 Found 561 hits with Last Name = 'van boeckel' and Initial = 'ca'
Found 561 hits with Last Name = 'van boeckel' and Initial = 'ca' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50118719
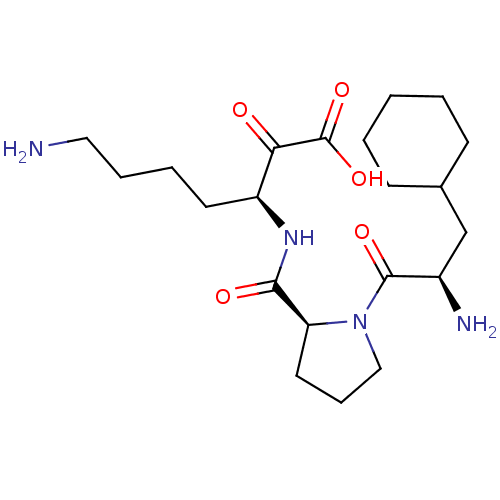
(7-Amino-3-{[1-(2-amino-3-cyclohexyl-propionyl)-pyr...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)C(O)=O Show InChI InChI=1S/C21H36N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h14-17H,1-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118730
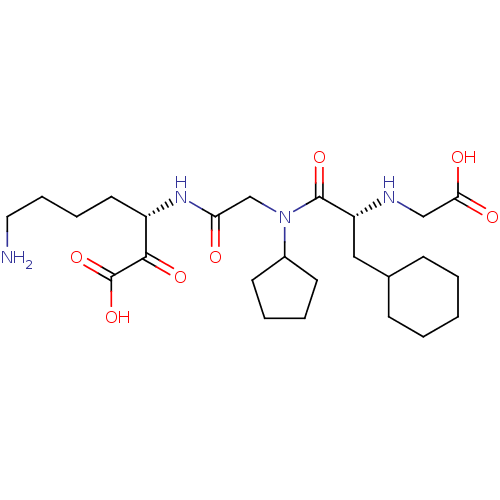
(7-Amino-3-(2-{[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CCCC1)C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C25H42N4O7/c26-13-7-6-12-19(23(33)25(35)36)28-21(30)16-29(18-10-4-5-11-18)24(34)20(27-15-22(31)32)14-17-8-2-1-3-9-17/h17-20,27H,1-16,26H2,(H,28,30)(H,31,32)(H,35,36)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118739
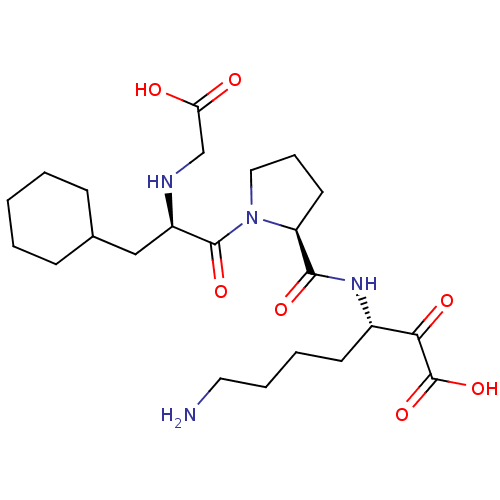
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C23H38N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h15-18,25H,1-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118728
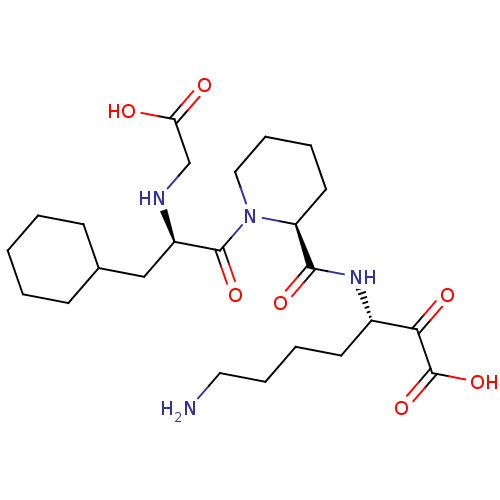
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C24H40N4O7/c25-12-6-4-10-17(21(31)24(34)35)27-22(32)19-11-5-7-13-28(19)23(33)18(26-15-20(29)30)14-16-8-2-1-3-9-16/h16-19,26H,1-15,25H2,(H,27,32)(H,29,30)(H,34,35)/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118732
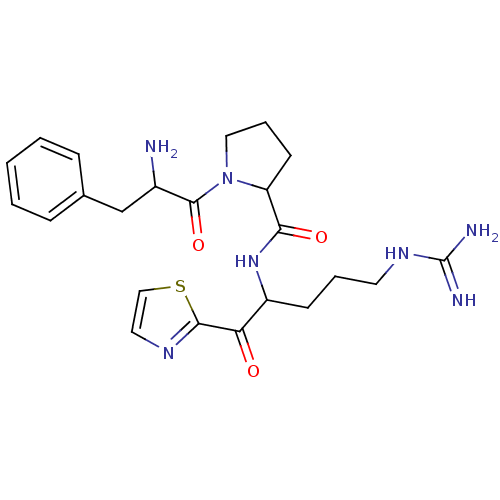
(1-(2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NC(Cc1ccccc1)C(=O)N1CCCC1C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118729
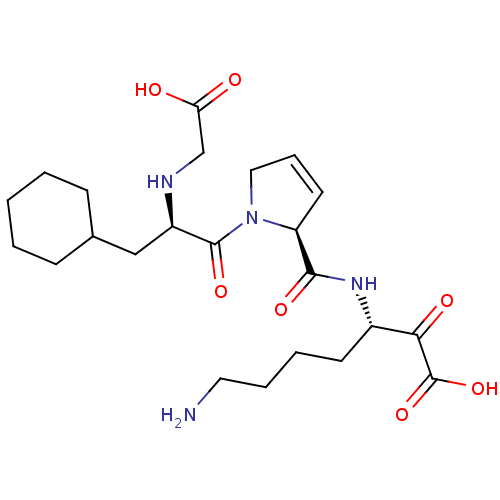
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1C=CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O |c:10| Show InChI InChI=1S/C23H36N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h6,10,15-18,25H,1-5,7-9,11-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118731
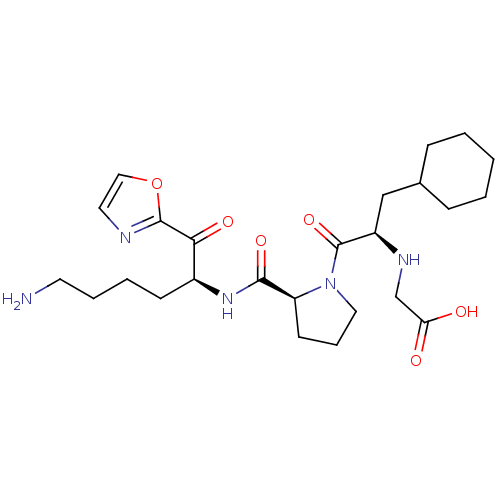
((2-{2-[5-Amino-1-(oxazole-2-carbonyl)-pentylcarbam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1ncco1 Show InChI InChI=1S/C25H39N5O6/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118735
