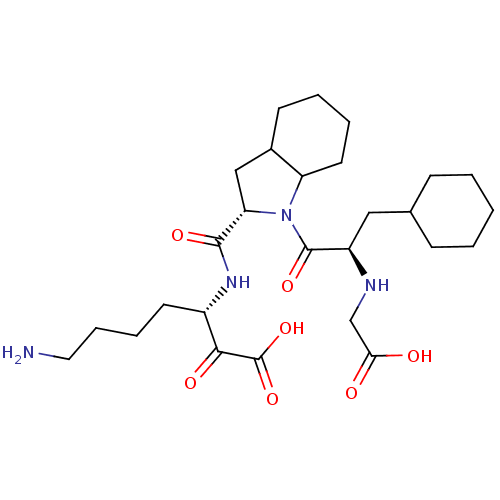
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C27H44N4O7/c28-13-7-6-11-19(24(34)27(37)38)30-25(35)22-15-18-10-4-5-12-21(18)31(22)26(36)20(29-16-23(32)33)14-17-8-2-1-3-9-17/h17-22,29H,1-16,28H2,(H,30,35)(H,32,33)(H,37,38)/t18?,19-,20+,21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118718
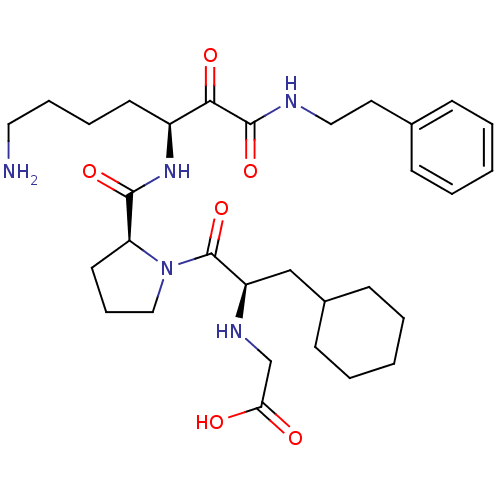
(CHEMBL343804 | {2-[2-(5-Amino-1-phenethylaminooxal...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H47N5O6/c32-17-8-7-14-24(28(39)30(41)33-18-16-22-10-3-1-4-11-22)35-29(40)26-15-9-19-36(26)31(42)25(34-21-27(37)38)20-23-12-5-2-6-13-23/h1,3-4,10-11,23-26,34H,2,5-9,12-21,32H2,(H,33,41)(H,35,40)(H,37,38)/t24-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118723
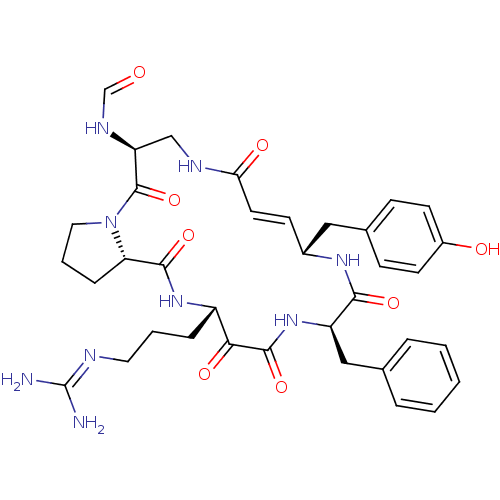
(CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-1=O)-[#7]-[#6]=O |r,t:24| Show InChI InChI=1S/C36H45N9O8/c37-36(38)39-16-4-8-26-31(49)34(52)44-27(19-22-6-2-1-3-7-22)32(50)42-24(18-23-10-13-25(47)14-11-23)12-15-30(48)40-20-28(41-21-46)35(53)45-17-5-9-29(45)33(51)43-26/h1-3,6-7,10-15,21,24,26-29,47H,4-5,8-9,16-20H2,(H,40,48)(H,41,46)(H,42,50)(H,43,51)(H,44,52)(H4,37,38,39)/b15-12+/t24-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118727
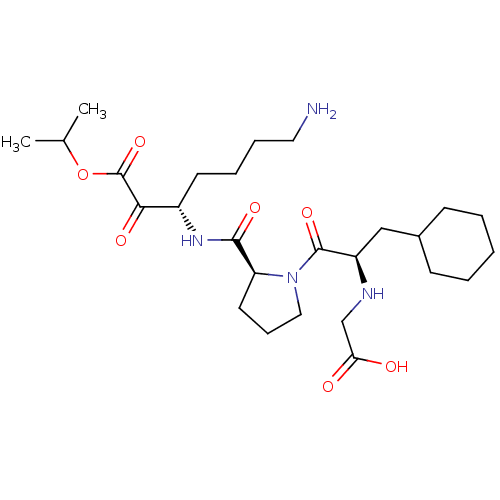
(2-((R)-1-((S)-2-(((S)-7-amino-1-isopropoxy-1,2-dio...)Show SMILES CC(C)OC(=O)C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C26H44N4O7/c1-17(2)37-26(36)23(33)19(11-6-7-13-27)29-24(34)21-12-8-14-30(21)25(35)20(28-16-22(31)32)15-18-9-4-3-5-10-18/h17-21,28H,3-16,27H2,1-2H3,(H,29,34)(H,31,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118720
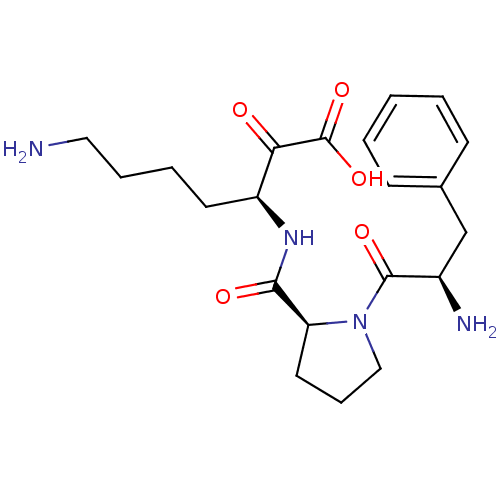
(7-Amino-3-{[1-(2-amino-3-phenyl-propionyl)-pyrroli...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C21H30N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h1-3,7-8,15-17H,4-6,9-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29388
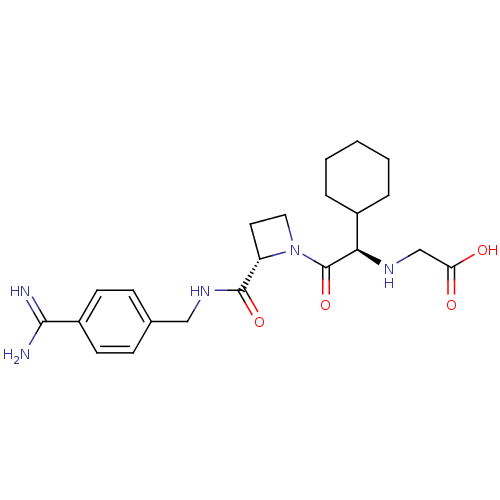
(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50118717
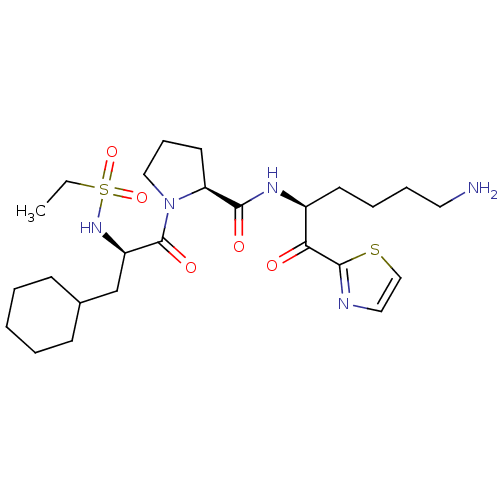
