 Found 290 hits with Last Name = 'giddings' and Initial = 'a'
Found 290 hits with Last Name = 'giddings' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50307292
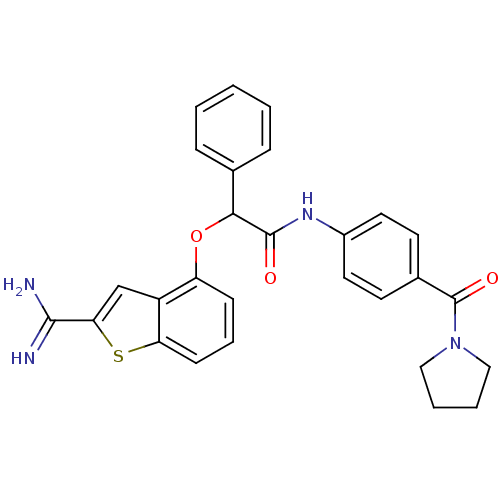
(2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)-2-phe...)Show SMILES NC(=N)c1cc2c(OC(C(=O)Nc3ccc(cc3)C(=O)N3CCCC3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C28H26N4O3S/c29-26(30)24-17-21-22(9-6-10-23(21)36-24)35-25(18-7-2-1-3-8-18)27(33)31-20-13-11-19(12-14-20)28(34)32-15-4-5-16-32/h1-3,6-14,17,25H,4-5,15-16H2,(H3,29,30)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50307291
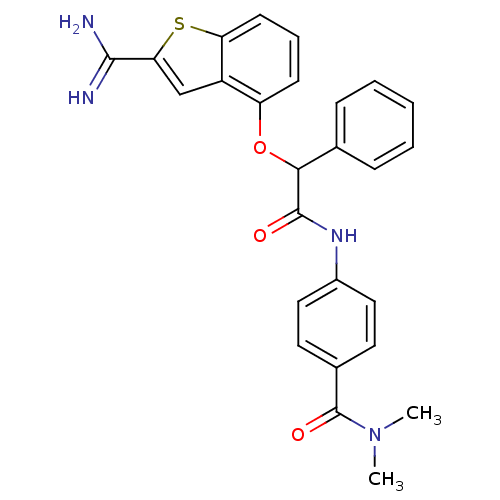
(4-[2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)-2-...)Show SMILES CN(C)C(=O)c1ccc(NC(=O)C(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 Show InChI InChI=1S/C26H24N4O3S/c1-30(2)26(32)17-11-13-18(14-12-17)29-25(31)23(16-7-4-3-5-8-16)33-20-9-6-10-21-19(20)15-22(34-21)24(27)28/h3-15,23H,1-2H3,(H3,27,28)(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050
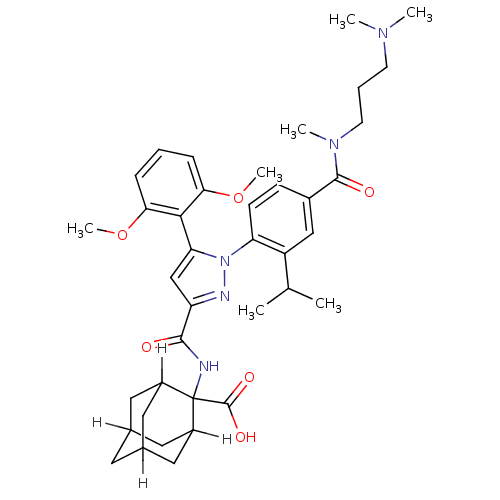
(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS1 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307314
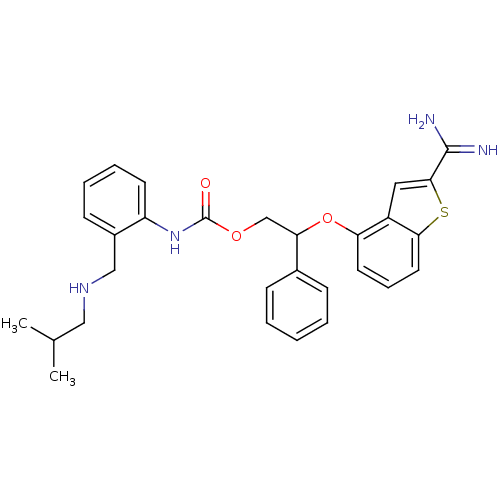
(CHEMBL597557 | [2-Isobutylamino-methyl)-phenyl]-ca...)Show SMILES CC(C)CNCc1ccccc1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C29H32N4O3S/c1-19(2)16-32-17-21-11-6-7-12-23(21)33-29(34)35-18-25(20-9-4-3-5-10-20)36-24-13-8-14-26-22(24)15-27(37-26)28(30)31/h3-15,19,25,32H,16-18H2,1-2H3,(H3,30,31)(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50307290
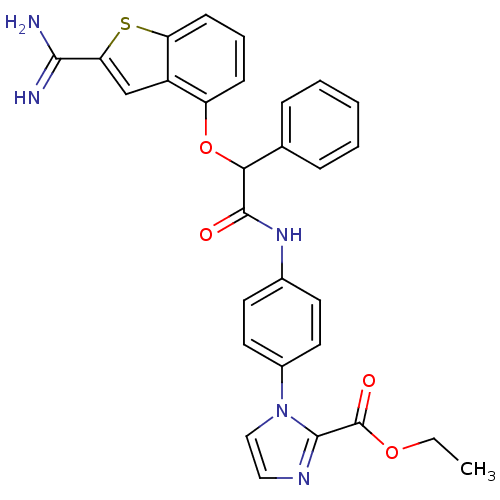
(1-{4-[2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)...)Show SMILES CCOC(=O)c1nccn1-c1ccc(NC(=O)C(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 Show InChI InChI=1S/C29H25N5O4S/c1-2-37-29(36)27-32-15-16-34(27)20-13-11-19(12-14-20)33-28(35)25(18-7-4-3-5-8-18)38-22-9-6-10-23-21(22)17-24(39-23)26(30)31/h3-17,25H,2H2,1H3,(H3,30,31)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307267
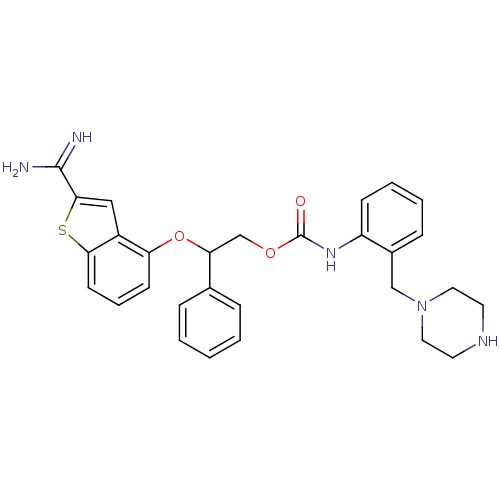
(CHEMBL597759 | [2-(Piperazin-1-ylmethyl)-phenyl]-c...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccccc3CN3CCNCC3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C29H31N5O3S/c30-28(31)27-17-22-24(11-6-12-26(22)38-27)37-25(20-7-2-1-3-8-20)19-36-29(35)33-23-10-5-4-9-21(23)18-34-15-13-32-14-16-34/h1-12,17,25,32H,13-16,18-19H2,(H3,30,31)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307308
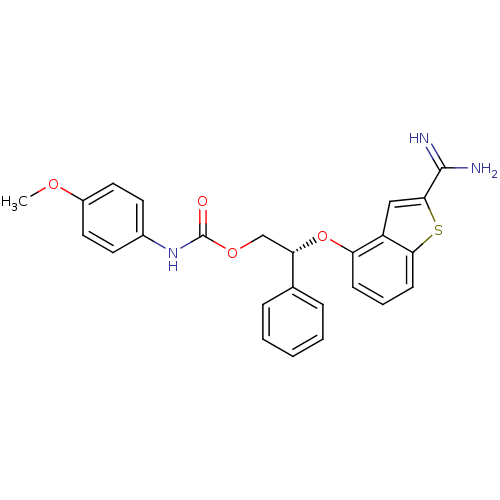
((R)-(4-Methoxy-phenyl)-carbamic acid 2-(2-carbamim...)Show SMILES COc1ccc(NC(=O)OC[C@H](Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H23N3O4S/c1-30-18-12-10-17(11-13-18)28-25(29)31-15-21(16-6-3-2-4-7-16)32-20-8-5-9-22-19(20)14-23(33-22)24(26)27/h2-14,21H,15H2,1H3,(H3,26,27)(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307313