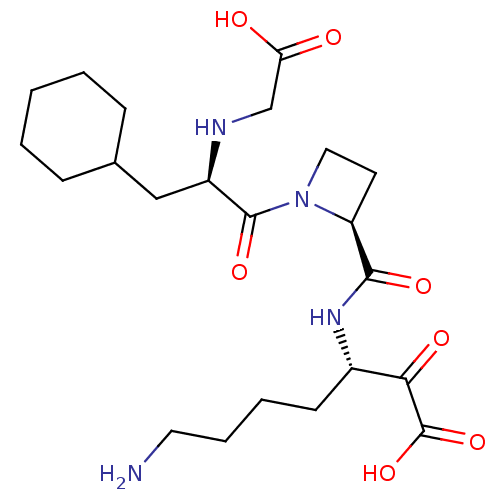
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C22H36N4O7/c23-10-5-4-8-15(19(29)22(32)33)25-20(30)17-9-11-26(17)21(31)16(24-13-18(27)28)12-14-6-2-1-3-7-14/h14-17,24H,1-13,23H2,(H,25,30)(H,27,28)(H,32,33)/t15-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118738
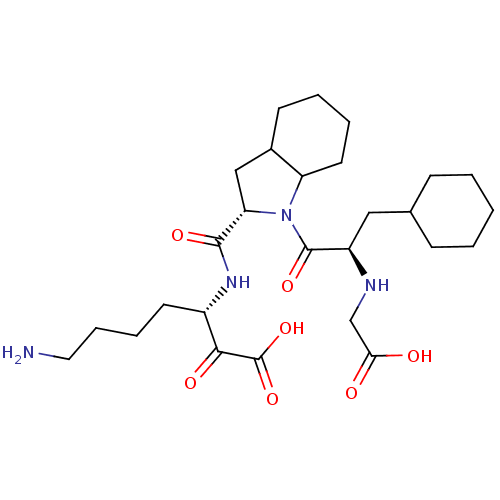
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C27H44N4O7/c28-13-7-6-11-19(24(34)27(37)38)30-25(35)22-15-18-10-4-5-12-21(18)31(22)26(36)20(29-16-23(32)33)14-17-8-2-1-3-9-17/h17-22,29H,1-16,28H2,(H,30,35)(H,32,33)(H,37,38)/t18?,19-,20+,21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118718
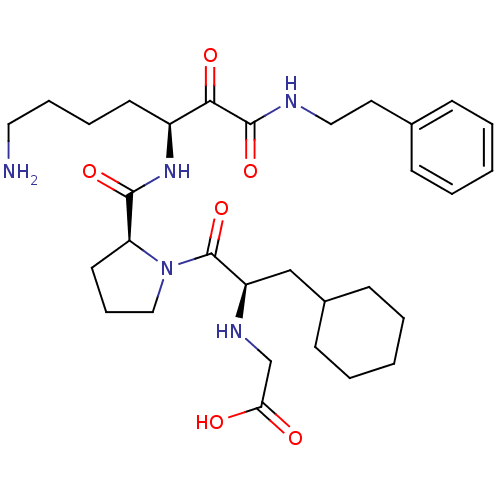
(CHEMBL343804 | {2-[2-(5-Amino-1-phenethylaminooxal...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H47N5O6/c32-17-8-7-14-24(28(39)30(41)33-18-16-22-10-3-1-4-11-22)35-29(40)26-15-9-19-36(26)31(42)25(34-21-27(37)38)20-23-12-5-2-6-13-23/h1,3-4,10-11,23-26,34H,2,5-9,12-21,32H2,(H,33,41)(H,35,40)(H,37,38)/t24-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118723
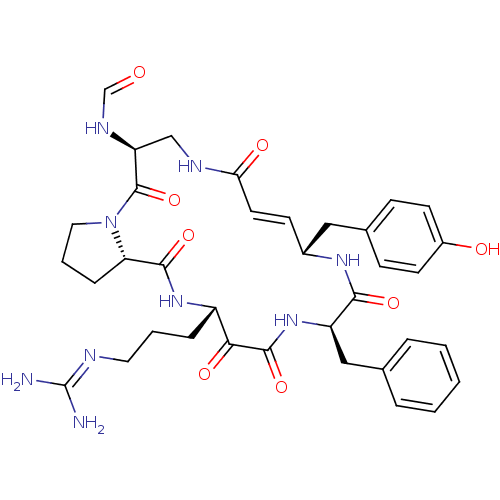
(CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-1=O)-[#7]-[#6]=O |r,t:24| Show InChI InChI=1S/C36H45N9O8/c37-36(38)39-16-4-8-26-31(49)34(52)44-27(19-22-6-2-1-3-7-22)32(50)42-24(18-23-10-13-25(47)14-11-23)12-15-30(48)40-20-28(41-21-46)35(53)45-17-5-9-29(45)33(51)43-26/h1-3,6-7,10-15,21,24,26-29,47H,4-5,8-9,16-20H2,(H,40,48)(H,41,46)(H,42,50)(H,43,51)(H,44,52)(H4,37,38,39)/b15-12+/t24-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118727
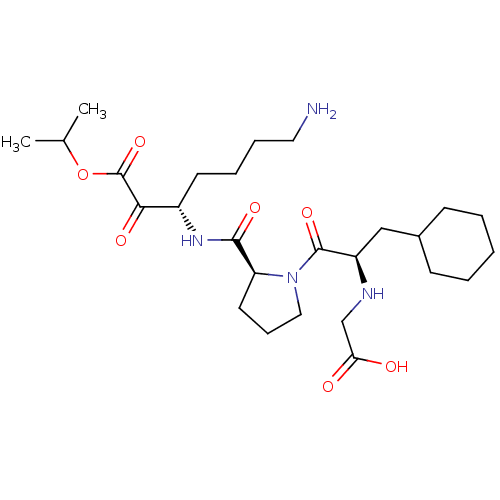
(2-((R)-1-((S)-2-(((S)-7-amino-1-isopropoxy-1,2-dio...)Show SMILES CC(C)OC(=O)C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C26H44N4O7/c1-17(2)37-26(36)23(33)19(11-6-7-13-27)29-24(34)21-12-8-14-30(21)25(35)20(28-16-22(31)32)15-18-9-4-3-5-10-18/h17-21,28H,3-16,27H2,1-2H3,(H,29,34)(H,31,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118720
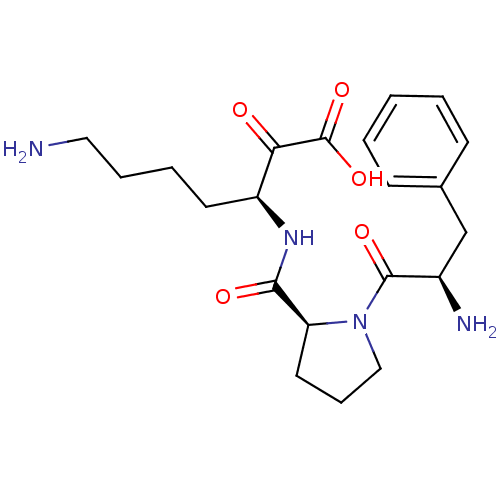