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES CCS(=O)(=O)N[C@H](CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)c1nccs1 Show InChI InChI=1S/C25H41N5O5S2/c1-2-37(34,35)29-20(17-18-9-4-3-5-10-18)25(33)30-15-8-12-21(30)23(32)28-19(11-6-7-13-26)22(31)24-27-14-16-36-24/h14,16,18-21,29H,2-13,15,17,26H2,1H3,(H,28,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118737
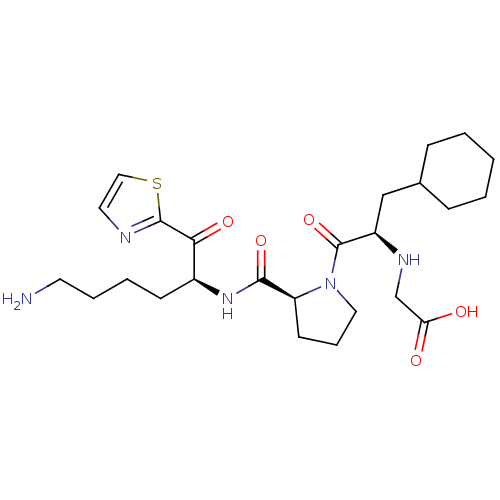
((2-{2-[5-Amino-1-(thiazole-2-carbonyl)-pentylcarba...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1nccs1 Show InChI InChI=1S/C25H39N5O5S/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50337250
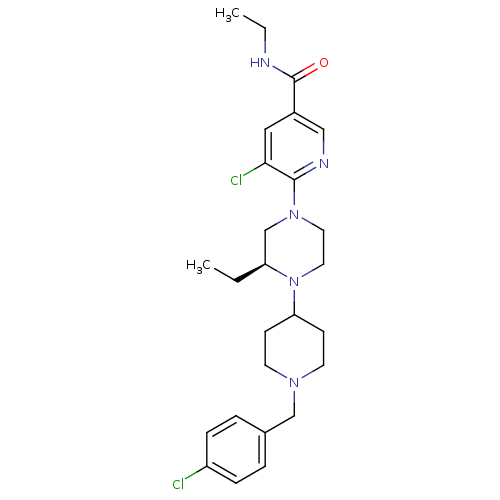
((S)-5-chloro-6-(4-(1-(4-chlorobenzyl)piperidin-4-y...)Show SMILES CCNC(=O)c1cnc(N2CCN([C@@H](CC)C2)C2CCN(Cc3ccc(Cl)cc3)CC2)c(Cl)c1 |r| Show InChI InChI=1S/C26H35Cl2N5O/c1-3-22-18-32(25-24(28)15-20(16-30-25)26(34)29-4-2)13-14-33(22)23-9-11-31(12-10-23)17-19-5-7-21(27)8-6-19/h5-8,15-16,22-23H,3-4,9-14,17-18H2,1-2H3,(H,29,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from human CXCR3 expressed in HEK293T cells |
Bioorg Med Chem Lett 19: 2252-7 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.093
BindingDB Entry DOI: 10.7270/Q2DJ5GW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118721
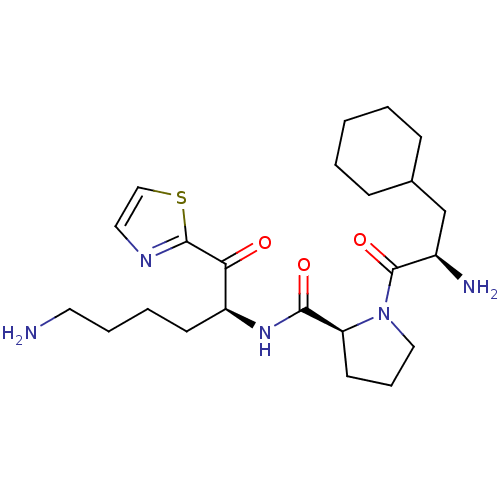
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h12,14,16-19H,1-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Mus musculus) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against thymidylate synthase |
J Med Chem 28: 1468-76 (1985)
BindingDB Entry DOI: 10.7270/Q2348JDZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50037996
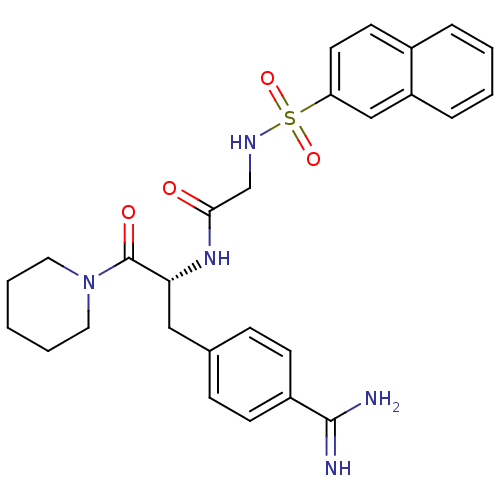
(1-[N-(naphthalen-2-ylsulfonyl)glycyl-4-carbamimido...)Show SMILES NC(=N)c1ccc(C[C@@H](NC(=O)CNS(=O)(=O)c2ccc3ccccc3c2)C(=O)N2CCCCC2)cc1 Show InChI InChI=1S/C27H31N5O4S/c28-26(29)21-10-8-19(9-11-21)16-24(27(34)32-14-4-1-5-15-32)31-25(33)18-30-37(35,36)23-13-12-20-6-2-3-7-22(20)17-23/h2-3,6-13,17,24,30H,1,4-5,14-16,18H2,(H3,28,29)(H,31,33)/t24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NV Organon
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for the thrombin inhibition. |
Bioorg Med Chem Lett 9: 2837-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SB468S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50060033