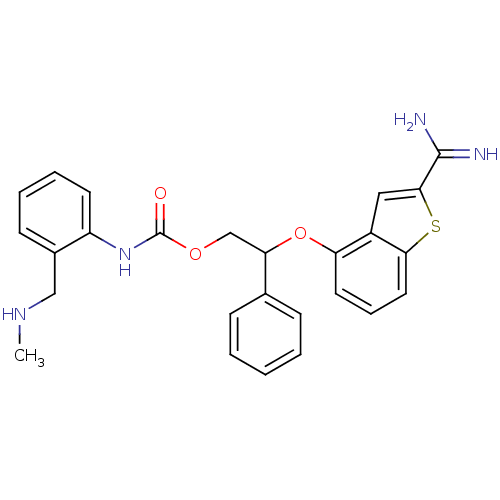
((2-Methylamino-methyl-phenyl)-carbamic acid 2-(2-c...)Show SMILES CNCc1ccccc1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C26H26N4O3S/c1-29-15-18-10-5-6-11-20(18)30-26(31)32-16-22(17-8-3-2-4-9-17)33-21-12-7-13-23-19(21)14-24(34-23)25(27)28/h2-14,22,29H,15-16H2,1H3,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307277
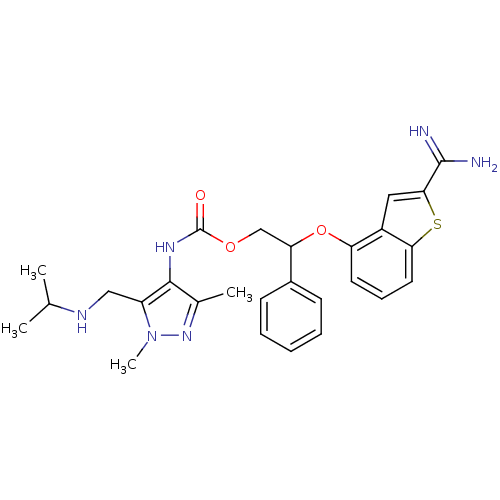
(CHEMBL604954 | [5-(Isopropylamino-methyl)-1,3-dime...)Show SMILES CC(C)NCc1c(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)c(C)nn1C Show InChI InChI=1S/C27H32N6O3S/c1-16(2)30-14-20-25(17(3)32-33(20)4)31-27(34)35-15-22(18-9-6-5-7-10-18)36-21-11-8-12-23-19(21)13-24(37-23)26(28)29/h5-13,16,22,30H,14-15H2,1-4H3,(H3,28,29)(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307312
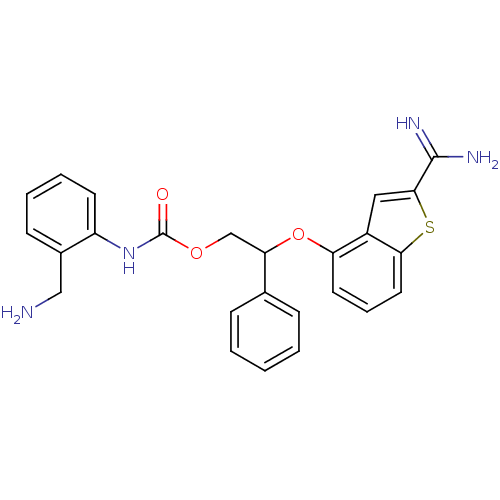
((2-Aminomethyl-phenyl)-carbamic acid 2-(2-carbamim...)Show SMILES NCc1ccccc1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C25H24N4O3S/c26-14-17-9-4-5-10-19(17)29-25(30)31-15-21(16-7-2-1-3-8-16)32-20-11-6-12-22-18(20)13-23(33-22)24(27)28/h1-13,21H,14-15,26H2,(H3,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307310
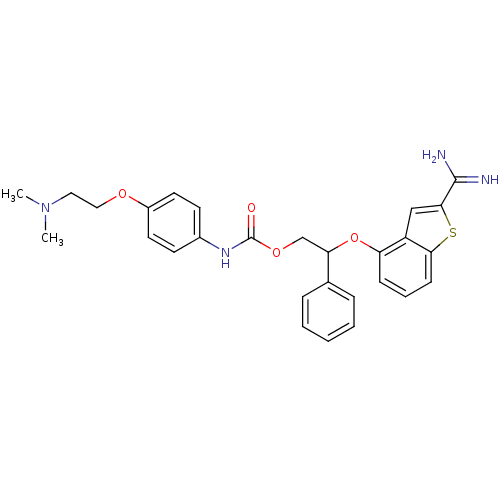
(CHEMBL597143 | [4-(2-Dimethylamino-ethoxy)-phenyl]...)Show SMILES CN(C)CCOc1ccc(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 Show InChI InChI=1S/C28H30N4O4S/c1-32(2)15-16-34-21-13-11-20(12-14-21)31-28(33)35-18-24(19-7-4-3-5-8-19)36-23-9-6-10-25-22(23)17-26(37-25)27(29)30/h3-14,17,24H,15-16,18H2,1-2H3,(H3,29,30)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307279
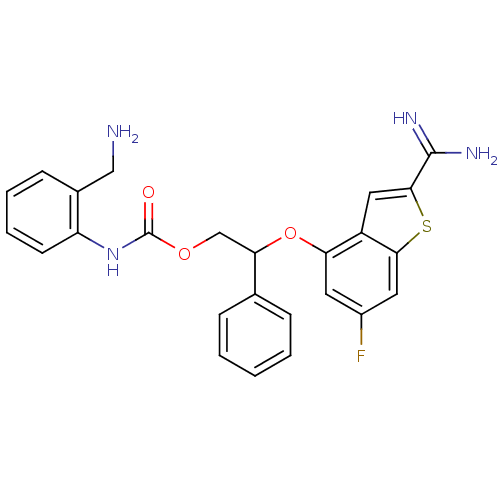
((2-Aminomethyl-phenyl)-carbamic acid 2-(2-carbamim...)Show SMILES NCc1ccccc1NC(=O)OCC(Oc1cc(F)cc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C25H23FN4O3S/c26-17-10-20(18-12-23(24(28)29)34-22(18)11-17)33-21(15-6-2-1-3-7-15)14-32-25(31)30-19-9-5-4-8-16(19)13-27/h1-12,21H,13-14,27H2,(H3,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307303

((2-Fluoro-phenyl)-carbamic acid 2-(2-carbamimidoyl...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccccc3F)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H20FN3O3S/c25-17-9-4-5-10-18(17)28-24(29)30-14-20(15-7-2-1-3-8-15)31-19-11-6-12-21-16(19)13-22(32-21)23(26)27/h1-13,20H,14H2,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307273
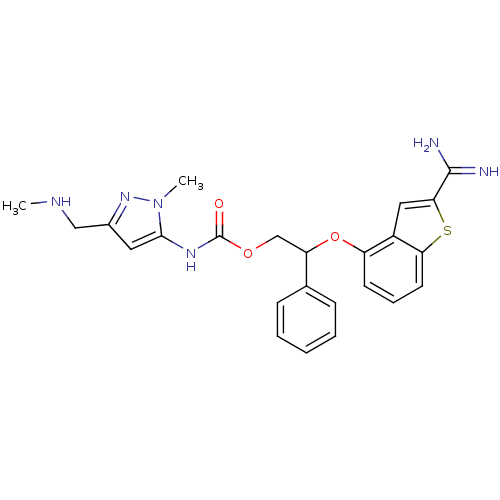
((5-Methaminomethyl-2-methyl-2H-pyrazol-3-yl)-carba...)Show SMILES CNCc1cc(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)n(C)n1 Show InChI InChI=1S/C24H26N6O3S/c1-27-13-16-11-22(30(2)29-16)28-24(31)32-14-19(15-7-4-3-5-8-15)33-18-9-6-10-20-17(18)12-21(34-20)23(25)26/h3-12,19,27H,13-14H2,1-2H3,(H3,25,26)(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307307
