 Found 108 hits with Last Name = 'lundeen' and Initial = 'k'
Found 108 hits with Last Name = 'lundeen' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377765
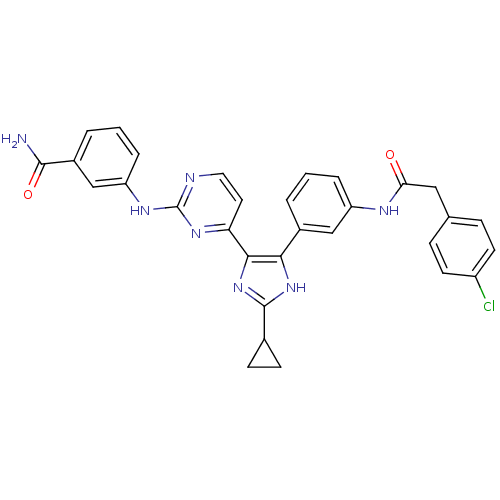
(CHEMBL254966)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C2CC2)c1 Show InChI InChI=1S/C31H26ClN7O2/c32-22-11-7-18(8-12-22)15-26(40)35-23-5-1-3-20(16-23)27-28(39-30(38-27)19-9-10-19)25-13-14-34-31(37-25)36-24-6-2-4-21(17-24)29(33)41/h1-8,11-14,16-17,19H,9-10,15H2,(H2,33,41)(H,35,40)(H,38,39)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377767

(CHEMBL429498)Show SMILES Clc1ccc(CC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1 Show InChI InChI=1S/C29H29ClN8O2/c30-21-8-4-18(5-9-21)16-24(39)34-22-3-1-2-20(17-22)25-26(37-27(36-25)19-6-7-19)23-10-11-31-28(35-23)32-12-14-38-15-13-33-29(38)40/h1-5,8-11,17,19H,6-7,12-16H2,(H,33,40)(H,34,39)(H,36,37)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377766
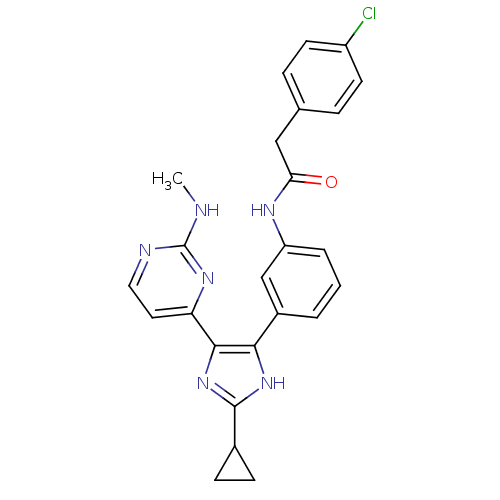
(CHEMBL403854)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C1CC1 Show InChI InChI=1S/C25H23ClN6O/c1-27-25-28-12-11-20(30-25)23-22(31-24(32-23)16-7-8-16)17-3-2-4-19(14-17)29-21(33)13-15-5-9-18(26)10-6-15/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,29,33)(H,31,32)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377763
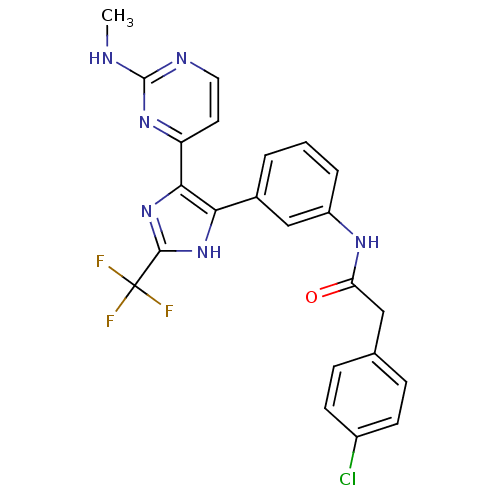
(CHEMBL258454)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C(F)(F)F Show InChI InChI=1S/C23H18ClF3N6O/c1-28-22-29-10-9-17(31-22)20-19(32-21(33-20)23(25,26)27)14-3-2-4-16(12-14)30-18(34)11-13-5-7-15(24)8-6-13/h2-10,12H,11H2,1H3,(H,30,34)(H,32,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377765

(CHEMBL254966)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C2CC2)c1 Show InChI InChI=1S/C31H26ClN7O2/c32-22-11-7-18(8-12-22)15-26(40)35-23-5-1-3-20(16-23)27-28(39-30(38-27)19-9-10-19)25-13-14-34-31(37-25)36-24-6-2-4-21(17-24)29(33)41/h1-8,11-14,16-17,19H,9-10,15H2,(H2,33,41)(H,35,40)(H,38,39)(H,34,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24206
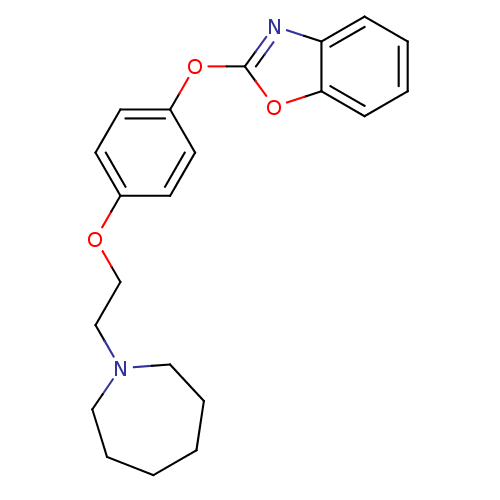