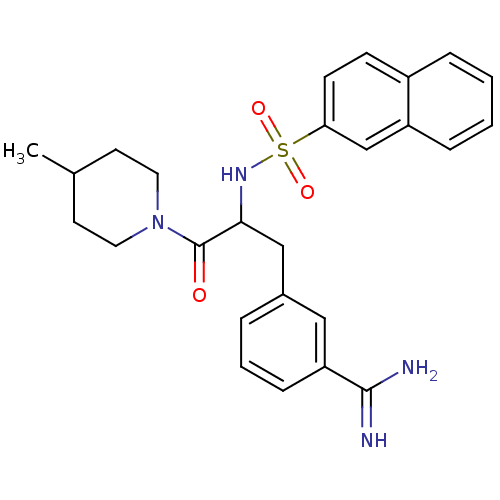
(3-[3-(4-Methyl-piperidin-1-yl)-2-(naphthalene-2-su...)Show SMILES CC1CCN(CC1)C(=O)C(Cc1cccc(c1)C(N)=N)NS(=O)(=O)c1ccc2ccccc2c1 Show InChI InChI=1S/C26H30N4O3S/c1-18-11-13-30(14-12-18)26(31)24(16-19-5-4-8-22(15-19)25(27)28)29-34(32,33)23-10-9-20-6-2-3-7-21(20)17-23/h2-10,15,17-18,24,29H,11-14,16H2,1H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NV Organon
Curated by ChEMBL
| Assay Description
The compound was evaluated in vitro for the thrombin inhibition. |
Bioorg Med Chem Lett 9: 2837-42 (1999)
BindingDB Entry DOI: 10.7270/Q2SB468S |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380130
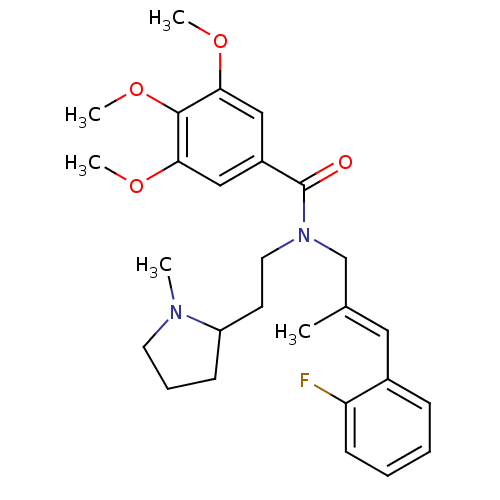
(CHEMBL2013230)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccccc1F Show InChI InChI=1S/C27H35FN2O4/c1-19(15-20-9-6-7-11-23(20)28)18-30(14-12-22-10-8-13-29(22)2)27(31)21-16-24(32-3)26(34-5)25(17-21)33-4/h6-7,9,11,15-17,22H,8,10,12-14,18H2,1-5H3/b19-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50310502
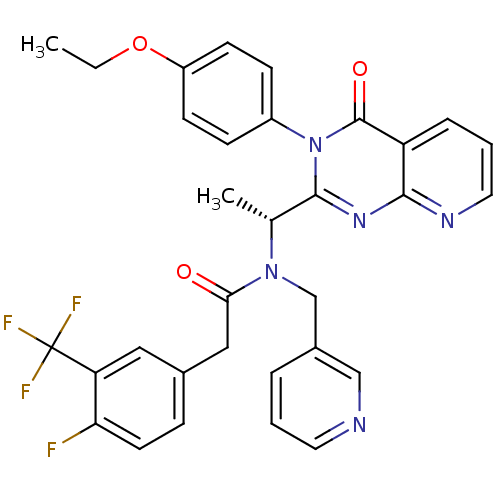
((R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydropyri...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(F)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C32H27F4N5O3/c1-3-44-24-11-9-23(10-12-24)41-30(39-29-25(31(41)43)7-5-15-38-29)20(2)40(19-22-6-4-14-37-18-22)28(42)17-21-8-13-27(33)26(16-21)32(34,35)36/h4-16,18,20H,3,17,19H2,1-2H3/t20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from CXCR3 expressed in HEK293 cells |
J Med Chem 55: 10572-83 (2012)
Article DOI: 10.1021/jm301240t
BindingDB Entry DOI: 10.7270/Q27P90J8 |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380114
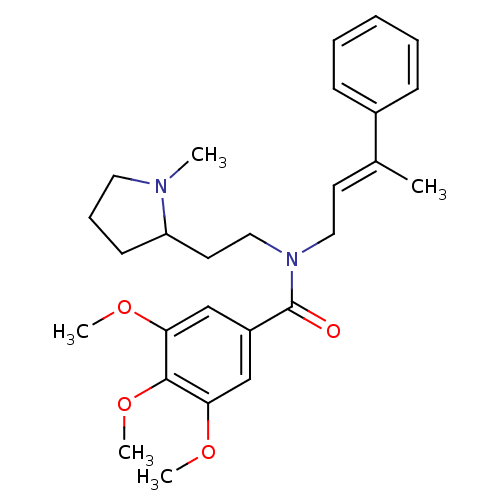
(CHEMBL2013216)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(CCC1CCCN1C)C\C=C(/C)c1ccccc1 Show InChI InChI=1S/C27H36N2O4/c1-20(21-10-7-6-8-11-21)13-16-29(17-14-23-12-9-15-28(23)2)27(30)22-18-24(31-3)26(33-5)25(19-22)32-4/h6-8,10-11,13,18-19,23H,9,12,14-17H2,1-5H3/b20-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118722