(7-Amino-3-{[1-(2-amino-3-phenyl-propionyl)-pyrroli...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C21H30N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h1-3,7-8,15-17H,4-6,9-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118717
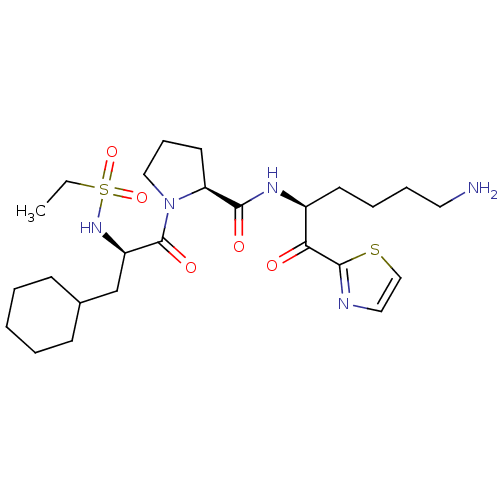
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES CCS(=O)(=O)N[C@H](CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)c1nccs1 Show InChI InChI=1S/C25H41N5O5S2/c1-2-37(34,35)29-20(17-18-9-4-3-5-10-18)25(33)30-15-8-12-21(30)23(32)28-19(11-6-7-13-26)22(31)24-27-14-16-36-24/h14,16,18-21,29H,2-13,15,17,26H2,1H3,(H,28,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29388
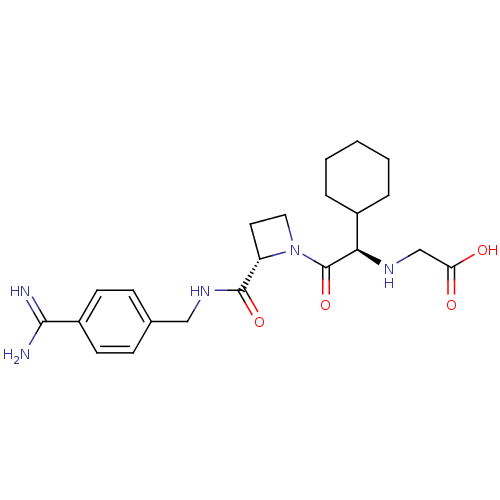
(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50118737
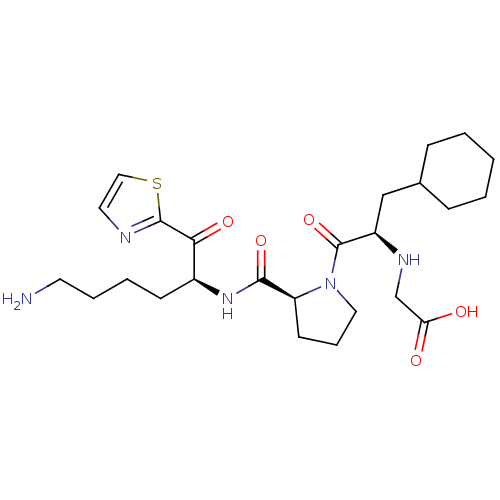
((2-{2-[5-Amino-1-(thiazole-2-carbonyl)-pentylcarba...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1nccs1 Show InChI InChI=1S/C25H39N5O5S/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118721
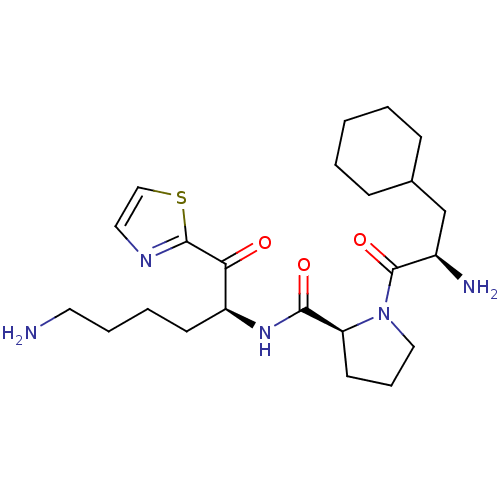
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h12,14,16-19H,1-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118722

(2-Amino-N-{[5-amino-1-(thiazole-2-carbonyl)-pentyl...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-8-19(21(30)22-26-12-13-32-22)27-20(29)15-28(17-9-10-17)23(31)18(25)14-16-6-2-1-3-7-16/h12-13,16-19H,1-11,14-15,24-25H2,(H,27,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118725
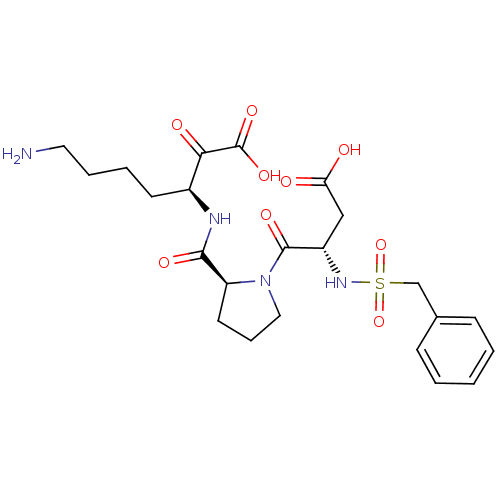
(7-Amino-3-{[1-(3-carboxy-2-phenylmethanesulfonylam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NS(=O)(=O)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C23H32N4O9S/c24-11-5-4-9-16(20(30)23(33)34)25-21(31)18-10-6-12-27(18)22(32)17(13-19(28)29)26-37(35,36)14-15-7-2-1-3-8-15/h1-3,7-8,16-18,26H,4-6,9-14,24H2,(H,25,31)(H,28,29)(H,33,34)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50038001
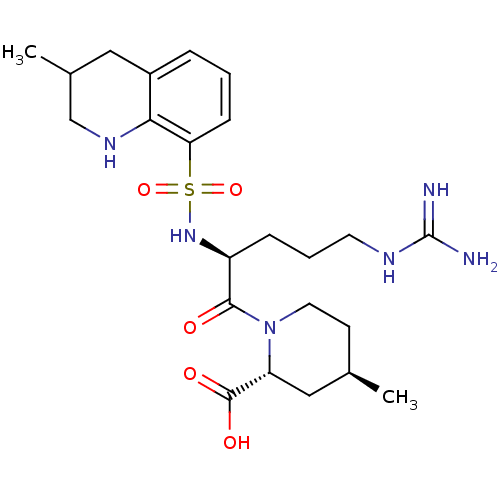
((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118734
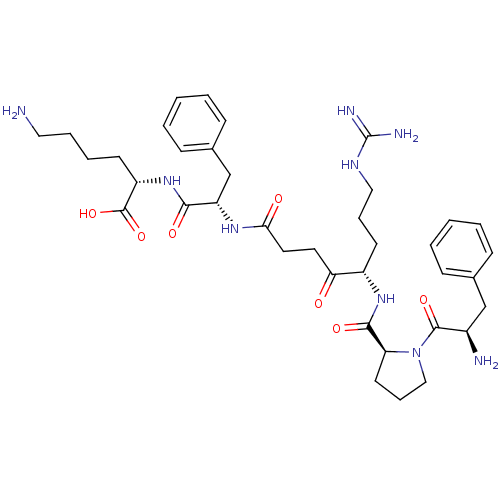