(2-{4-[2-(azepan-1-yl)ethoxy]phenoxy}-1,3-benzoxazo...)Show InChI InChI=1S/C21H24N2O3/c1-2-6-14-23(13-5-1)15-16-24-17-9-11-18(12-10-17)25-21-22-19-7-3-4-8-20(19)26-21/h3-4,7-12H,1-2,5-6,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377761

(CHEMBL430413)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1)C1CC1 Show InChI InChI=1S/C25H21ClF3N7O/c1-30-23-31-10-9-19(34-23)21-20(35-22(36-21)13-5-6-13)14-3-2-4-15(11-14)32-24(37)33-16-7-8-18(26)17(12-16)25(27,28)29/h2-4,7-13H,5-6H2,1H3,(H,35,36)(H,30,31,34)(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24216
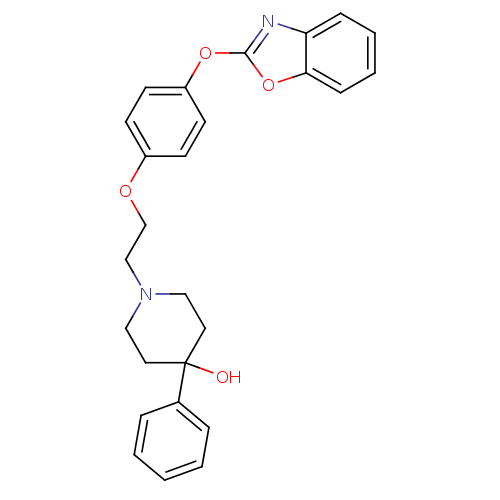
(1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}-4-p...)Show SMILES OC1(CCN(CCOc2ccc(Oc3nc4ccccc4o3)cc2)CC1)c1ccccc1 Show InChI InChI=1S/C26H26N2O4/c29-26(20-6-2-1-3-7-20)14-16-28(17-15-26)18-19-30-21-10-12-22(13-11-21)31-25-27-23-8-4-5-9-24(23)32-25/h1-13,29H,14-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377767

(CHEMBL429498)Show SMILES Clc1ccc(CC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1 Show InChI InChI=1S/C29H29ClN8O2/c30-21-8-4-18(5-9-21)16-24(39)34-22-3-1-2-20(17-22)25-26(37-27(36-25)19-6-7-19)23-10-11-31-28(35-23)32-12-14-38-15-13-33-29(38)40/h1-5,8-11,17,19H,6-7,12-16H2,(H,33,40)(H,34,39)(H,36,37)(H,31,32,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24199
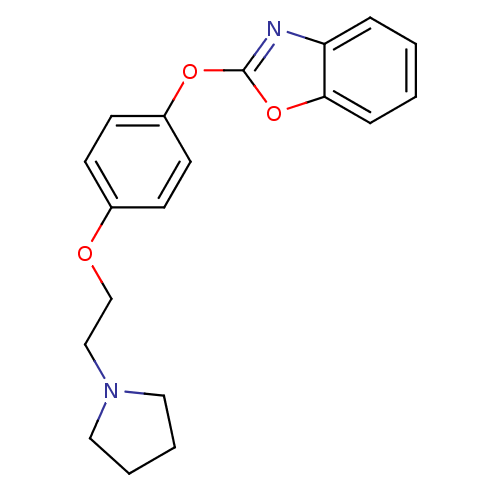
(2-{4-[2-(pyrrolidin-1-yl)ethoxy]phenoxy}-1,3-benzo...)Show InChI InChI=1S/C19H20N2O3/c1-2-6-18-17(5-1)20-19(24-18)23-16-9-7-15(8-10-16)22-14-13-21-11-3-4-12-21/h1-2,5-10H,3-4,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24224
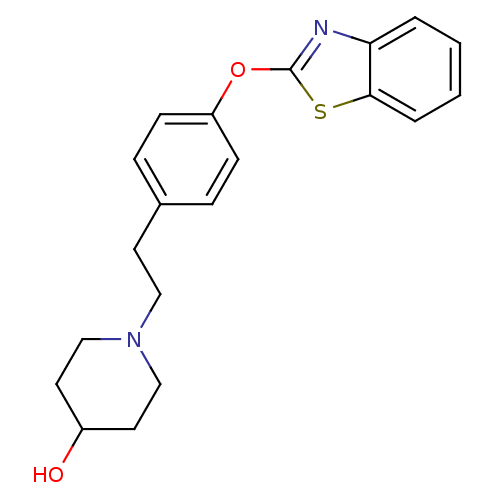
(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}pip...)Show InChI InChI=1S/C20H22N2O2S/c23-16-10-13-22(14-11-16)12-9-15-5-7-17(8-6-15)24-20-21-18-3-1-2-4-19(18)25-20/h1-8,16,23H,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24238
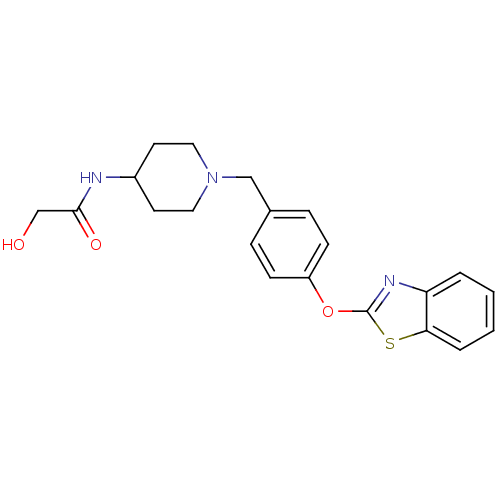
(Benzthiazole compound, 33p | N-(1-{[4-(1,3-benzoth...)Show SMILES OCC(=O)NC1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C21H23N3O3S/c25-14-20(26)22-16-9-11-24(12-10-16)13-15-5-7-17(8-6-15)27-21-23-18-3-1-2-4-19(18)28-21/h1-8,16,25H,9-14H2,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24212