(2-Amino-N-{[5-amino-1-(thiazole-2-carbonyl)-pentyl...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-8-19(21(30)22-26-12-13-32-22)27-20(29)15-28(17-9-10-17)23(31)18(25)14-16-6-2-1-3-7-16/h12-13,16-19H,1-11,14-15,24-25H2,(H,27,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184539

((2S)-2-(6-butyl-2-(4-(4-(trifluoromethyl)phenyl)-1...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C29H39F3N6O2/c1-5-7-9-23-17-26(36-24(16-20(3)4)27(39)33-14-8-15-40-6-2)37-28(35-23)38-18-25(34-19-38)21-10-12-22(13-11-21)29(30,31)32/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,33,39)(H,35,36,37)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Dihydrofolate reductase
(Mus musculus (Mouse)) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity was evaluated against dihydrofolate reductase |
J Med Chem 28: 1468-76 (1985)
BindingDB Entry DOI: 10.7270/Q2348JDZ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118725
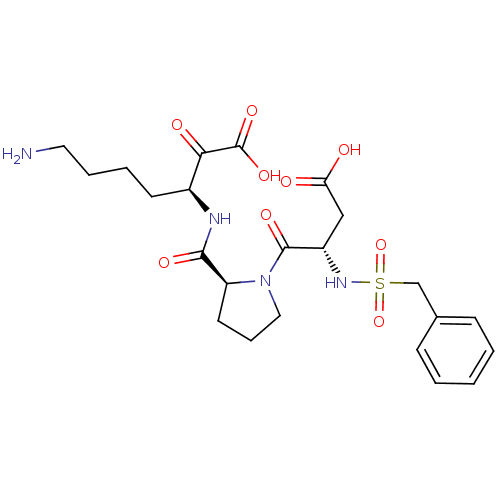
(7-Amino-3-{[1-(3-carboxy-2-phenylmethanesulfonylam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NS(=O)(=O)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C23H32N4O9S/c24-11-5-4-9-16(20(30)23(33)34)25-21(31)18-10-6-12-27(18)22(32)17(13-19(28)29)26-37(35,36)14-15-7-2-1-3-8-15/h1-3,7-8,16-18,26H,4-6,9-14,24H2,(H,25,31)(H,28,29)(H,33,34)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380134
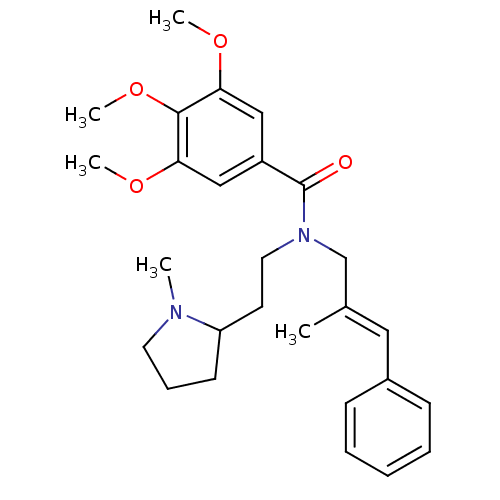
(CHEMBL2013215)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccccc1 Show InChI InChI=1S/C27H36N2O4/c1-20(16-21-10-7-6-8-11-21)19-29(15-13-23-12-9-14-28(23)2)27(30)22-17-24(31-3)26(33-5)25(18-22)32-4/h6-8,10-11,16-18,23H,9,12-15,19H2,1-5H3/b20-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184530
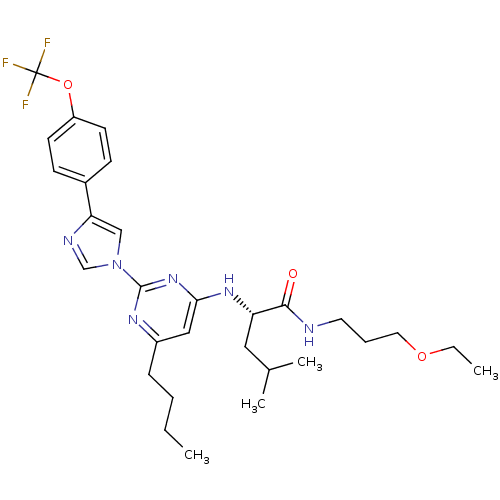
((2S)-2-(6-butyl-2-(4-(4-(trifluoromethoxy)phenyl)-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C29H39F3N6O3/c1-5-7-9-22-17-26(36-24(16-20(3)4)27(39)33-14-8-15-40-6-2)37-28(35-22)38-18-25(34-19-38)21-10-12-23(13-11-21)41-29(30,31)32/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,33,39)(H,35,36,37)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50038001
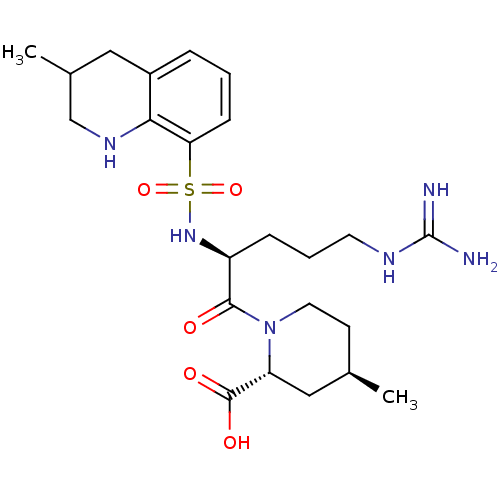
((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380131
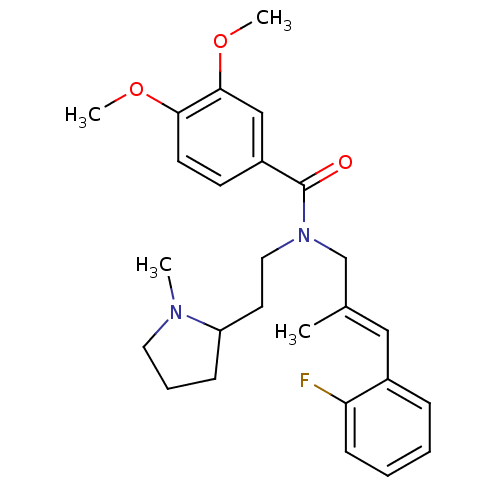
