 Found 1143 hits with Last Name = 'alonso-galicia' and Initial = 'm'
Found 1143 hits with Last Name = 'alonso-galicia' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50391763

(CHEMBL2146867)Show SMILES CC1CN(CCc2ccc(cc2)[N+]([O-])=O)CCN1CCc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-17-16-22(12-10-18-2-6-20(7-3-18)24(26)27)14-15-23(17)13-11-19-4-8-21(9-5-19)25(28)29/h2-9,17H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG |
ACS Med Chem Lett 3: 367-372 (2012)
Article DOI: 10.1021/ml3000066
BindingDB Entry DOI: 10.7270/Q2MK6DZV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50295535
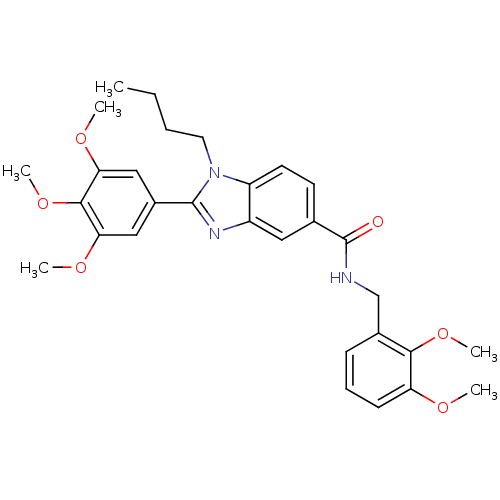
(1-butyl-N-(2,3-dimethoxybenzyl)-2-(3,4,5-trimethox...)Show SMILES CCCCn1c(nc2cc(ccc12)C(=O)NCc1cccc(OC)c1OC)-c1cc(OC)c(OC)c(OC)c1 Show InChI InChI=1S/C30H35N3O6/c1-7-8-14-33-23-13-12-19(30(34)31-18-20-10-9-11-24(35-2)27(20)38-5)15-22(23)32-29(33)21-16-25(36-3)28(39-6)26(17-21)37-4/h9-13,15-17H,7-8,14,18H2,1-6H3,(H,31,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH in HEK293 cells assessed as conversion of 14,15-epoxyeicosatrienoic acid to 14,15-dihydroepoxyeicosatrienoic acid |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268438

(CHEMBL524992 | spiro[chroman-2,4'-piperidine]-1'-y...)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCC2(CC1)CCc1ccccc1O2 Show InChI InChI=1S/C21H20F3NO2/c22-21(23,24)17-7-5-16(6-8-17)19(26)25-13-11-20(12-14-25)10-9-15-3-1-2-4-18(15)27-20/h1-8H,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268439
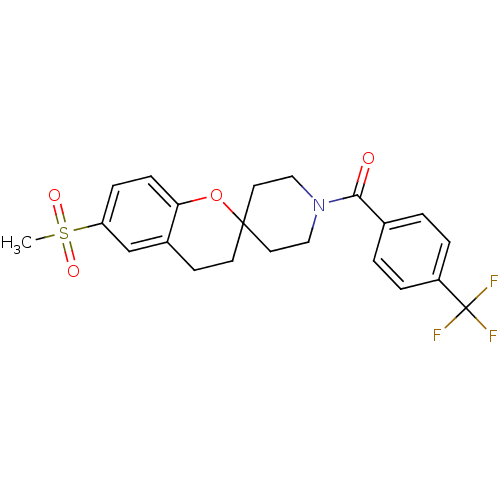
((6-(methylsulfonyl)spiro[chroman-2,4'-piperidine]-...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)c3ccc(cc3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C22H22F3NO4S/c1-31(28,29)18-6-7-19-16(14-18)8-9-21(30-19)10-12-26(13-11-21)20(27)15-2-4-17(5-3-15)22(23,24)25/h2-7,14H,8-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268434