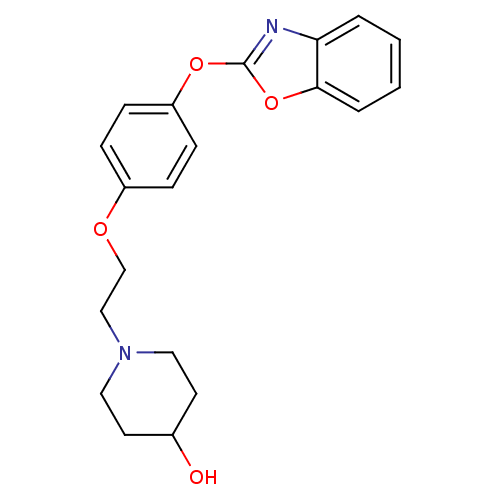
(1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}pipe...)Show InChI InChI=1S/C20H22N2O4/c23-15-9-11-22(12-10-15)13-14-24-16-5-7-17(8-6-16)25-20-21-18-3-1-2-4-19(18)26-20/h1-8,15,23H,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377766

(CHEMBL403854)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C1CC1 Show InChI InChI=1S/C25H23ClN6O/c1-27-25-28-12-11-20(30-25)23-22(31-24(32-23)16-7-8-16)17-3-2-4-19(14-17)29-21(33)13-15-5-9-18(26)10-6-15/h2-6,9-12,14,16H,7-8,13H2,1H3,(H,29,33)(H,31,32)(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24235
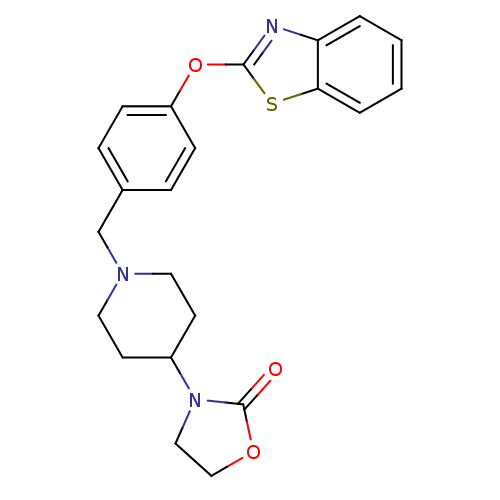
(3-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...)Show SMILES O=C1OCCN1C1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C22H23N3O3S/c26-22-25(13-14-27-22)17-9-11-24(12-10-17)15-16-5-7-18(8-6-16)28-21-23-19-3-1-2-4-20(19)29-21/h1-8,17H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377771
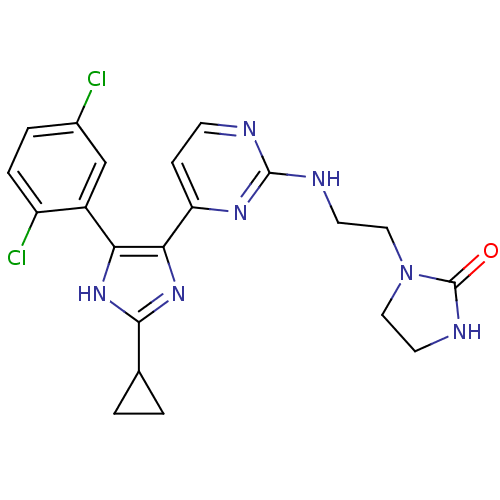
(CHEMBL401710)Show SMILES Clc1ccc(Cl)c(c1)-c1[nH]c(nc1-c1ccnc(NCCN2CCNC2=O)n1)C1CC1 Show InChI InChI=1S/C21H21Cl2N7O/c22-13-3-4-15(23)14(11-13)17-18(29-19(28-17)12-1-2-12)16-5-6-24-20(27-16)25-7-9-30-10-8-26-21(30)31/h3-6,11-12H,1-2,7-10H2,(H,26,31)(H,28,29)(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24203
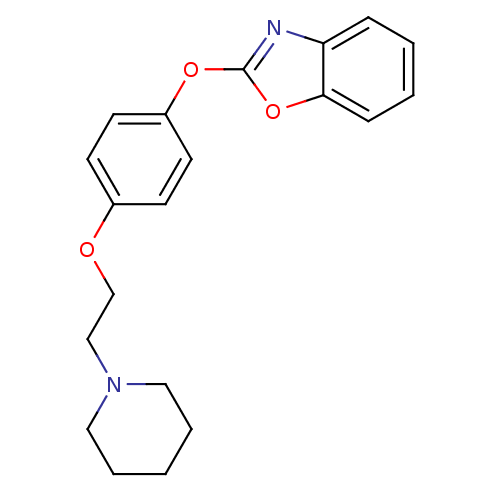
(2-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1,3-benzox...)Show InChI InChI=1S/C20H22N2O3/c1-4-12-22(13-5-1)14-15-23-16-8-10-17(11-9-16)24-20-21-18-6-2-3-7-19(18)25-20/h2-3,6-11H,1,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24239
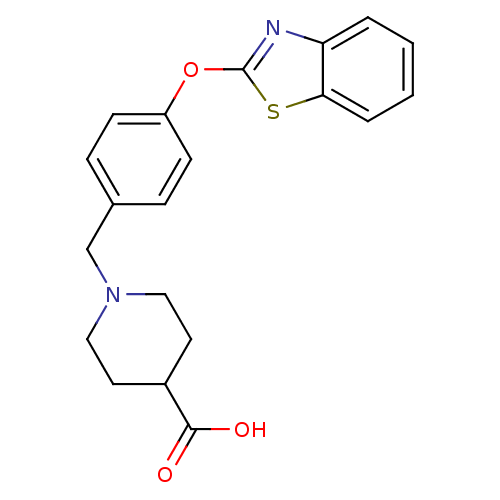
(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...)Show InChI InChI=1S/C20H20N2O3S/c23-19(24)15-9-11-22(12-10-15)13-14-5-7-16(8-6-14)25-20-21-17-3-1-2-4-18(17)26-20/h1-8,15H,9-13H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24222
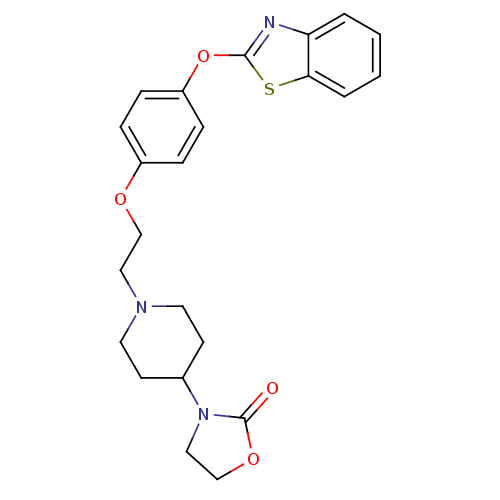
(3-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...)Show SMILES O=C1OCCN1C1CCN(CCOc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C23H25N3O4S/c27-23-26(14-16-29-23)17-9-11-25(12-10-17)13-15-28-18-5-7-19(8-6-18)30-22-24-20-3-1-2-4-21(20)31-22/h1-8,17H,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24236