(CHEMBL2013231)Show SMILES COc1ccc(cc1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccccc1F Show InChI InChI=1S/C26H33FN2O3/c1-19(16-20-8-5-6-10-23(20)27)18-29(15-13-22-9-7-14-28(22)2)26(30)21-11-12-24(31-3)25(17-21)32-4/h5-6,8,10-12,16-17,22H,7,9,13-15,18H2,1-4H3/b19-16+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184531

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C28H37F3N6O3/c1-5-8-21-16-25(35-23(15-19(3)4)26(38)32-13-7-14-39-6-2)36-27(34-21)37-17-24(33-18-37)20-9-11-22(12-10-20)40-28(29,30)31/h9-12,16-19,23H,5-8,13-15H2,1-4H3,(H,32,38)(H,34,35,36)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380121
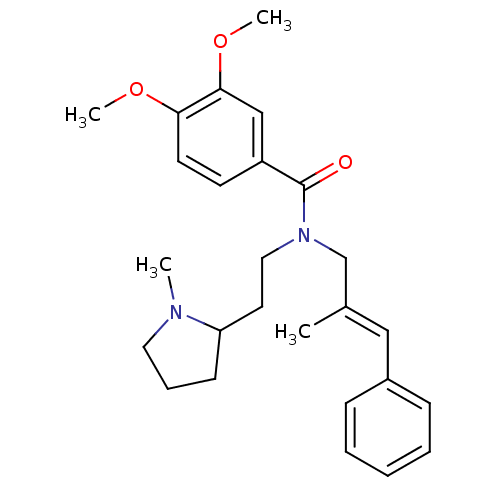
(CHEMBL2013219)Show SMILES COc1ccc(cc1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccccc1 Show InChI InChI=1S/C26H34N2O3/c1-20(17-21-9-6-5-7-10-21)19-28(16-14-23-11-8-15-27(23)2)26(29)22-12-13-24(30-3)25(18-22)31-4/h5-7,9-10,12-13,17-18,23H,8,11,14-16,19H2,1-4H3/b20-17+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184518

((2S)-2-(6-butyl-2-(4-(4-chlorophenyl)-1H-imidazol-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H39ClN6O2/c1-5-7-9-23-17-26(33-24(16-20(3)4)27(36)30-14-8-15-37-6-2)34-28(32-23)35-18-25(31-19-35)21-10-12-22(29)13-11-21/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,30,36)(H,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118734
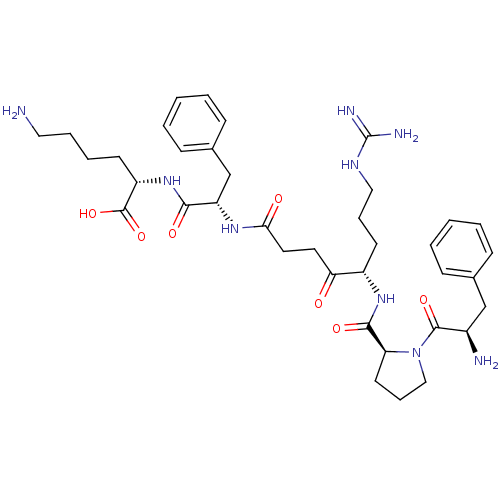
(6-Amino-2-[2-(5-{[1-(2-amino-3-phenyl-propionyl)-p...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H55N9O7/c39-20-8-7-15-29(37(53)54)46-34(50)30(24-26-13-5-2-6-14-26)44-33(49)19-18-32(48)28(16-9-21-43-38(41)42)45-35(51)31-17-10-22-47(31)36(52)27(40)23-25-11-3-1-4-12-25/h1-6,11-14,27-31H,7-10,15-24,39-40H2,(H,44,49)(H,45,51)(H,46,50)(H,53,54)(H4,41,42,43)/t27-,28+,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380123
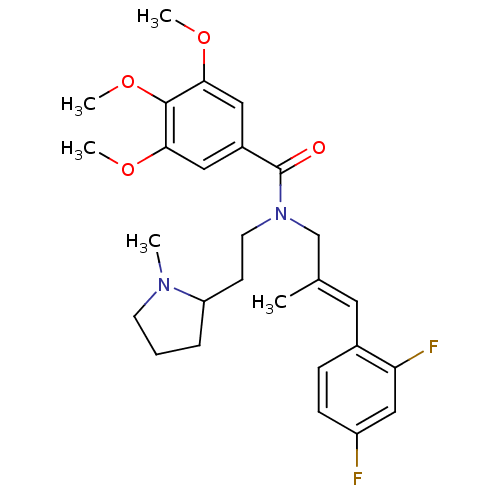
(CHEMBL2013222)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccc(F)cc1F Show InChI InChI=1S/C27H34F2N2O4/c1-18(13-19-8-9-21(28)16-23(19)29)17-31(12-10-22-7-6-11-30(22)2)27(32)20-14-24(33-3)26(35-5)25(15-20)34-4/h8-9,13-16,22H,6-7,10-12,17H2,1-5H3/b18-13+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184527

