

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131871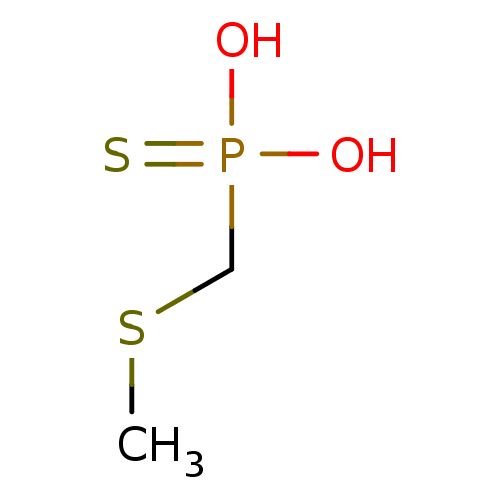 (CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131868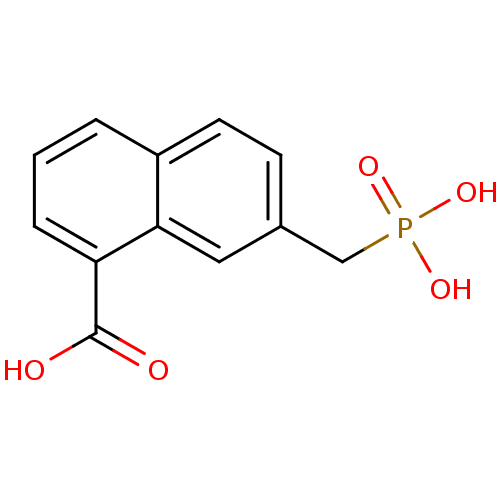 (7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131866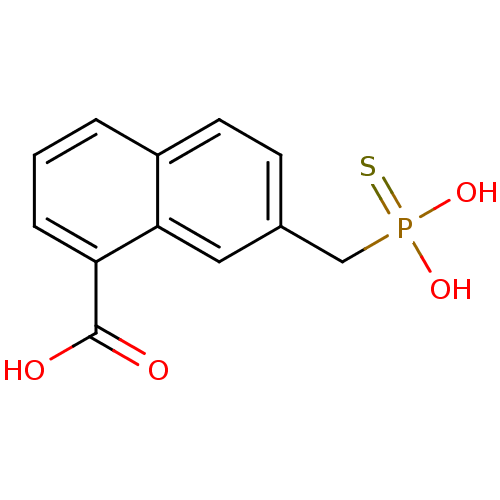 (7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131866 (7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131861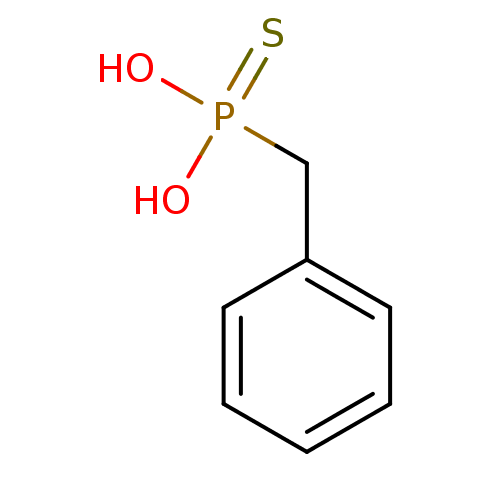 (Benzyl-phosphonothioic acid | CHEMBL123163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Rattus norvegicus) | BDBM50131868 (7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against E. coli Alkaline Phosphatase | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131865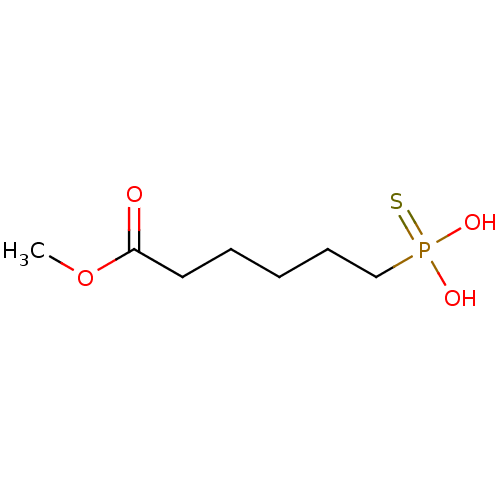 (6-Thiophosphono-hexanoic acid methyl ester | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131868 (7-Phosphonomethyl-naphthalene-1-carboxylic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131860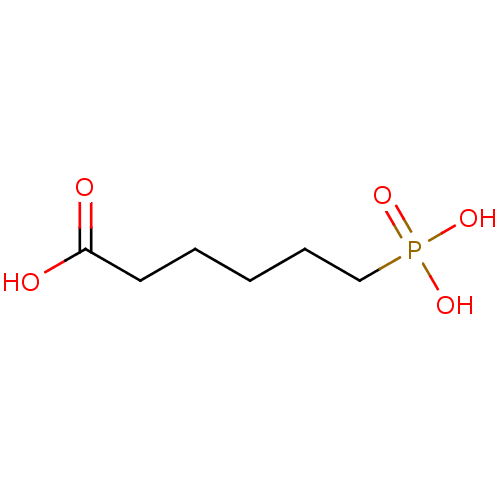 (6-Phosphono-hexanoic acid | CHEMBL122539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131863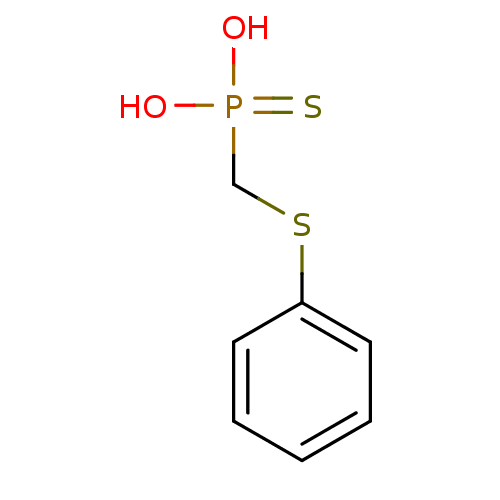 (CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131863 (CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131861 (Benzyl-phosphonothioic acid | CHEMBL123163) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50080274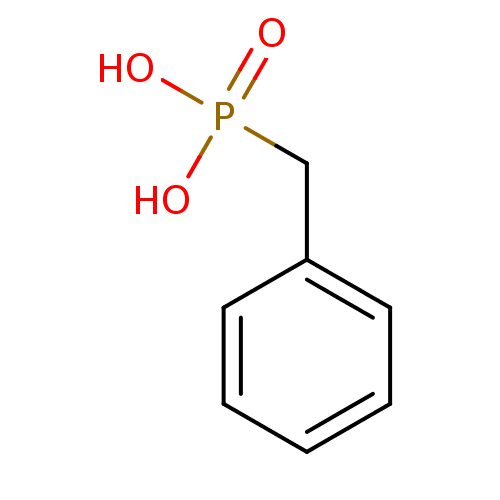 (Benzyl-phosphonic acid | CHEMBL299737 | Phenyl-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131870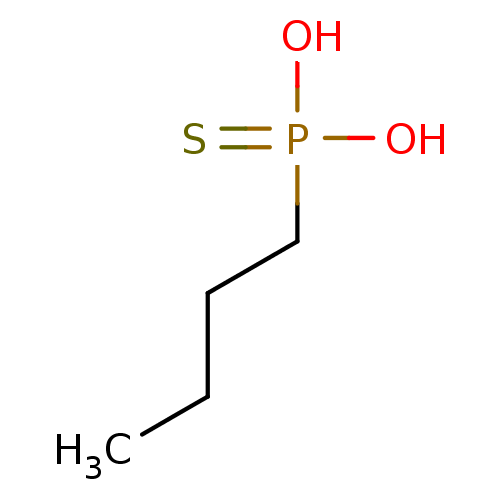 (Butyl-phosphonothioic acid | CHEMBL331046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, tissue-nonspecific isozyme (Rattus norvegicus) | BDBM50131864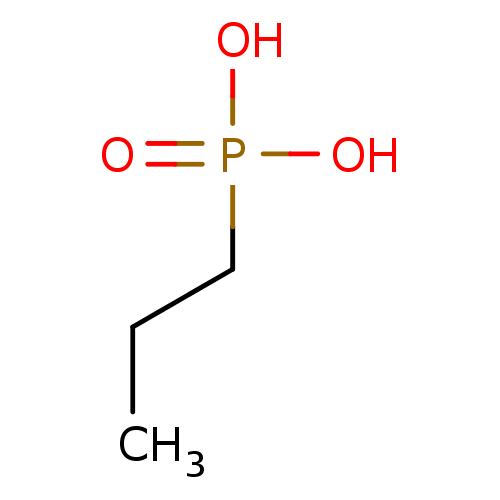 (CHEMBL125296 | Propyl-phosphonic acid) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 6.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against E. coli Alkaline Phosphatase | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131862 (CHEMBL122938 | methylphosphonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.01E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131865 (6-Thiophosphono-hexanoic acid methyl ester | CHEMB...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131871 (CHEMBL122097 | Methylsulfanylmethyl-phosphonothioi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.20E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131866 (7-Thiophosphonomethyl-naphthalene-1-carboxylic aci...