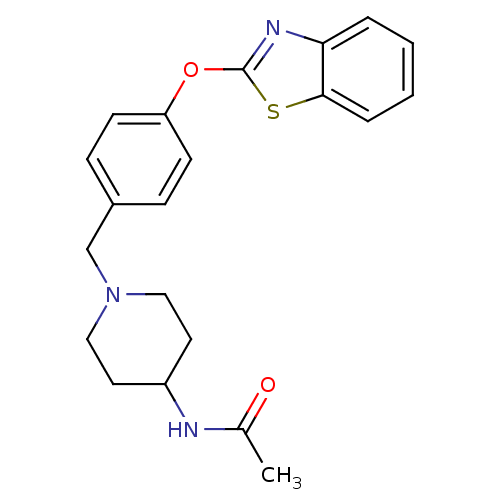
(Benzthiazole compound, 33n | N-(1-{[4-(1,3-benzoth...)Show InChI InChI=1S/C21H23N3O2S/c1-15(25)22-17-10-12-24(13-11-17)14-16-6-8-18(9-7-16)26-21-23-19-4-2-3-5-20(19)27-21/h2-9,17H,10-14H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24234
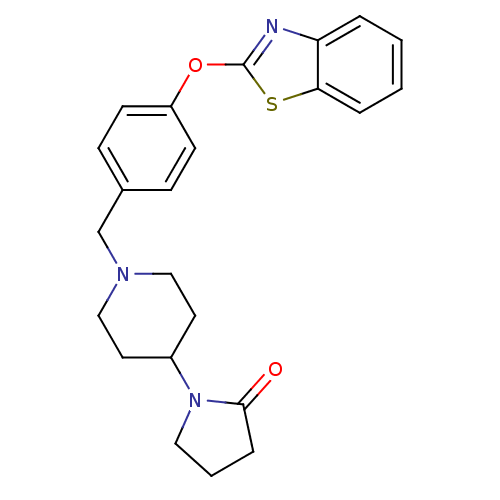
(1-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...)Show SMILES O=C1CCCN1C1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C23H25N3O2S/c27-22-6-3-13-26(22)18-11-14-25(15-12-18)16-17-7-9-19(10-8-17)28-23-24-20-4-1-2-5-21(20)29-23/h1-2,4-5,7-10,18H,3,6,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24218
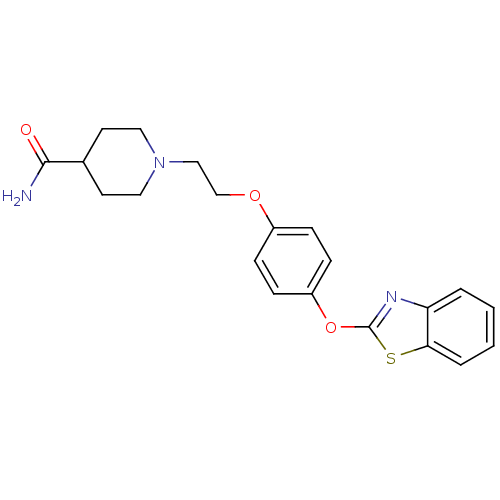
(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl}pi...)Show SMILES NC(=O)C1CCN(CCOc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C21H23N3O3S/c22-20(25)15-9-11-24(12-10-15)13-14-26-16-5-7-17(8-6-16)27-21-23-18-3-1-2-4-19(18)28-21/h1-8,15H,9-14H2,(H2,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24227
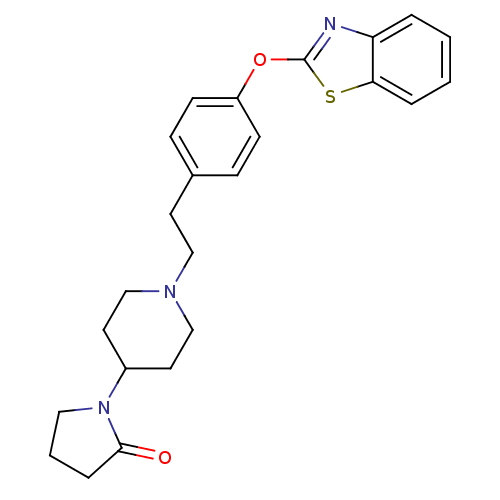
(1-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}...)Show SMILES O=C1CCCN1C1CCN(CCc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C24H27N3O2S/c28-23-6-3-14-27(23)19-12-16-26(17-13-19)15-11-18-7-9-20(10-8-18)29-24-25-21-4-1-2-5-22(21)30-24/h1-2,4-5,7-10,19H,3,6,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24215
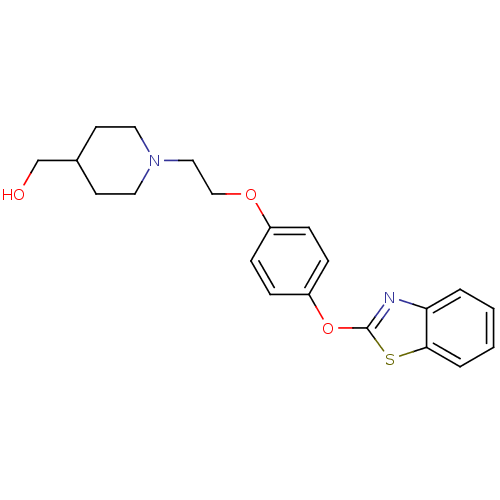
((1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl}p...)Show InChI InChI=1S/C21H24N2O3S/c24-15-16-9-11-23(12-10-16)13-14-25-17-5-7-18(8-6-17)26-21-22-19-3-1-2-4-20(19)27-21/h1-8,16,24H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24201
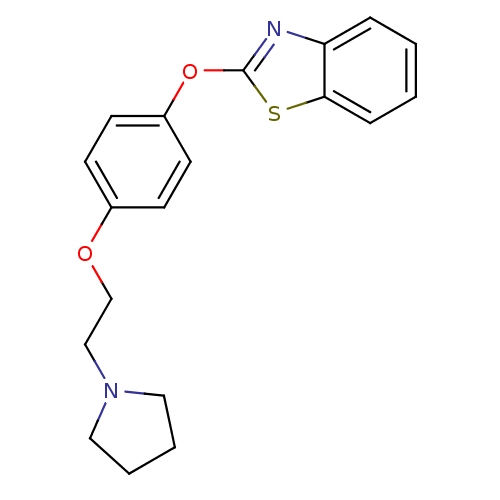
(2-{4-[2-(pyrrolidin-1-yl)ethoxy]phenoxy}-1,3-benzo...)Show InChI InChI=1S/C19H20N2O2S/c1-2-6-18-17(5-1)20-19(24-18)23-16-9-7-15(8-10-16)22-14-13-21-11-3-4-12-21/h1-2,5-10H,3-4,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377758
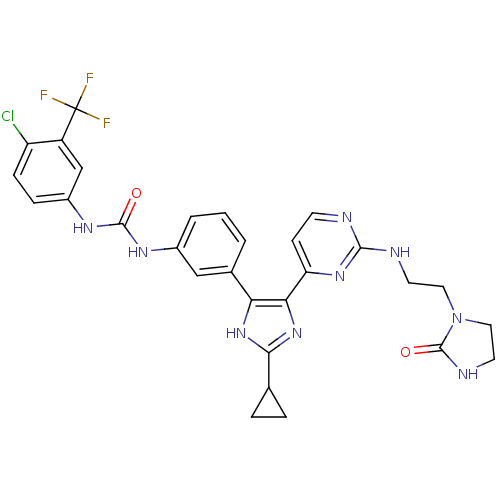