((2S)-2-(6-butyl-2-(4-(4-hydroxyphenyl)-1H-imidazol...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(O)cc1 Show InChI InChI=1S/C28H40N6O3/c1-5-7-9-22-17-26(32-24(16-20(3)4)27(36)29-14-8-15-37-6-2)33-28(31-22)34-18-25(30-19-34)21-10-12-23(35)13-11-21/h10-13,17-20,24,35H,5-9,14-16H2,1-4H3,(H,29,36)(H,31,32,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50121205

(CHEBI:18295 | Histamine)Show InChI InChI=1S/C5H9N3/c6-2-1-5-3-7-4-8-5/h3-4H,1-2,6H2,(H,7,8) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University
Curated by ChEMBL
| Assay Description
Histamine H3 receptor competition binding using [3H]Na-methylhistamine |
J Med Chem 46: 3162-5 (2003)
Article DOI: 10.1021/jm0300025
BindingDB Entry DOI: 10.7270/Q24B343F |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184540
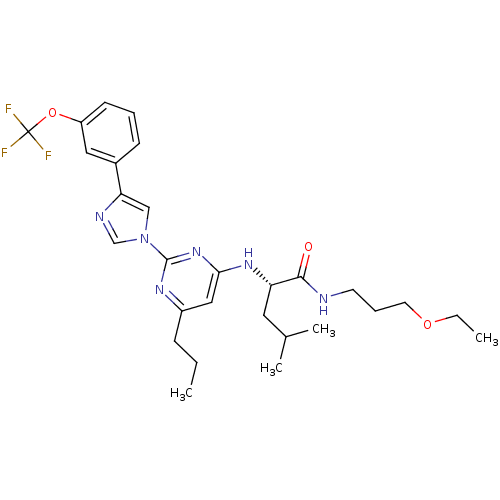
((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C28H37F3N6O3/c1-5-9-21-16-25(35-23(14-19(3)4)26(38)32-12-8-13-39-6-2)36-27(34-21)37-17-24(33-18-37)20-10-7-11-22(15-20)40-28(29,30)31/h7,10-11,15-19,23H,5-6,8-9,12-14H2,1-4H3,(H,32,38)(H,34,35,36)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184536
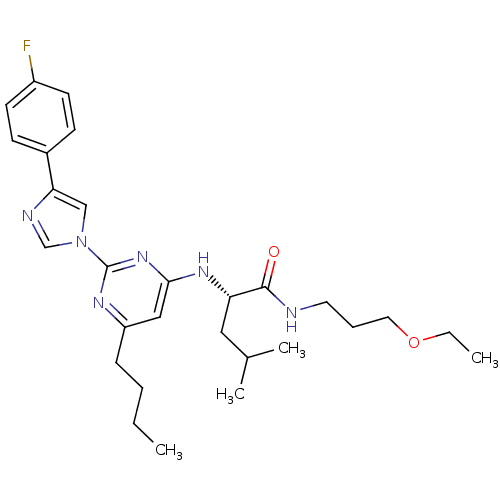
((2S)-2-(6-butyl-2-(4-(4-fluorophenyl)-1H-imidazol-...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C28H39FN6O2/c1-5-7-9-23-17-26(33-24(16-20(3)4)27(36)30-14-8-15-37-6-2)34-28(32-23)35-18-25(31-19-35)21-10-12-22(29)13-11-21/h10-13,17-20,24H,5-9,14-16H2,1-4H3,(H,30,36)(H,32,33,34)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50211114

((-)-(R)-N-(1-(3-(4-ethoxyphenyl)-4-oxo-3,4-dihydro...)Show SMILES CCOc1ccc(cc1)-n1c(nc2ncccc2c1=O)[C@@H](C)N(Cc1cccnc1)C(=O)Cc1ccc(OC(F)(F)F)cc1 |r| Show InChI InChI=1S/C32H28F3N5O4/c1-3-43-25-14-10-24(11-15-25)40-30(38-29-27(31(40)42)7-5-17-37-29)21(2)39(20-23-6-4-16-36-19-23)28(41)18-22-8-12-26(13-9-22)44-32(33,34)35/h4-17,19,21H,3,18,20H2,1-2H3/t21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL10 from human CXCR3 expressed in HEK293 cells |
Bioorg Med Chem 19: 3384-93 (2011)
Article DOI: 10.1016/j.bmc.2011.04.035
BindingDB Entry DOI: 10.7270/Q2RB75RG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50399980
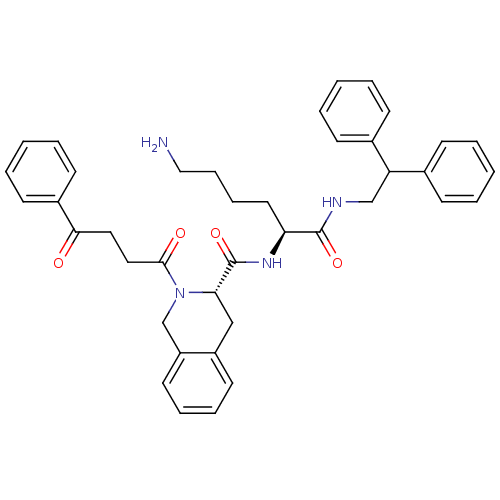