(6-Amino-2-[2-(5-{[1-(2-amino-3-phenyl-propionyl)-p...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H55N9O7/c39-20-8-7-15-29(37(53)54)46-34(50)30(24-26-13-5-2-6-14-26)44-33(49)19-18-32(48)28(16-9-21-43-38(41)42)45-35(51)31-17-10-22-47(31)36(52)27(40)23-25-11-3-1-4-12-25/h1-6,11-14,27-31H,7-10,15-24,39-40H2,(H,44,49)(H,45,51)(H,46,50)(H,53,54)(H4,41,42,43)/t27-,28+,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118724
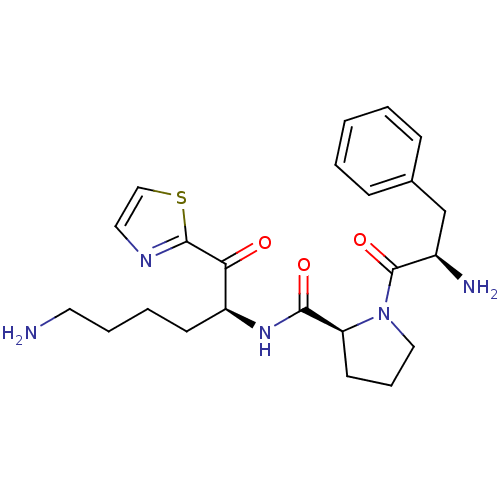
((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h1-3,7-8,12,14,17-19H,4-6,9-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118716

((S)-1-(3,3-diphenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CC(c1ccccc1)c1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C29H34N4O3S/c30-16-8-7-14-24(27(35)29-31-17-19-37-29)32-28(36)25-15-9-18-33(25)26(34)20-23(21-10-3-1-4-11-21)22-12-5-2-6-13-22/h1-6,10-13,17,19,23-25H,7-9,14-16,18,20,30H2,(H,32,36)/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118715
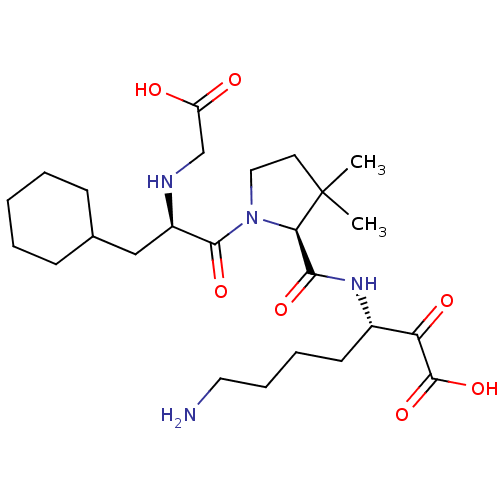
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES CC1(C)CCN([C@@H]1C(=O)N[C@@H](CCCCN)C(=O)C(O)=O)C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C25H42N4O7/c1-25(2)11-13-29(21(25)22(33)28-17(10-6-7-12-26)20(32)24(35)36)23(34)18(27-15-19(30)31)14-16-8-4-3-5-9-16/h16-18,21,27H,3-15,26H2,1-2H3,(H,28,33)(H,30,31)(H,35,36)/t17-,18+,21+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 376 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118714
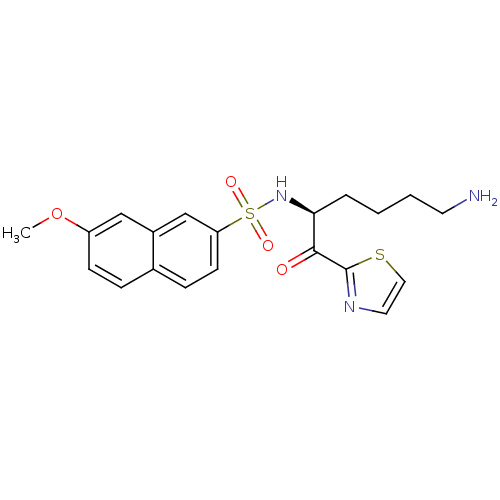
(7-Methoxy-naphthalene-2-sulfonic acid [5-amino-1-(...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H](CCCCN)C(=O)c1nccs1 Show InChI InChI=1S/C20H23N3O4S2/c1-27-16-7-5-14-6-8-17(13-15(14)12-16)29(25,26)23-18(4-2-3-9-21)19(24)20-22-10-11-28-20/h5-8,10-13,18,23H,2-4,9,21H2,1H3/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118733
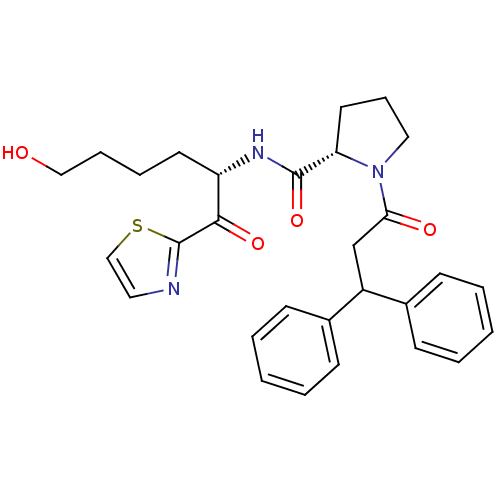
(1-(3,3-Diphenyl-propionyl)-pyrrolidine-2-carboxyli...)Show SMILES OCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)CC(c1ccccc1)c1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C29H33N3O4S/c33-18-8-7-14-24(27(35)29-30-16-19-37-29)31-28(36)25-15-9-17-32(25)26(34)20-23(21-10-3-1-4-11-21)22-12-5-2-6-13-22/h1-6,10-13,16,19,23-25,33H,7-9,14-15,17-18,20H2,(H,31,36)/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118726