(CHEMBL256921)Show SMILES FC(F)(F)c1cc(NC(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)ccc1Cl Show InChI InChI=1S/C29H27ClF3N9O2/c30-21-7-6-19(15-20(21)29(31,32)33)38-27(43)37-18-3-1-2-17(14-18)23-24(41-25(40-23)16-4-5-16)22-8-9-34-26(39-22)35-10-12-42-13-11-36-28(42)44/h1-3,6-9,14-16H,4-5,10-13H2,(H,36,44)(H,40,41)(H,34,35,39)(H2,37,38,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24214
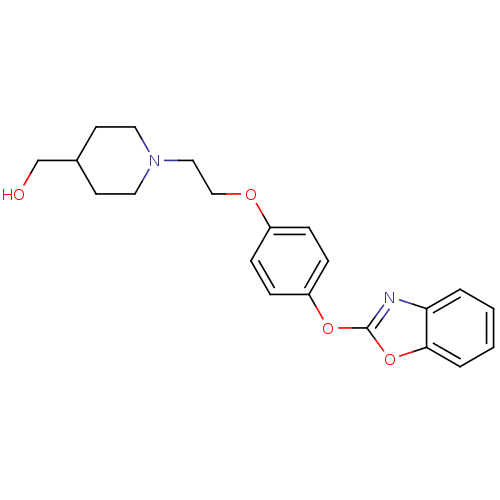
((1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}pip...)Show InChI InChI=1S/C21H24N2O4/c24-15-16-9-11-23(12-10-16)13-14-25-17-5-7-18(8-6-17)26-21-22-19-3-1-2-4-20(19)27-21/h1-8,16,24H,9-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24220
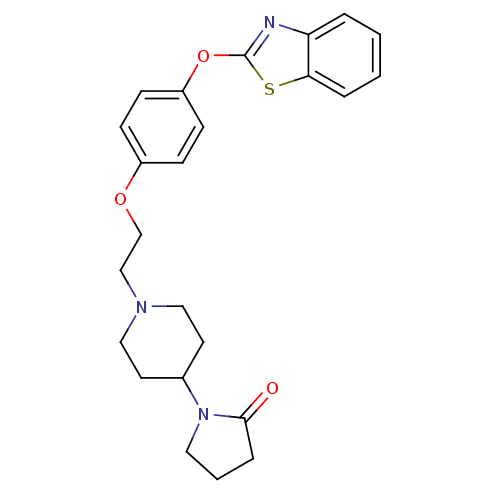
(1-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...)Show SMILES O=C1CCCN1C1CCN(CCOc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C24H27N3O3S/c28-23-6-3-13-27(23)18-11-14-26(15-12-18)16-17-29-19-7-9-20(10-8-19)30-24-25-21-4-1-2-5-22(21)31-24/h1-2,4-5,7-10,18H,3,6,11-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24233
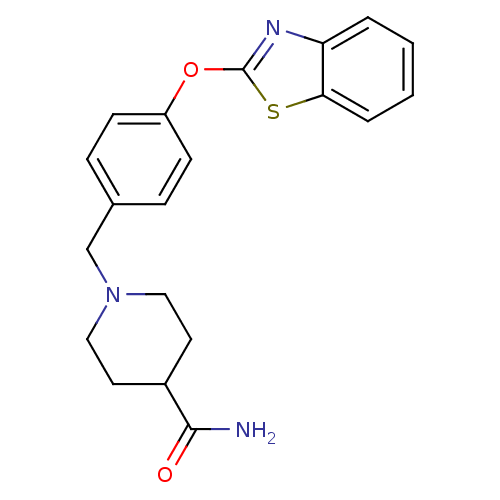
(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...)Show InChI InChI=1S/C20H21N3O2S/c21-19(24)15-9-11-23(12-10-15)13-14-5-7-16(8-6-14)25-20-22-17-3-1-2-4-18(17)26-20/h1-8,15H,9-13H2,(H2,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24223
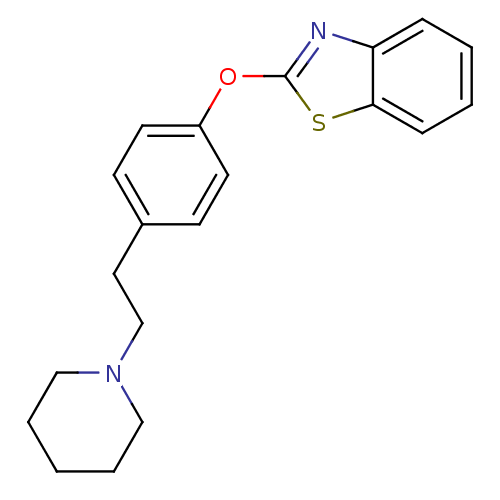
(2-{4-[2-(piperidin-1-yl)ethyl]phenoxy}-1,3-benzoth...)Show InChI InChI=1S/C20H22N2OS/c1-4-13-22(14-5-1)15-12-16-8-10-17(11-9-16)23-20-21-18-6-2-3-7-19(18)24-20/h2-3,6-11H,1,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377762
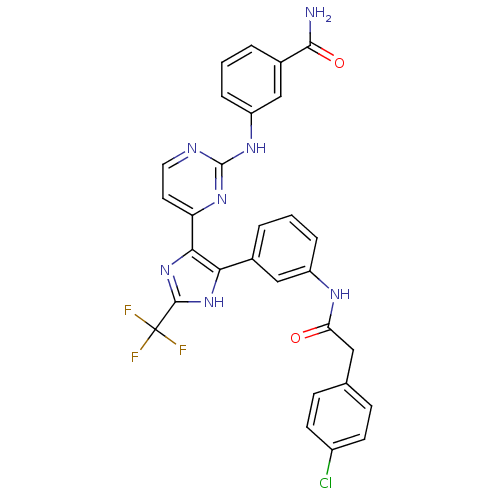
(CHEMBL256775)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Cc3ccc(Cl)cc3)c2)C(F)(F)F)c1 Show InChI InChI=1S/C29H21ClF3N7O2/c30-19-9-7-16(8-10-19)13-23(41)36-20-5-1-3-17(14-20)24-25(40-27(39-24)29(31,32)33)22-11-12-35-28(38-22)37-21-6-2-4-18(15-21)26(34)42/h1-12,14-15H,13H2,(H2,34,42)(H,36,41)(H,39,40)(H,35,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377764
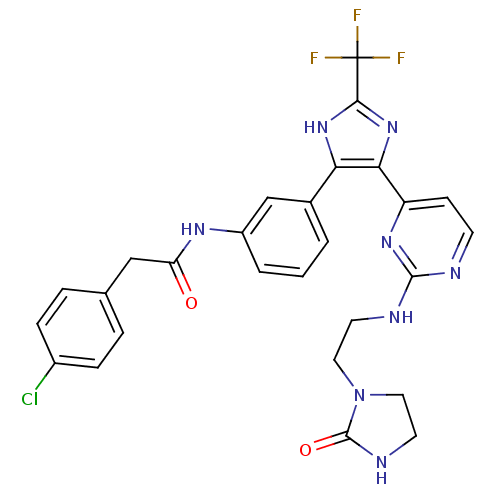