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131867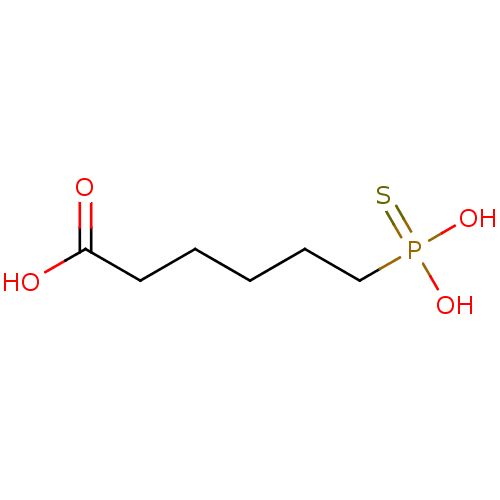 (6-Thiophosphono-hexanoic acid | CHEMBL123990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131867 (6-Thiophosphono-hexanoic acid | CHEMBL123990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131864 (CHEMBL125296 | Propyl-phosphonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.55E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131869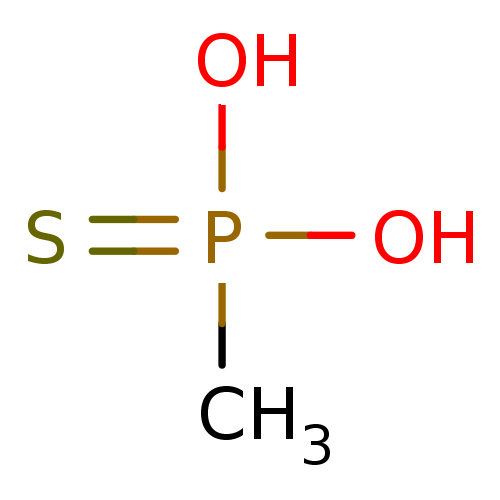 (CHEMBL122577 | Methyl-phosphonothioic acid) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131860 (6-Phosphono-hexanoic acid | CHEMBL122539) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 7.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131862 (CHEMBL122938 | methylphosphonic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131863 (CHEMBL331460 | Phenylsulfanylmethyl-phosphonothioi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.58E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131869 (CHEMBL122577 | Methyl-phosphonothioic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.70E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline phosphatase, germ cell type (Homo sapiens (Human)) | BDBM50131869 (CHEMBL122577 | Methyl-phosphonothioic acid) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibition of Human Placental alkaline Phosphatase (PLAP) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein phosphatase (Enterobacteria phage lambda) | BDBM50131870 (Butyl-phosphonothioic acid | CHEMBL331046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Serine/Threonine Protein Phosphatase (Lamda protein phosphatase) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131867 (6-Thiophosphono-hexanoic acid | CHEMBL123990) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131862 (CHEMBL122938 | methylphosphonic acid) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 3.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein tyrosine phosphatase receptor type C-associated protein (Homo sapiens (Human)) | BDBM50131860 (6-Phosphono-hexanoic acid | CHEMBL122539) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4.20E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Utah State University Curated by ChEMBL | Assay Description Inhibitory activity against Tyrosine phosphatase from Yersinia (Yop protein) | J Med Chem 46: 3703-8 (2003) Article DOI: 10.1021/jm030106f BindingDB Entry DOI: 10.7270/Q2MG7NXN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50161093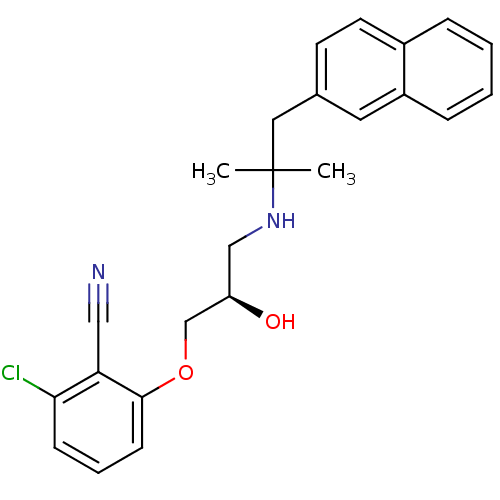 ((R)-2-chloro-6-(2-hydroxy-3-(2-methyl-1-(naphthale...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166511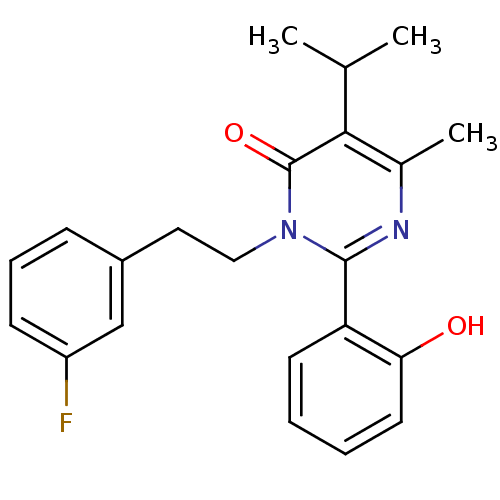 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166504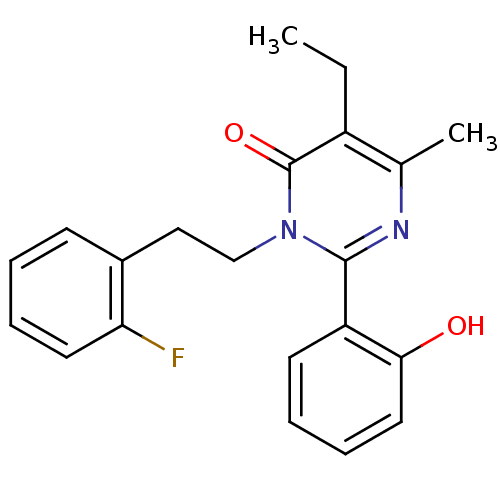 (5-Ethyl-3-[2-(2-fluoro-phenyl)-ethyl]-2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166510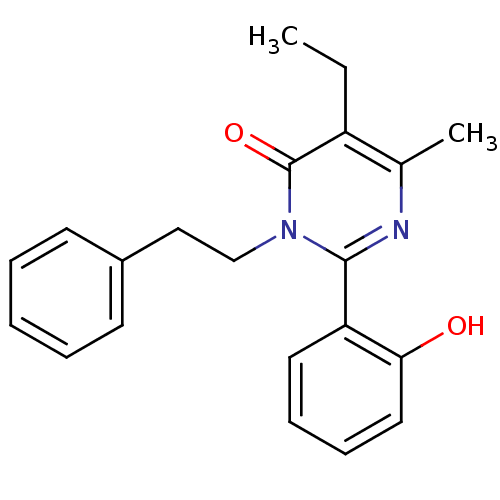 (5-Ethyl-2-(2-hydroxy-phenyl)-6-methyl-3-phenethyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166517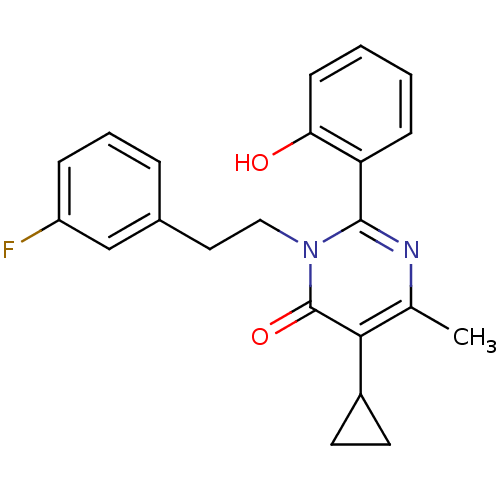 (5-Cyclopropyl-3-[2-(3-fluoro-phenyl)-ethyl]-2-(2-h...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166507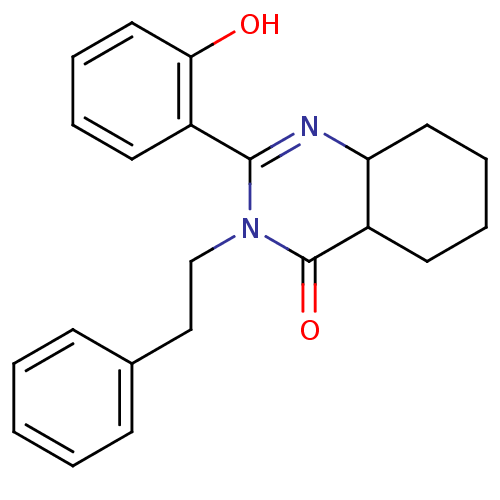 (2-(2-Hydroxy-phenyl)-3-phenethyl-4a,5,6,7,8,8a-hex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166503 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166505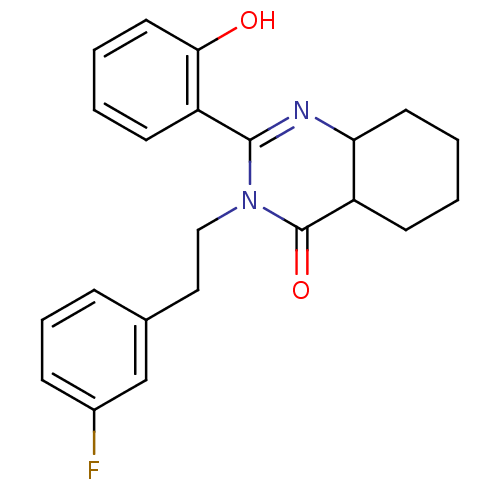 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166512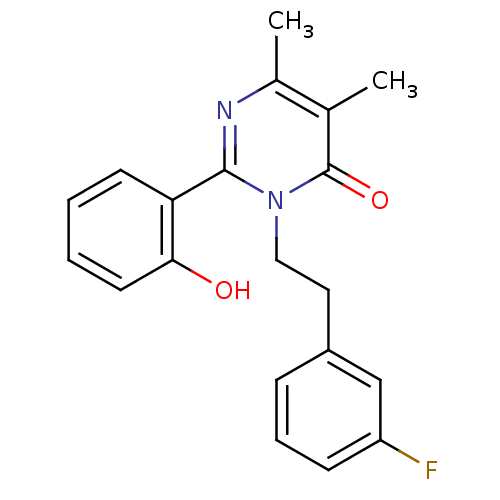 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166514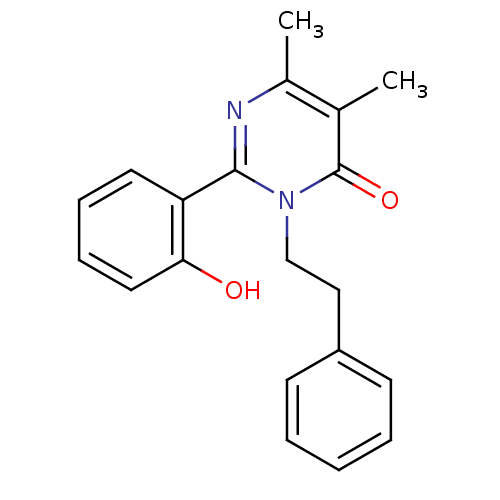 (2-(2-Hydroxy-phenyl)-5,6-dimethyl-3-phenethyl-3H-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166509 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166508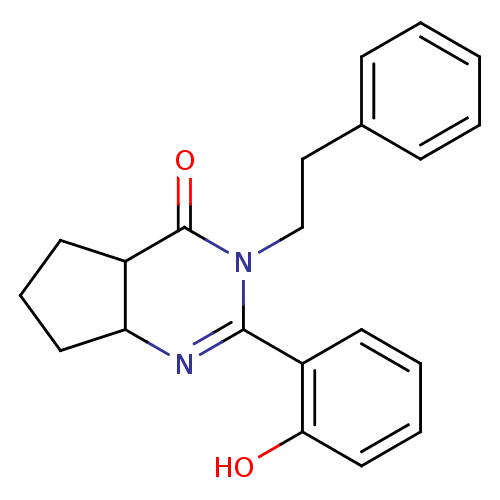 (2-(2-Hydroxy-phenyl)-3-phenethyl-3,4a,5,6,7,7a-hex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166516 (2-(2-Hydroxy-phenyl)-5-methyl-3-phenethyl-6-triflu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166506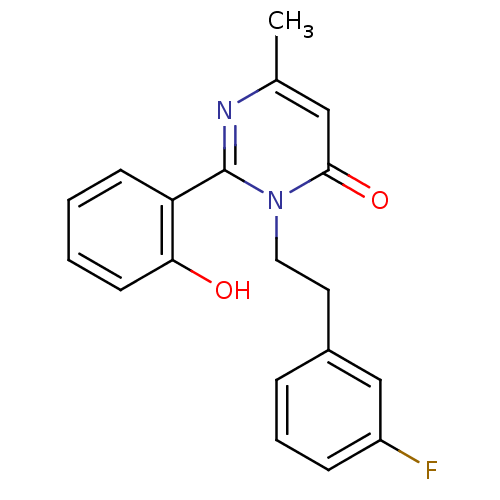 (3-[2-(3-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166515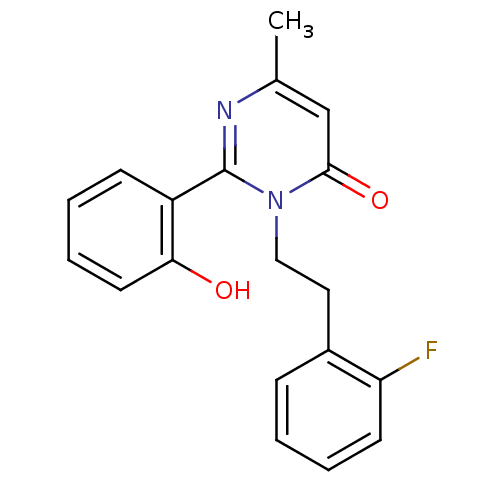 (3-[2-(2-Fluoro-phenyl)-ethyl]-2-(2-hydroxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50166513 (2-(2-Hydroxy-phenyl)-6-methyl-3-phenethyl-3H-pyrim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Extracellular calcium-sensing receptor (Homo sapiens (Human)) | BDBM50162542 (2-Furan-2-yl-3-phenethyl-3H-quinazolin-4-one | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibitory concentration was evaluated for in vitro calcilytic activity against human calcium receptor | Bioorg Med Chem Lett 15: 2537-40 (2005) Article DOI: 10.1016/j.bmcl.2005.03.054 BindingDB Entry DOI: 10.7270/Q26Q1WRQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||