(CHEMBL2181467)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)CCC(=O)c1ccccc1)C(=O)NCC(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C40H44N4O4/c41-25-13-12-22-35(39(47)42-27-34(29-14-4-1-5-15-29)30-16-6-2-7-17-30)43-40(48)36-26-32-20-10-11-21-33(32)28-44(36)38(46)24-23-37(45)31-18-8-3-9-19-31/h1-11,14-21,34-36H,12-13,22-28,41H2,(H,42,47)(H,43,48)/t35-,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 39.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from CXCR3 expressed in HEK293 cells |
J Med Chem 55: 10572-83 (2012)
Article DOI: 10.1021/jm301240t
BindingDB Entry DOI: 10.7270/Q27P90J8 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184535
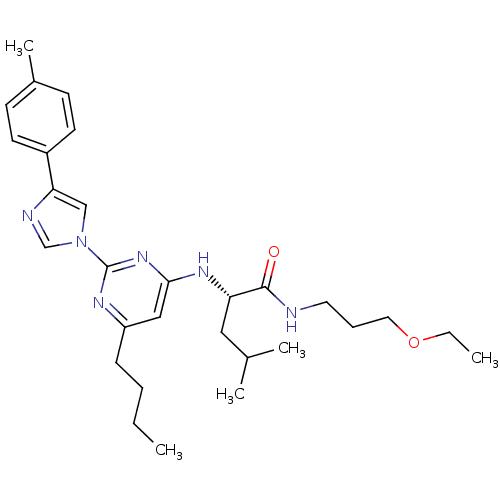
((2S)-2-(6-butyl-2-(4-p-tolyl-1H-imidazol-1-yl)pyri...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(C)cc1 Show InChI InChI=1S/C29H42N6O2/c1-6-8-10-24-18-27(33-25(17-21(3)4)28(36)30-15-9-16-37-7-2)34-29(32-24)35-19-26(31-20-35)23-13-11-22(5)12-14-23/h11-14,18-21,25H,6-10,15-17H2,1-5H3,(H,30,36)(H,32,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184521
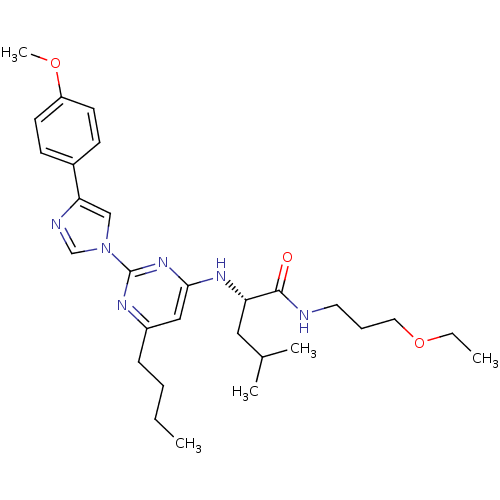
((2S)-2-(6-butyl-2-(4-(4-methoxyphenyl)-1H-imidazol...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(OC)cc1 Show InChI InChI=1S/C29H42N6O3/c1-6-8-10-23-18-27(33-25(17-21(3)4)28(36)30-15-9-16-38-7-2)34-29(32-23)35-19-26(31-20-35)22-11-13-24(37-5)14-12-22/h11-14,18-21,25H,6-10,15-17H2,1-5H3,(H,30,36)(H,32,33,34)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184523
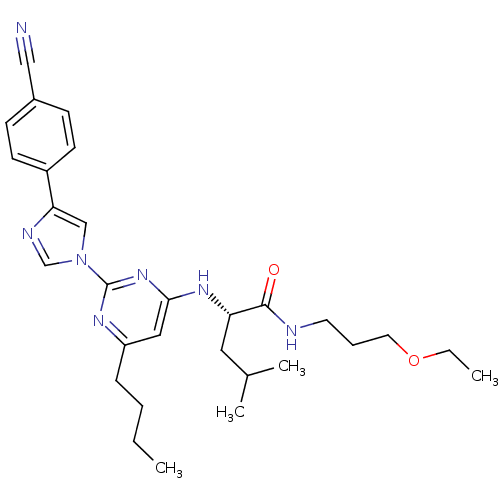
((2S)-2-(6-butyl-2-(4-(4-cyanophenyl)-1H-imidazol-1...)Show SMILES CCCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccc(cc1)C#N Show InChI InChI=1S/C29H39N7O2/c1-5-7-9-24-17-27(34-25(16-21(3)4)28(37)31-14-8-15-38-6-2)35-29(33-24)36-19-26(32-20-36)23-12-10-22(18-30)11-13-23/h10-13,17,19-21,25H,5-9,14-16H2,1-4H3,(H,31,37)(H,33,34,35)/t25-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184517

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-methyl-2-(4-...)Show SMILES CCOCCCNC(=O)[C@H](CC(C)C)Nc1cc(C)nc(n1)-n1cnc(c1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H33F3N6O3/c1-5-37-12-6-11-30-24(36)21(13-17(2)3)33-23-14-18(4)32-25(34-23)35-15-22(31-16-35)19-7-9-20(10-8-19)38-26(27,28)29/h7-10,14-17,21H,5-6,11-13H2,1-4H3,(H,30,36)(H,32,33,34)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50184537