(CHEMBL254965)Show SMILES FC(F)(F)c1nc(c([nH]1)-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)-c1ccnc(NCCN2CCNC2=O)n1 Show InChI InChI=1S/C27H24ClF3N8O2/c28-18-6-4-16(5-7-18)14-21(40)35-19-3-1-2-17(15-19)22-23(38-24(37-22)27(29,30)31)20-8-9-32-25(36-20)33-10-12-39-13-11-34-26(39)41/h1-9,15H,10-14H2,(H,34,41)(H,35,40)(H,37,38)(H,32,33,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24237

(Benzthiazole compound, 33o | [(1-{[4-(1,3-benzothi...)Show SMILES CC(=O)OCC(=O)NC1CCN(Cc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C23H25N3O4S/c1-16(27)29-15-22(28)24-18-10-12-26(13-11-18)14-17-6-8-19(9-7-17)30-23-25-20-4-2-3-5-21(20)31-23/h2-9,18H,10-15H2,1H3,(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377761

(CHEMBL430413)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)c1)C1CC1 Show InChI InChI=1S/C25H21ClF3N7O/c1-30-23-31-10-9-19(34-23)21-20(35-22(36-21)13-5-6-13)14-3-2-4-15(11-14)32-24(37)33-16-7-8-18(26)17(12-16)25(27,28)29/h2-4,7-13H,5-6H2,1H3,(H,35,36)(H,30,31,34)(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377769
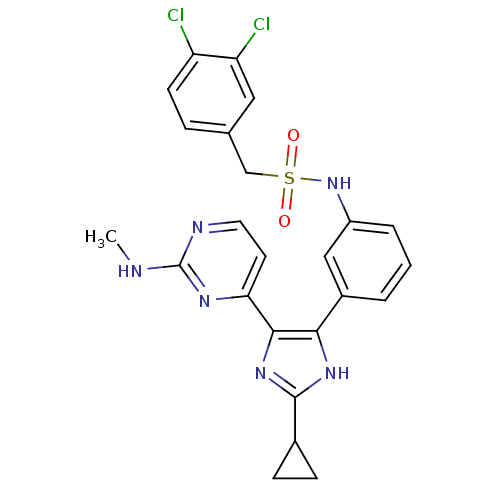
(CHEMBL402029)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NS(=O)(=O)Cc2ccc(Cl)c(Cl)c2)c1)C1CC1 Show InChI InChI=1S/C24H22Cl2N6O2S/c1-27-24-28-10-9-20(29-24)22-21(30-23(31-22)15-6-7-15)16-3-2-4-17(12-16)32-35(33,34)13-14-5-8-18(25)19(26)11-14/h2-5,8-12,15,32H,6-7,13H2,1H3,(H,30,31)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24240
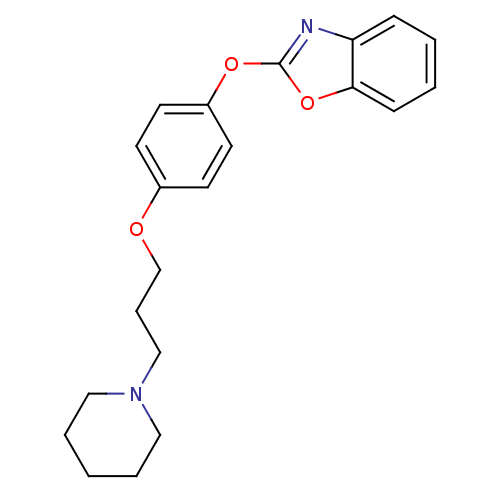
(2-{4-[3-(piperidin-1-yl)propoxy]phenoxy}-1,3-benzo...)Show InChI InChI=1S/C21H24N2O3/c1-4-13-23(14-5-1)15-6-16-24-17-9-11-18(12-10-17)25-21-22-19-7-2-3-8-20(19)26-21/h2-3,7-12H,1,4-6,13-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24225
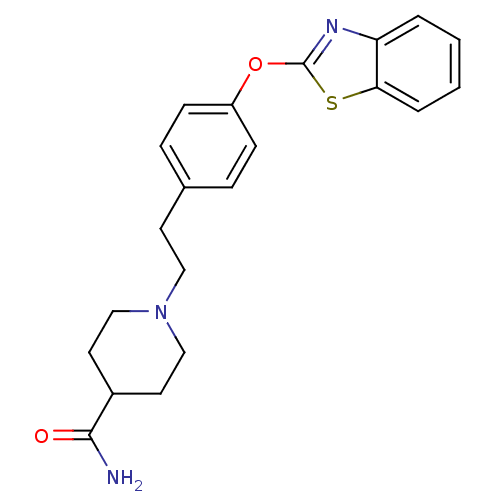
(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}pip...)Show InChI InChI=1S/C21H23N3O2S/c22-20(25)16-10-13-24(14-11-16)12-9-15-5-7-17(8-6-15)26-21-23-18-3-1-2-4-19(18)27-21/h1-8,16H,9-14H2,(H2,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
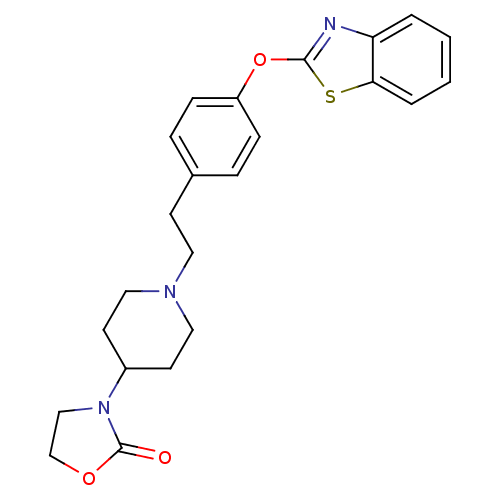
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24228