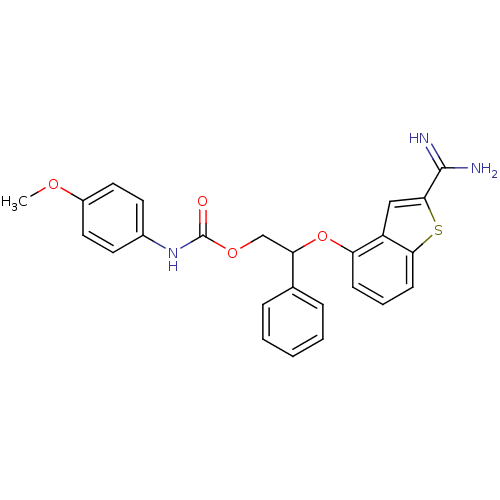
((4-Methoxy-phenyl)-carbamic acid 2-(2-carbamimidoy...)Show SMILES COc1ccc(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 Show InChI InChI=1S/C25H23N3O4S/c1-30-18-12-10-17(11-13-18)28-25(29)31-15-21(16-6-3-2-4-7-16)32-20-8-5-9-22-19(20)14-23(33-22)24(26)27/h2-14,21H,15H2,1H3,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307276
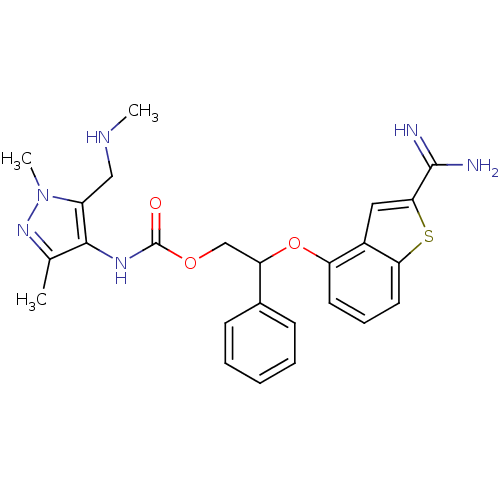
((5-Ethylaminomethyl-1,3-dimethyl-1H-pyrazol-4-yl)-...)Show SMILES CNCc1c(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)c(C)nn1C Show InChI InChI=1S/C25H28N6O3S/c1-15-23(18(13-28-2)31(3)30-15)29-25(32)33-14-20(16-8-5-4-6-9-16)34-19-10-7-11-21-17(19)12-22(35-21)24(26)27/h4-12,20,28H,13-14H2,1-3H3,(H3,26,27)(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS2 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM85050

(CAS_184162-64-9 | SR 142948A | SR142948 | SR142948...)Show SMILES [H]C12CC3([H])CC([H])(C1)C(NC(=O)c1cc(-c4c(OC)cccc4OC)n(n1)-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)C)(C(O)=O)C([H])(C2)C3 |TLB:8:6:53:1.52.2,8:1:6.9.5:53,10:9:3.5.53:1.8.52,THB:47:9:3.5.53:1.8.52,47:9:53:1.52.2,2:1:9:3.5.53,10:9:53:1.52.2,(4.26,2.59,;4.59,4.1,;3.08,4.03,;4.37,4.77,;5.38,3.61,;5.66,4.3,;6.94,4.77,;8.42,4.31,;5.99,3.36,;6.94,6.26,;8.48,6.12,;9.13,4.73,;8.25,3.46,;10.66,4.59,;11.67,5.75,;13.09,5.15,;14.41,5.94,;15.76,5.2,;16.31,3.76,;17.83,3.52,;17.08,5.99,;17.05,7.53,;15.7,8.28,;14.38,7.48,;13.04,8.23,;13.01,9.77,;12.96,3.62,;11.46,3.27,;13.7,2.27,;15.24,2.24,;15.99,.9,;15.2,-.42,;13.66,-.4,;12.91,.95,;11.37,.98,;10.58,-.34,;10.62,2.32,;15.94,-1.77,;17.48,-1.8,;15.15,-3.09,;13.61,-3.06,;15.9,-4.44,;15.1,-5.76,;15.85,-7.1,;15.06,-8.42,;15.8,-9.77,;13.52,-8.4,;7.34,7.74,;6.25,8.83,;8.6,8.63,;5.66,7,;5.66,8.54,;4.59,5.71,;4.37,6.26,)| Show InChI InChI=1S/C39H51N5O6/c1-23(2)29-21-26(37(46)43(5)15-9-14-42(3)4)12-13-31(29)44-32(35-33(49-6)10-8-11-34(35)50-7)22-30(41-44)36(45)40-39(38(47)48)27-17-24-16-25(19-27)20-28(39)18-24/h8,10-13,21-25,27-28H,9,14-20H2,1-7H3,(H,40,45)(H,47,48) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 2 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307268
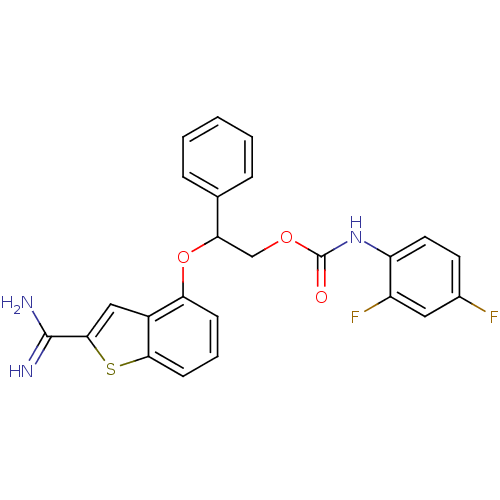
((2,4-Difluoro-phenyl)-carbamic acid 2-(2-carbamimi...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccc(F)cc3F)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H19F2N3O3S/c25-15-9-10-18(17(26)11-15)29-24(30)31-13-20(14-5-2-1-3-6-14)32-19-7-4-8-21-16(19)12-22(33-21)23(27)28/h1-12,20H,13H2,(H3,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307269