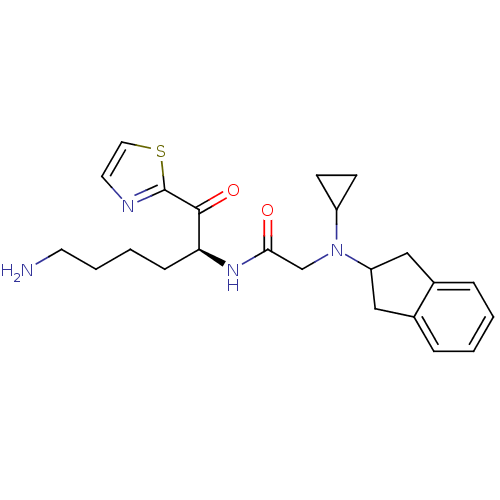
(CHEMBL334701 | N-[5-Amino-1-(thiazole-2-carbonyl)-...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C1Cc2ccccc2C1)C(=O)c1nccs1 Show InChI InChI=1S/C23H30N4O2S/c24-10-4-3-7-20(22(29)23-25-11-12-30-23)26-21(28)15-27(18-8-9-18)19-13-16-5-1-2-6-17(16)14-19/h1-2,5-6,11-12,18-20H,3-4,7-10,13-15,24H2,(H,26,28)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118736
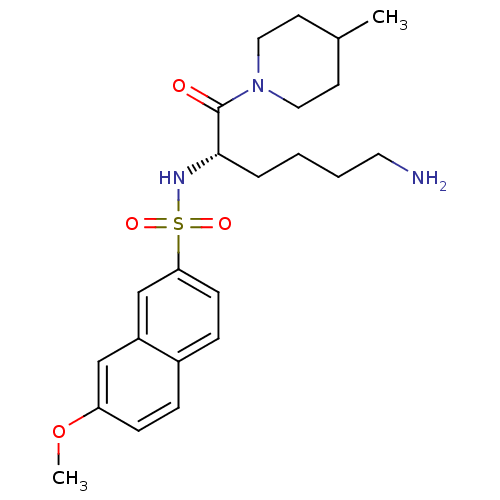
(7-Methoxy-naphthalene-2-sulfonic acid [5-amino-1-(...)Show SMILES COc1ccc2ccc(cc2c1)S(=O)(=O)N[C@@H](CCCCN)C(=O)N1CCC(C)CC1 Show InChI InChI=1S/C23H33N3O4S/c1-17-10-13-26(14-11-17)23(27)22(5-3-4-12-24)25-31(28,29)21-9-7-18-6-8-20(30-2)15-19(18)16-21/h6-9,15-17,22,25H,3-5,10-14,24H2,1-2H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028179
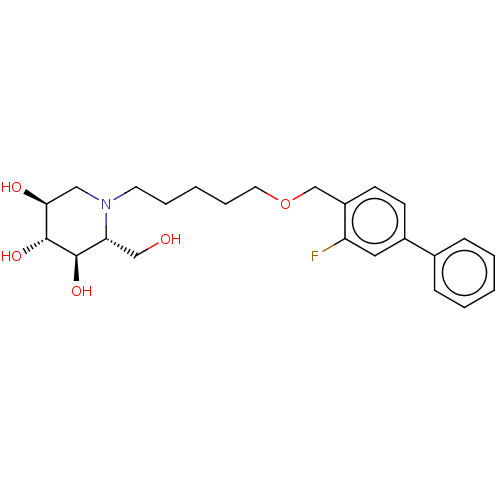
(CHEMBL3354624)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1F)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22+,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028244
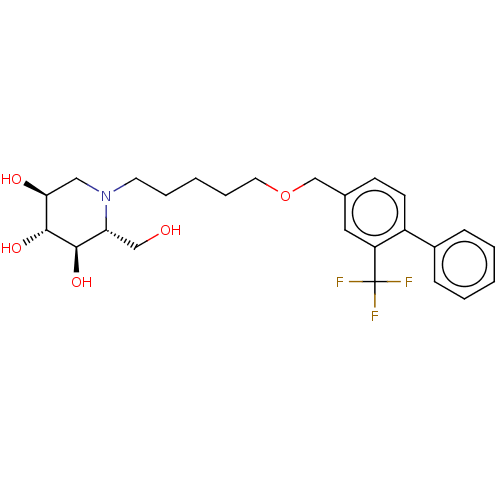
(CHEMBL3354627)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22+,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028180

(CHEMBL3354625)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(c(F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22+,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028255
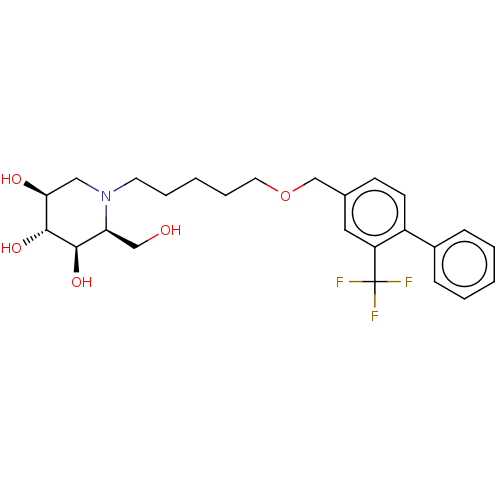
(CHEMBL3354635)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(-c2ccccc2)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028254
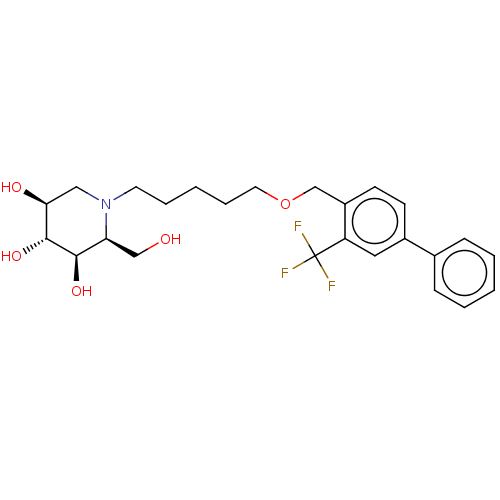
(CHEMBL3354634)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028181
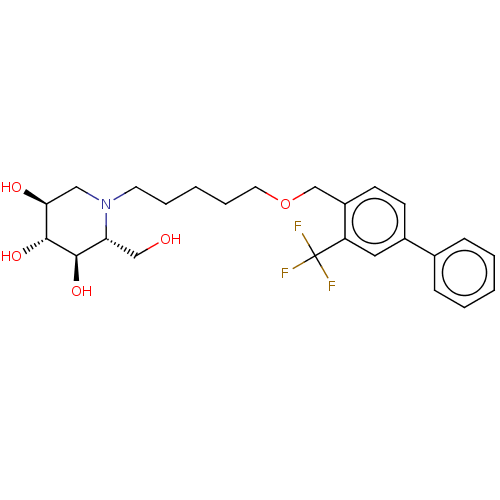
(CHEMBL3354626)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C25H32F3NO5/c26-25(27,28)20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-34-12-6-2-5-11-29-14-22(31)24(33)23(32)21(29)15-30/h1,3-4,7-10,13,21-24,30-33H,2,5-6,11-12,14-16H2/t21-,22+,23-,24-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530205