(3-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}...)Show SMILES O=C1OCCN1C1CCN(CCc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C23H25N3O3S/c27-23-26(15-16-28-23)18-10-13-25(14-11-18)12-9-17-5-7-19(8-6-17)29-22-24-20-3-1-2-4-21(20)30-22/h1-8,18H,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377763

(CHEMBL258454)Show SMILES CNc1nccc(n1)-c1nc([nH]c1-c1cccc(NC(=O)Cc2ccc(Cl)cc2)c1)C(F)(F)F Show InChI InChI=1S/C23H18ClF3N6O/c1-28-22-29-10-9-17(31-22)20-19(32-21(33-20)23(25,26)27)14-3-2-4-16(12-14)30-18(34)11-13-5-7-15(24)8-6-13/h2-10,12H,11H2,1H3,(H,30,34)(H,32,33)(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24213
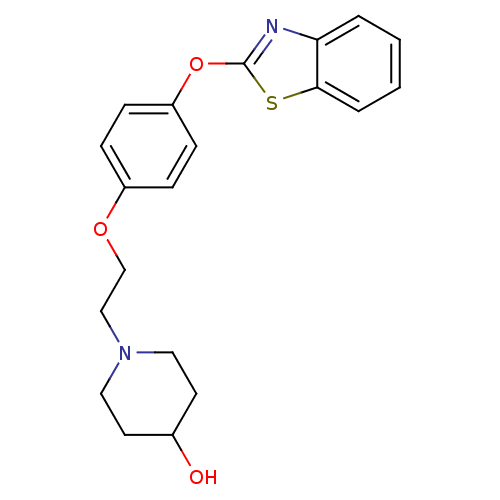
(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl}pi...)Show InChI InChI=1S/C20H22N2O3S/c23-15-9-11-22(12-10-15)13-14-24-16-5-7-17(8-6-16)25-20-21-18-3-1-2-4-19(18)26-20/h1-8,15,23H,9-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377760
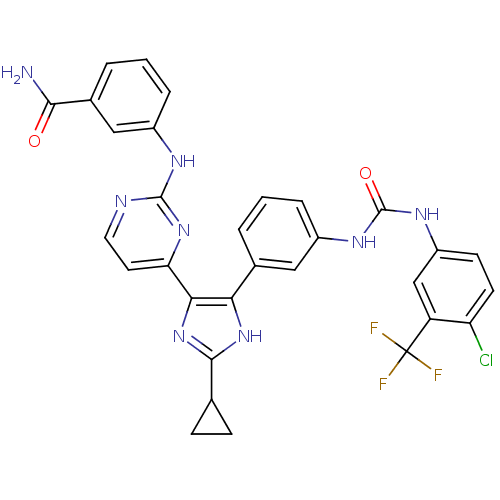
(CHEMBL255496)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c2)C2CC2)c1 Show InChI InChI=1S/C31H24ClF3N8O2/c32-23-10-9-21(15-22(23)31(33,34)35)40-30(45)39-20-6-1-3-17(13-20)25-26(43-28(42-25)16-7-8-16)24-11-12-37-29(41-24)38-19-5-2-4-18(14-19)27(36)44/h1-6,9-16H,7-8H2,(H2,36,44)(H,42,43)(H,37,38,41)(H2,39,40,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24232
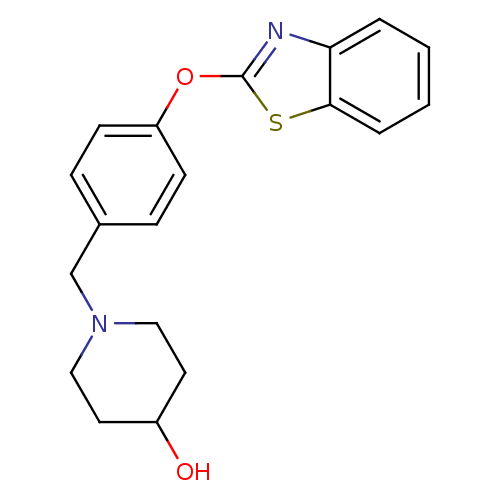
(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...)Show InChI InChI=1S/C19H20N2O2S/c22-15-9-11-21(12-10-15)13-14-5-7-16(8-6-14)23-19-20-17-3-1-2-4-18(17)24-19/h1-8,15,22H,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24229
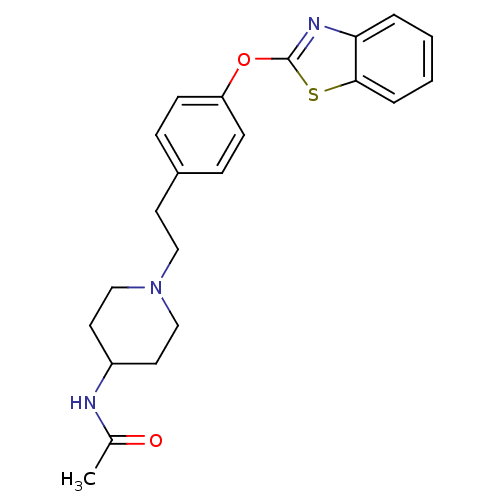
(Benzthiazole compound, 27n | N-(1-{2-[4-(1,3-benzo...)Show SMILES CC(=O)NC1CCN(CCc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C22H25N3O2S/c1-16(26)23-18-11-14-25(15-12-18)13-10-17-6-8-19(9-7-17)27-22-24-20-4-2-3-5-21(20)28-22/h2-9,18H,10-15H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50377760

(CHEMBL255496)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c2)C2CC2)c1 Show InChI InChI=1S/C31H24ClF3N8O2/c32-23-10-9-21(15-22(23)31(33,34)35)40-30(45)39-20-6-1-3-17(13-20)25-26(43-28(42-25)16-7-8-16)24-11-12-37-29(41-24)38-19-5-2-4-18(14-19)27(36)44/h1-6,9-16H,7-8H2,(H2,36,44)(H,42,43)(H,37,38,41)(H2,39,40,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of B-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377759