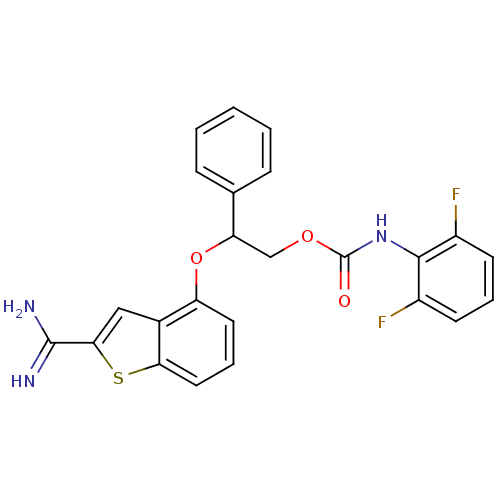
((2,6-Difluoro-phenyl)-carbamic acid 2-(2-carbamimi...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3c(F)cccc3F)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H19F2N3O3S/c25-16-8-4-9-17(26)22(16)29-24(30)31-13-19(14-6-2-1-3-7-14)32-18-10-5-11-20-15(18)12-21(33-20)23(27)28/h1-12,19H,13H2,(H3,27,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307306
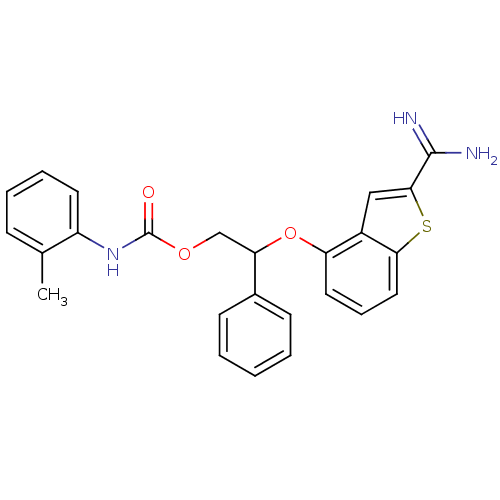
(CHEMBL604289 | o-Tolyl-carbamic acid 2-(2-carbamim...)Show SMILES Cc1ccccc1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C25H23N3O3S/c1-16-8-5-6-11-19(16)28-25(29)30-15-21(17-9-3-2-4-10-17)31-20-12-7-13-22-18(20)14-23(32-22)24(26)27/h2-14,21H,15H2,1H3,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307305
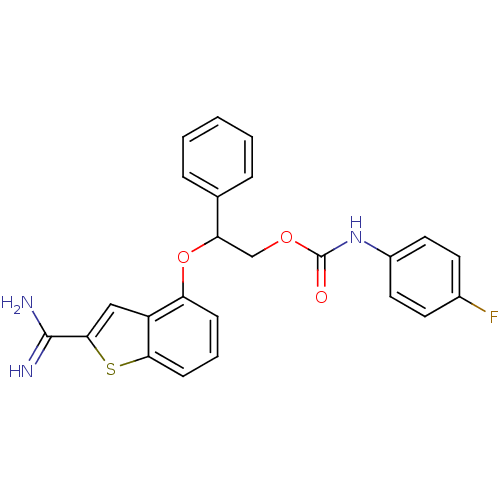
((4-Fluoro-phenyl)-carbamic acid 2-(2-carbamimidoyl...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccc(F)cc3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H20FN3O3S/c25-16-9-11-17(12-10-16)28-24(29)30-14-20(15-5-2-1-3-6-15)31-19-7-4-8-21-18(19)13-22(32-21)23(26)27/h1-13,20H,14H2,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307315
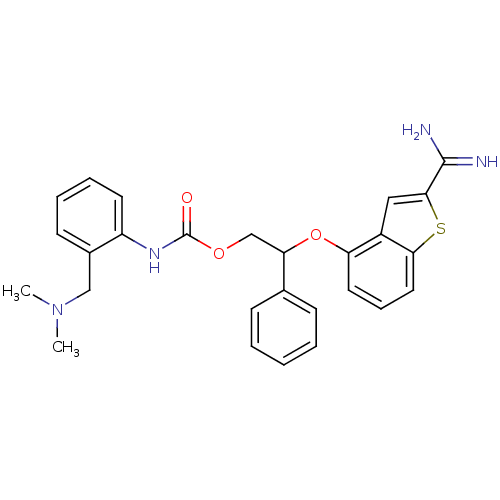
((2-Dimethylaminomethyl-phenyl)-carbamic acid 2-(2-...)Show SMILES CN(C)Cc1ccccc1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C27H28N4O3S/c1-31(2)16-19-11-6-7-12-21(19)30-27(32)33-17-23(18-9-4-3-5-10-18)34-22-13-8-14-24-20(22)15-25(35-24)26(28)29/h3-15,23H,16-17H2,1-2H3,(H3,28,29)(H,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307274
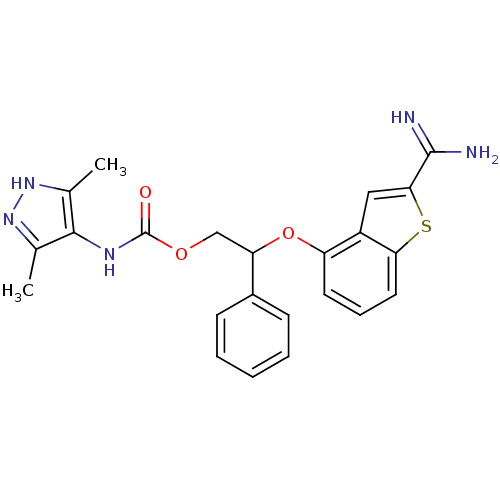
((3,5-Dimethyl-1H-pyrazol-4-yl)-carbamic acid 2-(2-...)Show SMILES Cc1n[nH]c(C)c1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C23H23N5O3S/c1-13-21(14(2)28-27-13)26-23(29)30-12-18(15-7-4-3-5-8-15)31-17-9-6-10-19-16(17)11-20(32-19)22(24)25/h3-11,18H,12H2,1-2H3,(H3,24,25)(H,26,29)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307280
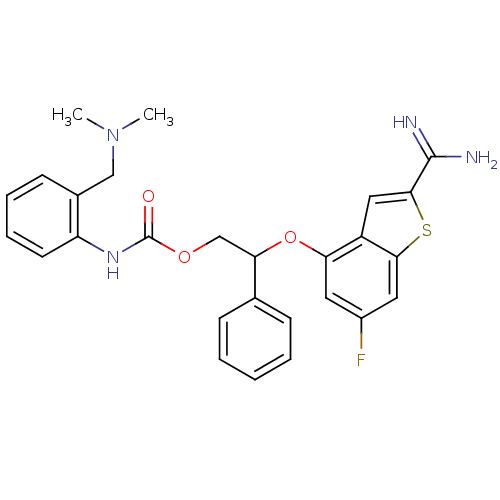
((2-Dimethylaminomethyl-phenyl)-carbamic acid 2-(2-...)Show SMILES CN(C)Cc1ccccc1NC(=O)OCC(Oc1cc(F)cc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C27H27FN4O3S/c1-32(2)15-18-10-6-7-11-21(18)31-27(33)34-16-23(17-8-4-3-5-9-17)35-22-12-19(28)13-24-20(22)14-25(36-24)26(29)30/h3-14,23H,15-16H2,1-2H3,(H3,29,30)(H,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307272
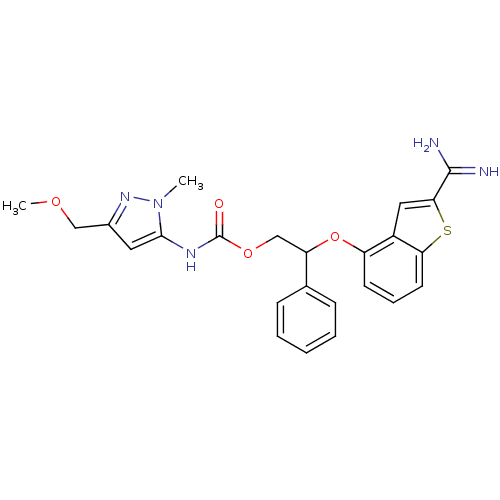
((5-Methoxymethyl-2-methyl-2H-pyrazol-3-yl)-carbami...)Show SMILES COCc1cc(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)n(C)n1 Show InChI InChI=1S/C24H25N5O4S/c1-29-22(11-16(28-29)13-31-2)27-24(30)32-14-19(15-7-4-3-5-8-15)33-18-9-6-10-20-17(18)12-21(34-20)23(25)26/h3-12,19H,13-14H2,1-2H3,(H3,25,26)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307271