((2S)-N-(3-ethoxypropyl)-4-methyl-2-(6-propyl-2-(4-...)Show SMILES CCCc1cc(N[C@@H](CC(C)C)C(=O)NCCCOCC)nc(n1)-n1cnc(c1)-c1ccccc1OC(F)(F)F Show InChI InChI=1S/C28H37F3N6O3/c1-5-10-20-16-25(35-22(15-19(3)4)26(38)32-13-9-14-39-6-2)36-27(34-20)37-17-23(33-18-37)21-11-7-8-12-24(21)40-28(29,30)31/h7-8,11-12,16-19,22H,5-6,9-10,13-15H2,1-4H3,(H,32,38)(H,34,35,36)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacopeia Drug Discovery, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human CXCR2 receptor transfected in CHO cell |
Bioorg Med Chem Lett 16: 2724-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.028
BindingDB Entry DOI: 10.7270/Q2KK9BBS |
More data for this
Ligand-Target Pair | |
Atypical chemokine receptor 3
(Homo sapiens (Human)) | BDBM50380125
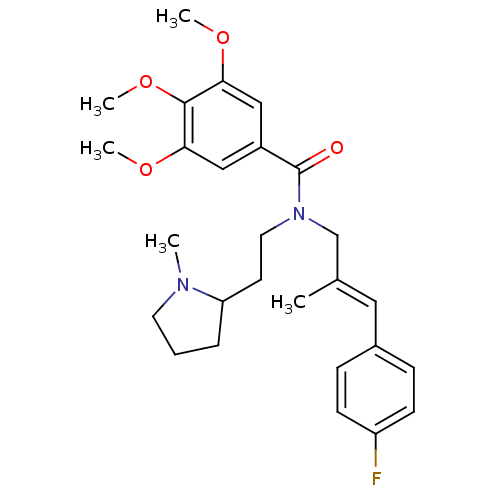
(CHEMBL2013224)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(CCC1CCCN1C)C\C(C)=C\c1ccc(F)cc1 Show InChI InChI=1S/C27H35FN2O4/c1-19(15-20-8-10-22(28)11-9-20)18-30(14-12-23-7-6-13-29(23)2)27(31)21-16-24(32-3)26(34-5)25(17-21)33-4/h8-11,15-17,23H,6-7,12-14,18H2,1-5H3/b19-15+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]-CXCL12 from human CXCR7 expressed in HEK293 cells after 3 hrs |
Eur J Med Chem 51: 184-92 (2012)
Article DOI: 10.1016/j.ejmech.2012.02.041
BindingDB Entry DOI: 10.7270/Q2PZ59SD |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 3
(Homo sapiens (Human)) | BDBM50399981

(CHEMBL2181450)Show SMILES CC1(C)[C@H]2C[C@@H]1C(C[N+](C)(C)Cc1ccc(cc1)-c1ccccc1I)=CC2 |r,c:27| Show InChI InChI=1S/C25H31IN/c1-25(2)21-14-13-20(23(25)15-21)17-27(3,4)16-18-9-11-19(12-10-18)22-7-5-6-8-24(22)26/h5-13,21,23H,14-17H2,1-4H3/q+1/t21-,23-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
VU University Amsterdam
Curated by ChEMBL
| Assay Description
Displacement of [125I]CXCL10 from CXCR3 expressed in HEK293 cells |
J Med Chem 55: 10572-83 (2012)
Article DOI: 10.1021/jm301240t
BindingDB Entry DOI: 10.7270/Q27P90J8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data