(CHEMBL255497)Show SMILES Clc1ccc(CS(=O)(=O)Nc2cccc(c2)-c2[nH]c(nc2-c2ccnc(NCCN3CCNC3=O)n2)C2CC2)cc1Cl Show InChI InChI=1S/C28H28Cl2N8O3S/c29-21-7-4-17(14-22(21)30)16-42(40,41)37-20-3-1-2-19(15-20)24-25(36-26(35-24)18-5-6-18)23-8-9-31-27(34-23)32-10-12-38-13-11-33-28(38)39/h1-4,7-9,14-15,18,37H,5-6,10-13,16H2,(H,33,39)(H,35,36)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50377768
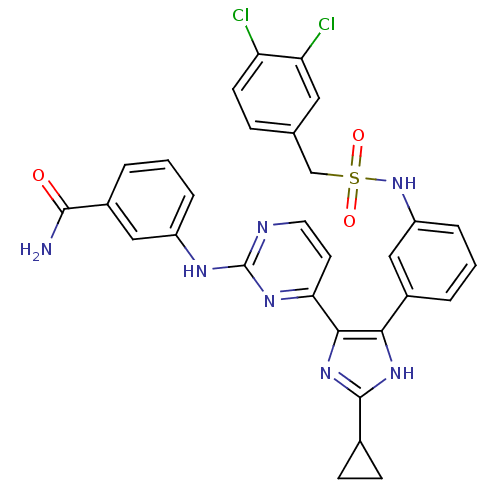
(CHEMBL402424)Show SMILES NC(=O)c1cccc(Nc2nccc(n2)-c2nc([nH]c2-c2cccc(NS(=O)(=O)Cc3ccc(Cl)c(Cl)c3)c2)C2CC2)c1 Show InChI InChI=1S/C30H25Cl2N7O3S/c31-23-10-7-17(13-24(23)32)16-43(41,42)39-22-6-1-3-19(14-22)26-27(38-29(37-26)18-8-9-18)25-11-12-34-30(36-25)35-21-5-2-4-20(15-21)28(33)40/h1-7,10-15,18,39H,8-9,16H2,(H2,33,40)(H,37,38)(H,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of C-RAF kinase |
Bioorg Med Chem Lett 18: 2825-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.002
BindingDB Entry DOI: 10.7270/Q2DJ5GH8 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24230
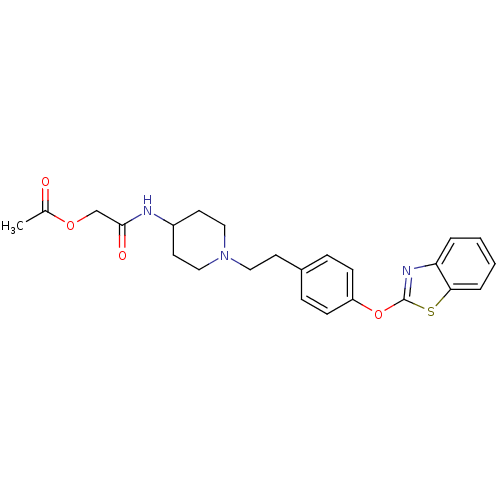
(Benzthiazole compound, 27o | [(1-{2-[4-(1,3-benzot...)Show SMILES CC(=O)OCC(=O)NC1CCN(CCc2ccc(Oc3nc4ccccc4s3)cc2)CC1 Show InChI InChI=1S/C24H27N3O4S/c1-17(28)30-16-23(29)25-19-11-14-27(15-12-19)13-10-18-6-8-20(9-7-18)31-24-26-21-4-2-3-5-22(21)32-24/h2-9,19H,10-16H2,1H3,(H,25,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM24204
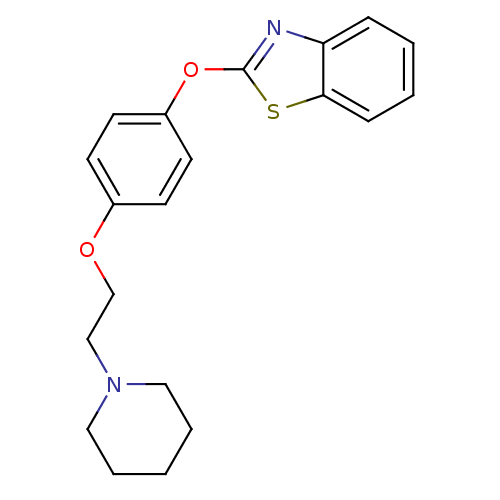
(2-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1,3-benzot...)Show InChI InChI=1S/C20H22N2O2S/c1-4-12-22(13-5-1)14-15-23-16-8-10-17(11-9-16)24-20-21-18-6-2-3-7-19(18)25-20/h2-3,6-11H,1,4-5,12-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical
| Assay Description
Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... |
J Med Chem 51: 4150-69 (2008)
Article DOI: 10.1021/jm701575k
BindingDB Entry DOI: 10.7270/Q2GB22CX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data