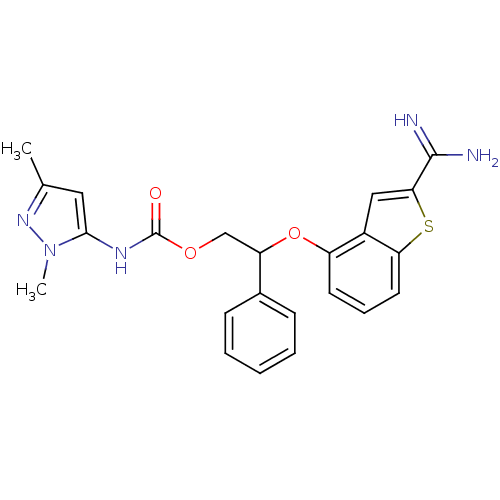
((2,5-Dimethyl-2H-pyrazol-3-yl)-carbamic acid 2-(2-...)Show SMILES Cc1cc(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)n(C)n1 Show InChI InChI=1S/C23H23N5O3S/c1-14-11-21(28(2)27-14)26-23(29)30-13-18(15-7-4-3-5-8-15)31-17-9-6-10-19-16(17)12-20(32-19)22(24)25/h3-12,18H,13H2,1-2H3,(H3,24,25)(H,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307311
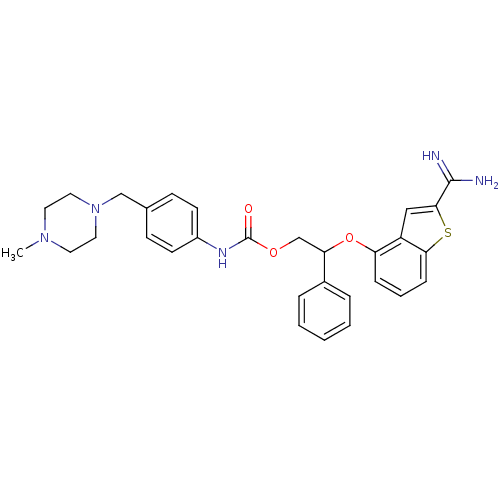
(CHEMBL597144 | [4-(4-Methyl-piperazin-1-ylmethyl)-...)Show SMILES CN1CCN(Cc2ccc(NC(=O)OCC(Oc3cccc4sc(cc34)C(N)=N)c3ccccc3)cc2)CC1 Show InChI InChI=1S/C30H33N5O3S/c1-34-14-16-35(17-15-34)19-21-10-12-23(13-11-21)33-30(36)37-20-26(22-6-3-2-4-7-22)38-25-8-5-9-27-24(25)18-28(39-27)29(31)32/h2-13,18,26H,14-17,19-20H2,1H3,(H3,31,32)(H,33,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307301
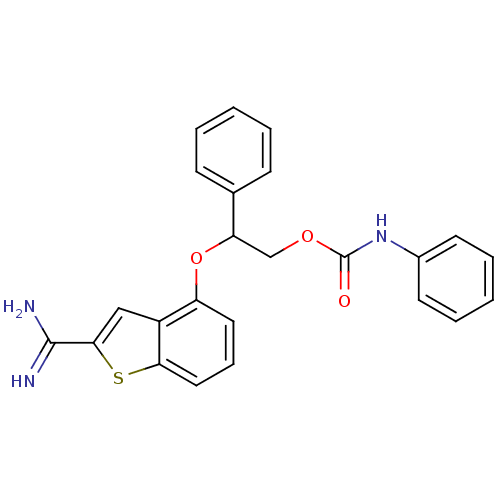
(CHEMBL598400 | Phenyl-carbamic acid 2-(2-carbamimi...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccccc3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H21N3O3S/c25-23(26)22-14-18-19(12-7-13-21(18)31-22)30-20(16-8-3-1-4-9-16)15-29-24(28)27-17-10-5-2-6-11-17/h1-14,20H,15H2,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307275
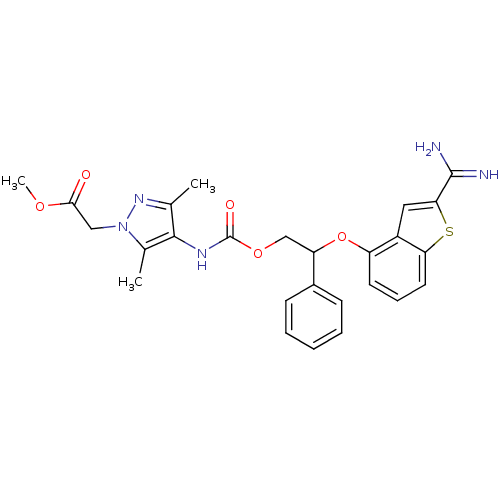
(CHEMBL601231 | {4-[2-(2-Carbamimidoyl-benzo[b]thio...)Show SMILES COC(=O)Cn1nc(C)c(NC(=O)OCC(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)c1C Show InChI InChI=1S/C26H27N5O5S/c1-15-24(16(2)31(30-15)13-23(32)34-3)29-26(33)35-14-20(17-8-5-4-6-9-17)36-19-10-7-11-21-18(19)12-22(37-21)25(27)28/h4-12,20H,13-14H2,1-3H3,(H3,27,28)(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307270
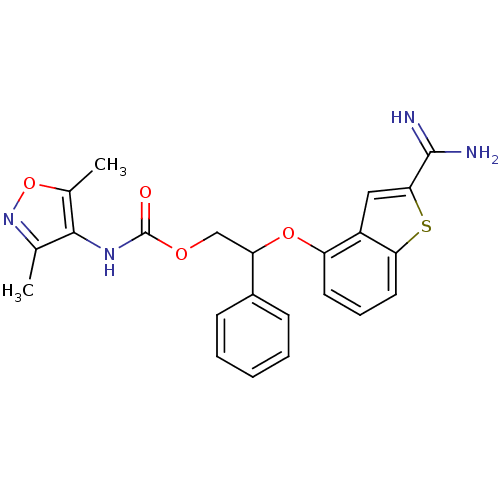
((3,5-Dimethyl-isoxazol-4-yl)-carbamic acid 2-(2-ca...)Show SMILES Cc1noc(C)c1NC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C23H22N4O4S/c1-13-21(14(2)31-27-13)26-23(28)29-12-18(15-7-4-3-5-8-15)30-17-9-6-10-19-16(17)11-20(32-19)22(24)25/h3-11,18H,12H2,1-2H3,(H3,24,25)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307278
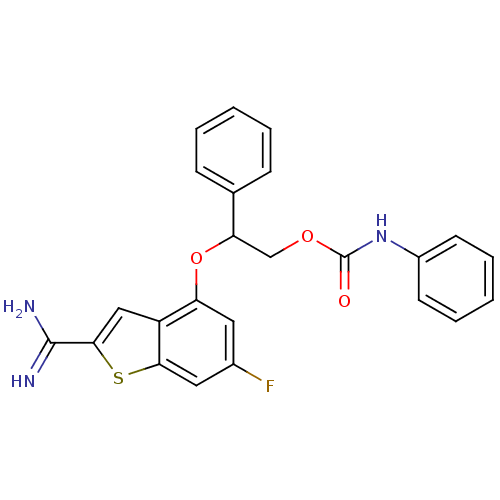
(CHEMBL602083 | Phenyl-carbamic acid 2-(2-carbamimi...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3ccccc3)c3ccccc3)cc(F)cc2s1 Show InChI InChI=1S/C24H20FN3O3S/c25-16-11-19(18-13-22(23(26)27)32-21(18)12-16)31-20(15-7-3-1-4-8-15)14-30-24(29)28-17-9-5-2-6-10-17/h1-13,20H,14H2,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 2 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307304
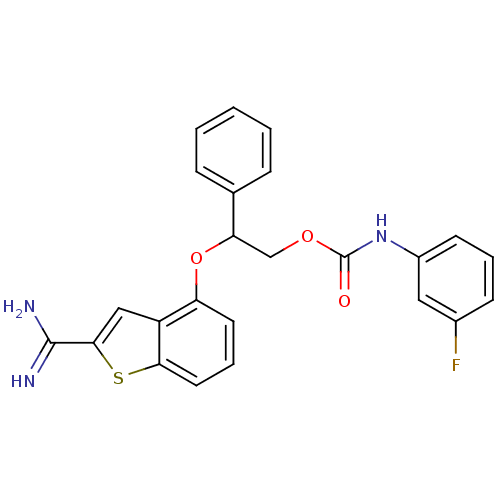
((3-Fluoro-phenyl)-carbamic acid 2-(2-carbamimidoyl...)Show SMILES NC(=N)c1cc2c(OC(COC(=O)Nc3cccc(F)c3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H20FN3O3S/c25-16-8-4-9-17(12-16)28-24(29)30-14-20(15-6-2-1-3-7-15)31-19-10-5-11-21-18(19)13-22(32-21)23(26)27/h1-13,20H,14H2,(H3,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041429