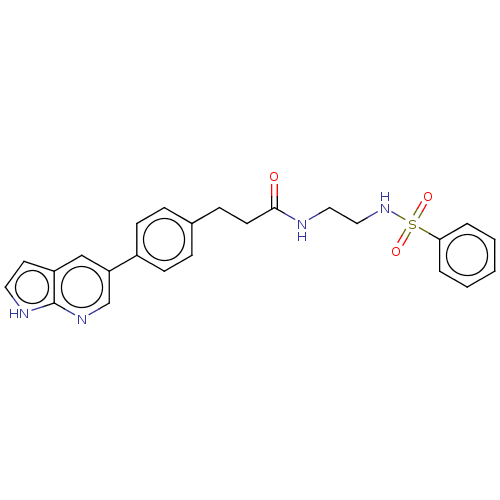
(CHEMBL4561847)Show SMILES O=C(CCc1ccc(cc1)-c1cnc2[nH]ccc2c1)NCCNS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H24N4O3S/c29-23(25-14-15-28-32(30,31)22-4-2-1-3-5-22)11-8-18-6-9-19(10-7-18)21-16-20-12-13-26-24(20)27-17-21/h1-7,9-10,12-13,16-17,28H,8,11,14-15H2,(H,25,29)(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530205

(CHEMBL4561847)Show SMILES O=C(CCc1ccc(cc1)-c1cnc2[nH]ccc2c1)NCCNS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C24H24N4O3S/c29-23(25-14-15-28-32(30,31)22-4-2-1-3-5-22)11-8-18-6-9-19(10-7-18)21-16-20-12-13-26-24(20)27-17-21/h1-7,9-10,12-13,16-17,28H,8,11,14-15H2,(H,25,29)(H,26,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028256

(CHEMBL3354636)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(cc1F)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-18(17-7-3-1-4-8-17)9-10-19(20)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530172
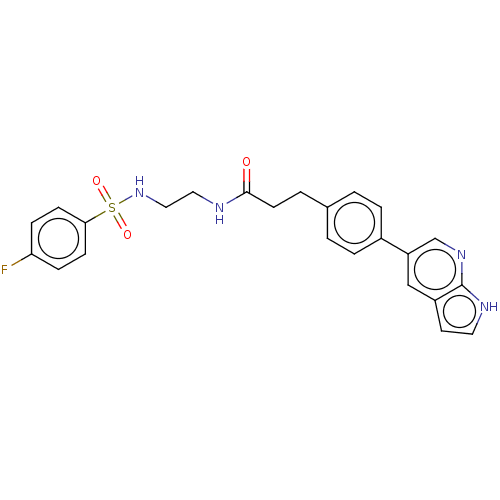
(CHEMBL4547323)Show SMILES Fc1ccc(cc1)S(=O)(=O)NCCNC(=O)CCc1ccc(cc1)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C24H23FN4O3S/c25-21-6-8-22(9-7-21)33(31,32)29-14-13-26-23(30)10-3-17-1-4-18(5-2-17)20-15-19-11-12-27-24(19)28-16-20/h1-2,4-9,11-12,15-16,29H,3,10,13-14H2,(H,26,30)(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530172

(CHEMBL4547323)Show SMILES Fc1ccc(cc1)S(=O)(=O)NCCNC(=O)CCc1ccc(cc1)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C24H23FN4O3S/c25-21-6-8-22(9-7-21)33(31,32)29-14-13-26-23(30)10-3-17-1-4-18(5-2-17)20-15-19-11-12-27-24(19)28-16-20/h1-2,4-9,11-12,15-16,29H,3,10,13-14H2,(H,26,30)(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530204

(CHEMBL4483002)Show SMILES Clc1ccccc1S(=O)(=O)NCCNC(=O)CCc1ccc(cc1)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C24H23ClN4O3S/c25-21-3-1-2-4-22(21)33(31,32)29-14-13-26-23(30)10-7-17-5-8-18(9-6-17)20-15-19-11-12-27-24(19)28-16-20/h1-6,8-9,11-12,15-16,29H,7,10,13-14H2,(H,26,30)(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50530204

(CHEMBL4483002)Show SMILES Clc1ccccc1S(=O)(=O)NCCNC(=O)CCc1ccc(cc1)-c1cnc2[nH]ccc2c1 Show InChI InChI=1S/C24H23ClN4O3S/c25-21-3-1-2-4-22(21)33(31,32)29-14-13-26-23(30)10-7-17-5-8-18(9-6-17)20-15-19-11-12-27-24(19)28-16-20/h1-6,8-9,11-12,15-16,29H,7,10,13-14H2,(H,26,30)(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.479 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST/His6-fused FLT3 ITD mutant (R571 to S993 residues) expressed in Sf9 insect cells assessed as residual ... |
Bioorg Med Chem 27: 692-699 (2019)
Article DOI: 10.1016/j.bmc.2019.01.006
BindingDB Entry DOI: 10.7270/Q2Z89GWX |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028221
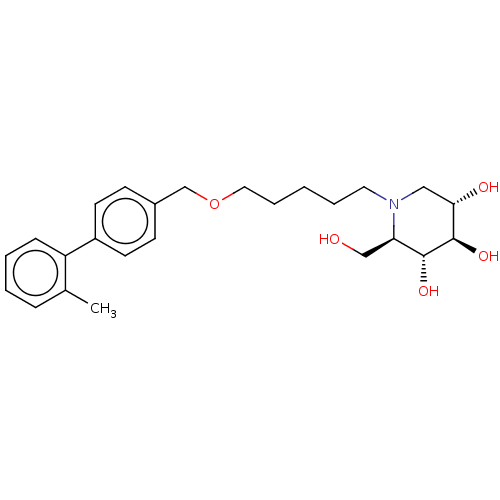
(CHEMBL3354041)Show SMILES Cc1ccccc1-c1ccc(COCCCCCN2C[C@H](O)[C@@H](O)[C@H](O)[C@H]2CO)cc1 |r| Show InChI InChI=1S/C25H35NO5/c1-18-7-3-4-8-21(18)20-11-9-19(10-12-20)17-31-14-6-2-5-13-26-15-23(28)25(30)24(29)22(26)16-27/h3-4,7-12,22-25,27-30H,2,5-6,13-17H2,1H3/t22-,23+,24-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028257
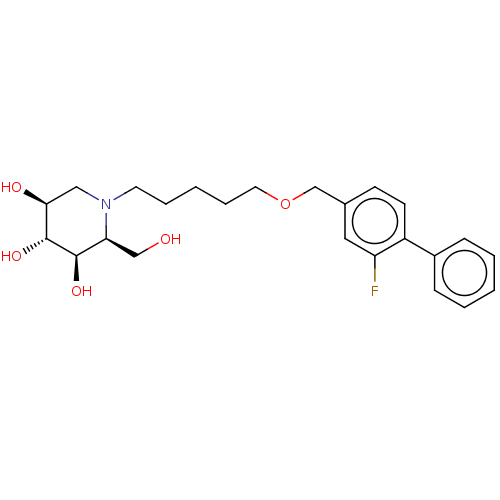
(CHEMBL3354637)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc(c(F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C24H32FNO5/c25-20-13-17(9-10-19(20)18-7-3-1-4-8-18)16-31-12-6-2-5-11-26-14-22(28)24(30)23(29)21(26)15-27/h1,3-4,7-10,13,21-24,27-30H,2,5-6,11-12,14-16H2/t21-,22-,23+,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312527
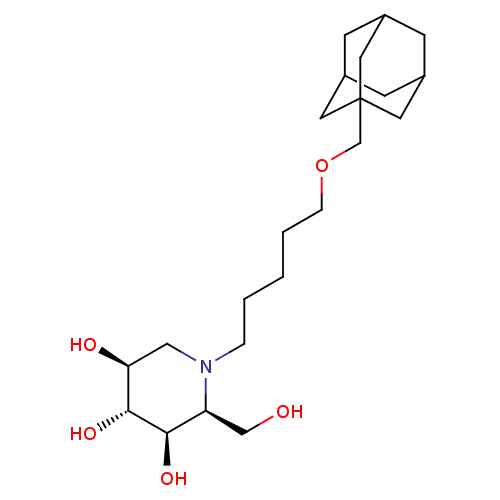
(CHEMBL1086996 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19-,20+,21+,22?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028176

(CHEMBL3354621)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCC(F)(F)COCc1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C24H31F2NO5/c25-24(26,11-4-12-27-13-21(29)23(31)22(30)20(27)14-28)16-32-15-17-7-9-19(10-8-17)18-5-2-1-3-6-18/h1-3,5-10,20-23,28-31H,4,11-16H2/t20-,21+,22-,23-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50312529
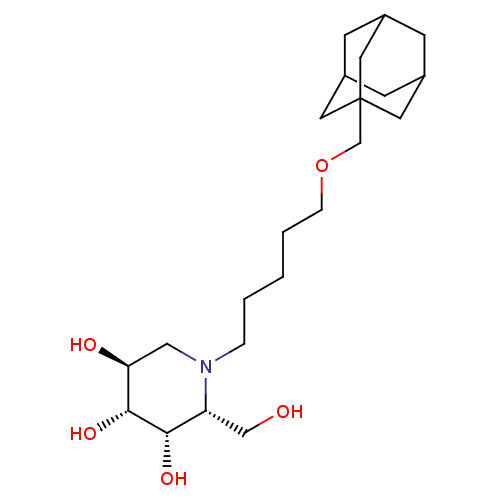