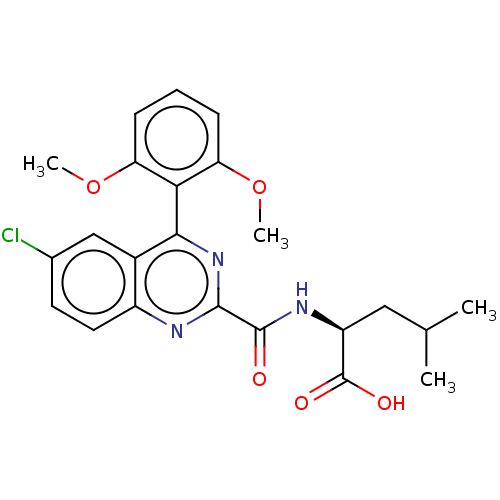
(CHEMBL3356854)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](CC(C)C)C(O)=O |r,wU:24.27,(2.86,-22.1,;4.19,-22.87,;4.2,-24.41,;2.87,-25.18,;2.87,-26.72,;4.2,-27.49,;5.54,-26.72,;6.87,-27.49,;6.87,-29.03,;5.53,-25.17,;6.86,-24.4,;8.19,-25.17,;9.52,-24.39,;9.52,-22.85,;8.17,-22.09,;8.16,-20.57,;6.83,-19.81,;5.51,-20.59,;4.17,-19.84,;5.52,-22.09,;6.85,-22.87,;10.86,-25.16,;10.87,-26.7,;12.19,-24.38,;13.53,-25.15,;14.86,-24.38,;16.2,-25.14,;17.53,-24.37,;16.2,-26.68,;13.54,-26.69,;12.2,-27.47,;14.87,-27.46,)| Show InChI InChI=1S/C23H24ClN3O5/c1-12(2)10-16(23(29)30)26-22(28)21-25-15-9-8-13(24)11-14(15)20(27-21)19-17(31-3)6-5-7-18(19)32-4/h5-9,11-12,16H,10H2,1-4H3,(H,26,28)(H,29,30)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041428

(CHEMBL3356855)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)N[C@@H](C1CCCCC1)C(O)=O |r,wU:24.27,(22.57,-23.73,;23.91,-24.49,;23.91,-26.03,;22.58,-26.8,;22.58,-28.35,;23.92,-29.12,;25.25,-28.35,;26.59,-29.11,;26.59,-30.65,;25.25,-26.8,;26.57,-26.02,;27.91,-26.79,;29.24,-26.02,;29.24,-24.48,;27.88,-23.72,;27.87,-22.19,;26.55,-21.44,;25.22,-22.22,;23.88,-21.46,;25.24,-23.72,;26.57,-24.49,;30.58,-26.78,;30.58,-28.32,;31.91,-26.01,;33.24,-26.77,;34.58,-26,;35.91,-26.77,;37.24,-26.01,;37.24,-24.46,;35.91,-23.69,;34.57,-24.47,;33.25,-28.32,;31.92,-29.09,;34.59,-29.08,)| Show InChI InChI=1S/C25H26ClN3O5/c1-33-18-9-6-10-19(34-2)20(18)22-16-13-15(26)11-12-17(16)27-23(28-22)24(30)29-21(25(31)32)14-7-4-3-5-8-14/h6,9-14,21H,3-5,7-8H2,1-2H3,(H,29,30)(H,31,32)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Rattus norvegicus) | BDBM50041427
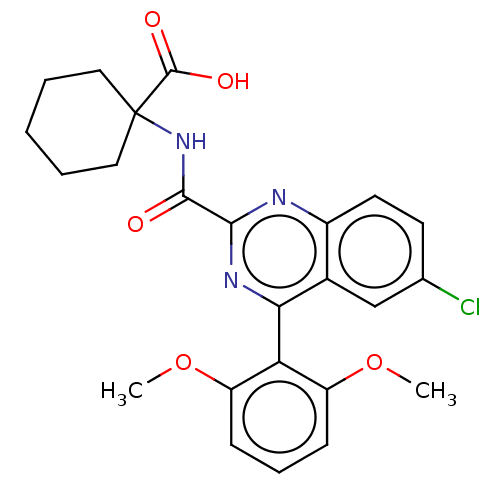
(CHEMBL3356853)Show SMILES COc1cccc(OC)c1-c1nc(nc2ccc(Cl)cc12)C(=O)NC1(CCCCC1)C(O)=O |(41.35,-22.47,;42.69,-23.24,;42.69,-24.79,;41.36,-25.56,;41.36,-27.11,;42.7,-27.88,;44.04,-27.1,;45.37,-27.87,;45.38,-29.42,;44.03,-25.55,;45.36,-24.78,;46.7,-25.55,;48.03,-24.77,;48.03,-23.23,;46.67,-22.47,;46.66,-20.94,;45.33,-20.18,;44.01,-20.96,;42.66,-20.21,;44.02,-22.47,;45.35,-23.24,;49.37,-25.54,;49.38,-27.08,;50.71,-24.76,;52.04,-25.53,;53.38,-26.3,;54.7,-25.53,;54.71,-23.99,;53.38,-23.22,;52.04,-23.99,;52.05,-27.08,;50.72,-27.85,;53.39,-27.84,)| Show InChI InChI=1S/C24H24ClN3O5/c1-32-17-7-6-8-18(33-2)19(17)20-15-13-14(25)9-10-16(15)26-21(27-20)22(29)28-24(23(30)31)11-4-3-5-12-24/h6-10,13H,3-5,11-12H2,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125L]NT from rat neurotensin receptor type 1 expressed in CHOK1 cells by radioligand binding assay |
Bioorg Med Chem Lett 25: 292-6 (2014)