(CHEMBL1088158 | N-[5-(Adamantan-1-yl-methoxy)-pent...)Show SMILES OC[C@@H]1[C@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCC12CC3CC(CC(C3)C1)C2 |r,TLB:21:22:20.25.19:26,THB:21:20:22.23.27:26,23:22:19:25.24.26,23:24:21.22.27:19| Show InChI InChI=1S/C22H39NO5/c24-13-18-20(26)21(27)19(25)12-23(18)4-2-1-3-5-28-14-22-9-15-6-16(10-22)8-17(7-15)11-22/h15-21,24-27H,1-14H2/t15?,16?,17?,18-,19+,20+,21-,22?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028249

(CHEMBL3354629)Show SMILES C[C@@H](OCCCCCN1C[C@H](O)[C@@H](O)[C@H](O)[C@@H]1CO)c1ccc(cc1)-c1ccccc1 |r| Show InChI InChI=1S/C25H35NO5/c1-18(19-10-12-21(13-11-19)20-8-4-2-5-9-20)31-15-7-3-6-14-26-16-23(28)25(30)24(29)22(26)17-27/h2,4-5,8-13,18,22-25,27-30H,3,6-7,14-17H2,1H3/t18-,22+,23+,24-,25-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
(Homo sapiens (Human)) | BDBM50028217
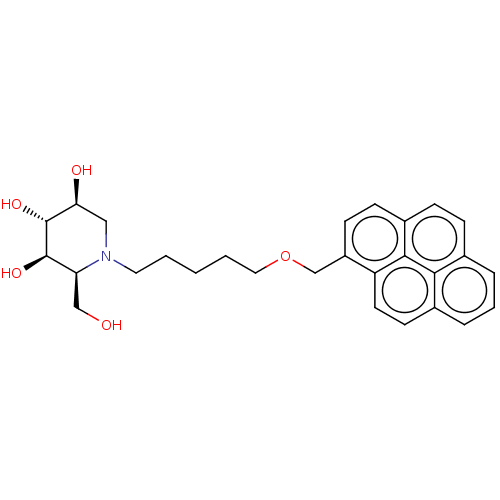
(CHEMBL3354037)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccc3cccc4ccc1c2c34 |r| Show InChI InChI=1S/C28H33NO5/c30-16-23-27(32)28(33)24(31)15-29(23)13-2-1-3-14-34-17-21-10-9-20-8-7-18-5-4-6-19-11-12-22(21)26(20)25(18)19/h4-12,23-24,27-28,30-33H,1-3,13-17H2/t23-,24-,27+,28+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
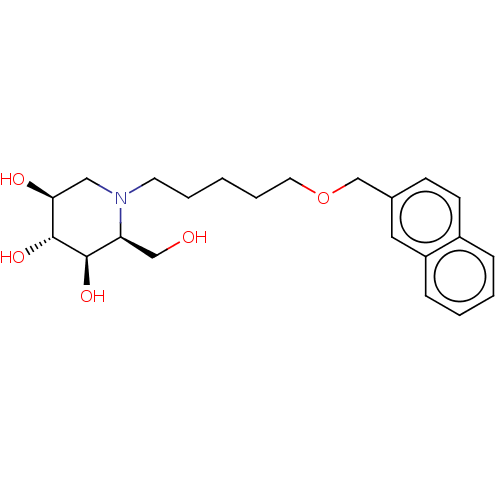
(Homo sapiens (Human)) | BDBM50028215
(CHEMBL3354035)Show SMILES OC[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H31NO5/c24-14-19-21(26)22(27)20(25)13-23(19)10-4-1-5-11-28-15-16-8-9-17-6-2-3-7-18(17)12-16/h2-3,6-9,12,19-22,24-27H,1,4-5,10-11,13-15H2/t19-,20-,21+,22+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |
Non-lysosomal glucosylceramidase
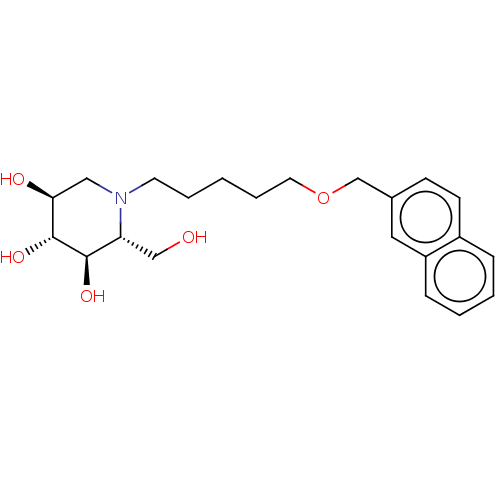
(Homo sapiens (Human)) | BDBM50028204
(CHEMBL3354022)Show SMILES OC[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1CCCCCOCc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C22H31NO5/c24-14-19-21(26)22(27)20(25)13-23(19)10-4-1-5-11-28-15-16-8-9-17-6-2-3-7-18(17)12-16/h2-3,6-9,12,19-22,24-27H,1,4-5,10-11,13-15H2/t19-,20+,21-,22-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Inhibition of GBA2 (unknown origin) |
J Med Chem 57: 9096-104 (2014)
Article DOI: 10.1021/jm501181z
BindingDB Entry DOI: 10.7270/Q20003PT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data