Article DOI: 10.1016/j.bmcl.2014.11.047
BindingDB Entry DOI: 10.7270/Q2M90B9G |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307300
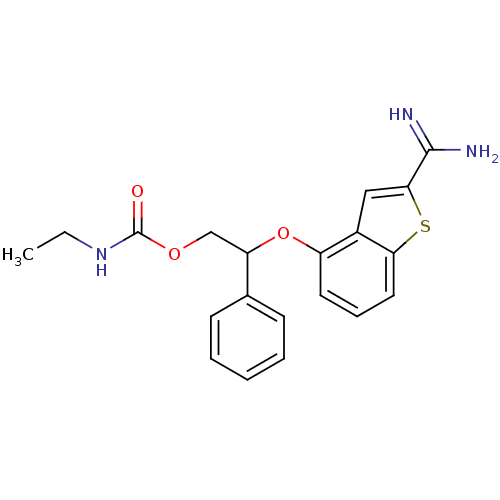
(CHEMBL606194 | Ethyl-carbamic acid 2-(2-carbamimid...)Show SMILES CCNC(=O)OCC(Oc1cccc2sc(cc12)C(N)=N)c1ccccc1 Show InChI InChI=1S/C20H21N3O3S/c1-2-23-20(24)25-12-16(13-7-4-3-5-8-13)26-15-9-6-10-17-14(15)11-18(27-17)19(21)22/h3-11,16H,2,12H2,1H3,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50130880

(CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...)Show SMILES [#6]-[#6]-[#6@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](/[#7])-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#6]-[#7])-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6](-[#8])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6](=O)-[#7]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C78H121N21O20/c1-7-43(6)63(73(115)96-57(76(118)119)37-42(4)5)97-70(112)55(39-45-21-25-47(101)26-22-45)95-72(114)59-18-13-35-99(59)75(117)52(16-11-33-86-78(83)84)90-64(106)48(15-10-32-85-77(81)82)89-71(113)58-17-12-34-98(58)74(116)51(14-8-9-31-79)91-69(111)56(40-60(80)102)94-66(108)50(28-30-62(104)105)88-68(110)54(38-44-19-23-46(100)24-20-44)93-67(109)53(36-41(2)3)92-65(107)49-27-29-61(103)87-49/h19-26,41-43,48-59,63,100-101H,7-18,27-40,79H2,1-6H3,(H2,80,102)(H,87,103)(H,88,110)(H,89,113)(H,90,106)(H,91,111)(H,92,107)(H,93,109)(H,94,108)(H,95,114)(H,96,115)(H,97,112)(H,104,105)(H,118,119)(H4,81,82,85)(H4,83,84,86)/t43-,48-,49-,50-,51-,52-,53-,54-,55-,56-,57-,58-,59-,63-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]neurotensin from rat NTS2 receptor expressed in CHO-K1 cells by competitive binding assay |
J Med Chem 57: 7472-7 (2014)
Article DOI: 10.1021/jm500857r
BindingDB Entry DOI: 10.7270/Q2BP04C6 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307297
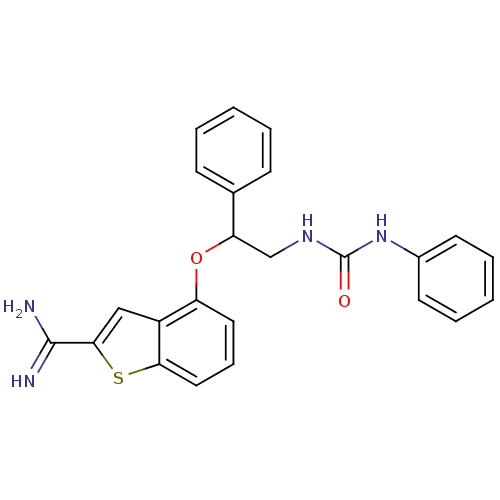
(4-[1-Phenyl-2-(3-phenyl-ureido)-ethoxy]-benzo[b]th...)Show SMILES NC(=N)c1cc2c(OC(CNC(=O)Nc3ccccc3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H22N4O2S/c25-23(26)22-14-18-19(12-7-13-21(18)31-22)30-20(16-8-3-1-4-9-16)15-27-24(29)28-17-10-5-2-6-11-17/h1-14,20H,15H2,(H3,25,26)(H2,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50240845
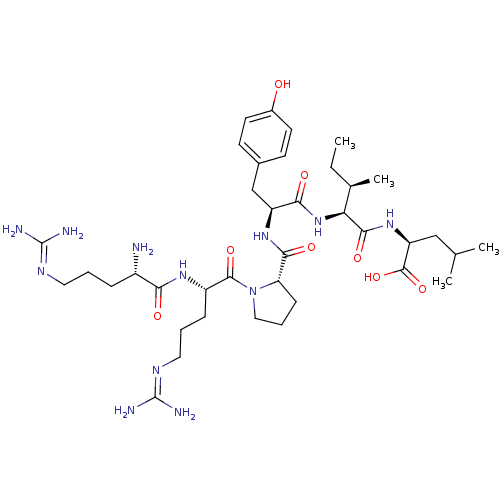
((S)-2-{(2S,3R)-2-[(S)-2-({(S)-1-[(S)-2-((S)-2-Amin...)Show SMILES [#6]-[#6]-[#6@@H](-[#6])-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@@H]-1-[#6]-[#6]-[#6]-[#7]-1-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#7]-[#6](=O)-[#6@@H](-[#7])-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6](-[#6])-[#6])-[#6](-[#8])=O |r| Show InChI InChI=1S/C38H64N12O8/c1-5-22(4)30(34(55)48-28(36(57)58)19-21(2)3)49-32(53)27(20-23-12-14-24(51)15-13-23)47-33(54)29-11-8-18-50(29)35(56)26(10-7-17-45-38(42)43)46-31(52)25(39)9-6-16-44-37(40)41/h12-15,21-22,25-30,51H,5-11,16-20,39H2,1-4H3,(H,46,52)(H,47,54)(H,48,55)(H,49,53)(H,57,58)(H4,40,41,44)(H4,42,43,45)/t22-,25+,26+,27+,28+,29+,30+/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 2
(Rattus norvegicus) | BDBM50019405

(LEVOCABASTINE | R-50547)Show SMILES C[C@@H]1CN(CC[C@]1(C(O)=O)c1ccccc1)[C@H]1CC[C@](CC1)(C#N)c1ccc(F)cc1 |wU:6.7,16.17,19.24,wD:1.0,6.10,19.26,(9.61,3.85,;8.28,3.08,;8.28,1.54,;6.95,.77,;5.61,1.54,;5.61,3.08,;6.95,3.85,;7.67,5.21,;9.21,5.26,;7.78,6.75,;6.22,5.21,;4.68,5.26,;3.96,6.62,;4.78,7.93,;6.32,7.88,;7.04,6.52,;6.95,-.77,;8.28,-1.54,;8.28,-3.08,;6.95,-3.85,;5.61,-3.08,;5.61,-1.54,;6.22,-5.21,;5.5,-6.57,;7.67,-5.21,;9.21,-5.26,;9.93,-6.62,;9.11,-7.93,;9.84,-9.29,;7.57,-7.88,;6.85,-6.52,)| Show InChI InChI=1S/C26H29FN2O2/c1-19-17-29(16-15-26(19,24(30)31)21-5-3-2-4-6-21)23-11-13-25(18-28,14-12-23)20-7-9-22(27)10-8-20/h2-10,19,23H,11-17H2,1H3,(H,30,31)/t19-,23-,25-,26-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute
Curated by ChEMBL
| Assay Description
Displacement of [125I]NT at rat NTS2 overexpressed in CHOK1 cells after 30 mins by gamma counting |
J Med Chem 57: 5318-32 (2014)
Article DOI: 10.1021/jm5003843
BindingDB Entry DOI: 10.7270/Q2PR7XJD |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307309
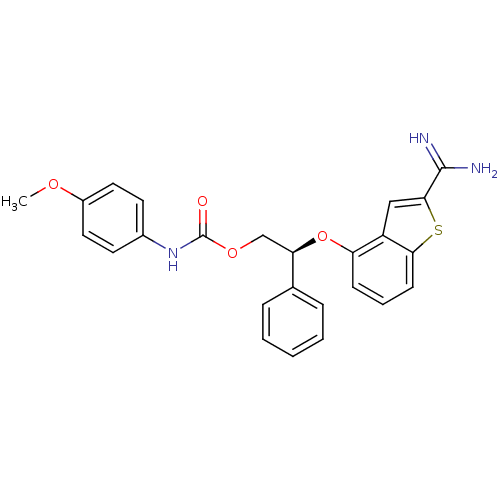
((S)-(4-Methoxy-phenyl)-carbamic acid 2-(2-carbamim...)Show SMILES COc1ccc(NC(=O)OC[C@@H](Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 |r| Show InChI InChI=1S/C25H23N3O4S/c1-30-18-12-10-17(11-13-18)28-25(29)31-15-21(16-6-3-2-4-7-16)32-20-8-5-9-22-19(20)14-23(33-22)24(26)27/h2-14,21H,15H2,1H3,(H3,26,27)(H,28,29)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50307296
(CHEMBL597370 | [2-(2-Carbamimidoyl-benzo[b]thiophe...)Show SMILES NC(=N)c1cc2c(OC(CNC(=O)Oc3ccccc3)c3ccccc3)cccc2s1 Show InChI InChI=1S/C24H21N3O3S/c25-23(26)22-14-18-19(12-7-13-21(18)31-22)30-20(16-8-3-1-4-9-16)15-27-24(28)29-17-10-5-2-6-11-17/h1-14,20H,15H2,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant factor 9a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50307289
(2-(2-Carbamimidoyl-benzo[b]thiophen-4-yloxy)-N-(4-...)Show SMILES COc1ccc(NC(=O)C(Oc2cccc3sc(cc23)C(N)=N)c2ccccc2)cc1 Show InChI InChI=1S/C24H21N3O3S/c1-29-17-12-10-16(11-13-17)27-24(28)22(15-6-3-2-4-7-15)30-19-8-5-9-20-18(19)14-21(31-20)23(25)26/h2-14,22H,1H3,(H3,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trigen Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of factor 10a by amidolytic assay |
J Med Chem 53: 1473-82 (2010)
Article DOI: 10.1021/jm901476x
BindingDB Entry DOI: 10.7270/Q2P84C0N |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data