((+/-)-6-(1-methyl-1H-pyrazol-5-yl)-4-oxo-N-((tans)...)Show SMILES Cn1nccc1-c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H28N4O3/c1-30-23(9-12-28-30)19-7-8-25-21(15-19)24(32)17-27(34-25)10-13-31(14-11-27)26(33)29-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22H,10-11,13-14,16-17H2,1H3,(H,29,33)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268435
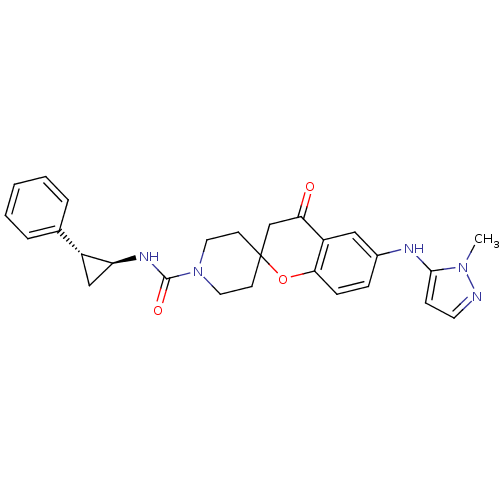
((+/-)-6-(1-methyl-1H-pyrazol-5-ylamino)-4-oxo-N-((...)Show SMILES Cn1nccc1Nc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H29N5O3/c1-31-25(9-12-28-31)29-19-7-8-24-21(15-19)23(33)17-27(35-24)10-13-32(14-11-27)26(34)30-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22,29H,10-11,13-14,16-17H2,1H3,(H,30,34)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310787

(CHEMBL1079360 | N-(4-chlorophenyl)-3-methyl-3-phen...)Show InChI InChI=1S/C19H21ClN2O/c1-19(15-6-3-2-4-7-15)12-5-13-22(14-19)18(23)21-17-10-8-16(20)9-11-17/h2-4,6-11H,5,12-14H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268321
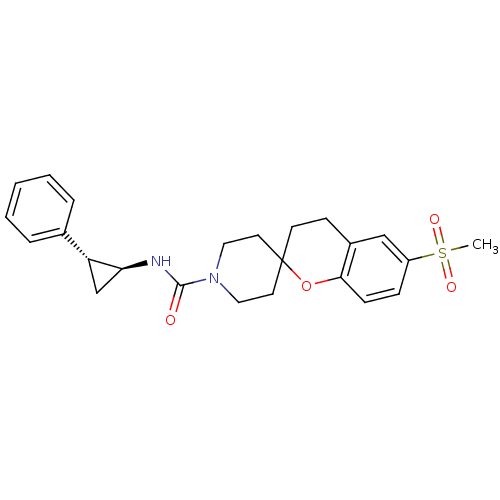
(6-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl)...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C24H28N2O4S/c1-31(28,29)19-7-8-22-18(15-19)9-10-24(30-22)11-13-26(14-12-24)23(27)25-21-16-20(21)17-5-3-2-4-6-17/h2-8,15,20-21H,9-14,16H2,1H3,(H,25,27)/t20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268320

((+/-)-6-fluoro-N-((trans)-2-phenylcyclopropyl)spir...)Show SMILES Fc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C23H25FN2O2/c24-18-6-7-21-17(14-18)8-9-23(28-21)10-12-26(13-11-23)22(27)25-20-15-19(20)16-4-2-1-3-5-16/h1-7,14,19-20H,8-13,15H2,(H,25,27)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268381
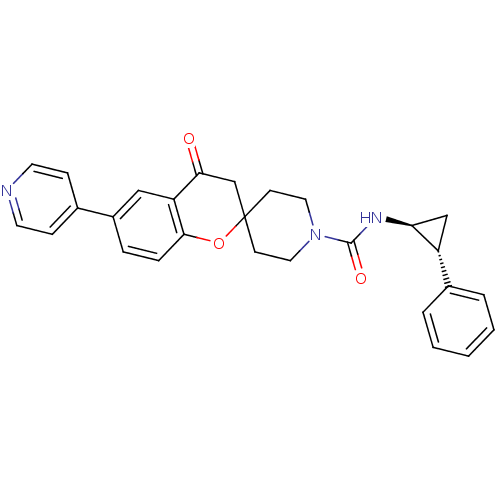
(4-oxo-N-((trans)-2-phenylcyclopropyl)-6-(pyridin-4...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1ccncc1 |r| Show InChI InChI=1S/C28H27N3O3/c32-25-18-28(34-26-7-6-21(16-23(25)26)19-8-12-29-13-9-19)10-14-31(15-11-28)27(33)30-24-17-22(24)20-4-2-1-3-5-20/h1-9,12-13,16,22,24H,10-11,14-15,17-18H2,(H,30,33)/t22-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310818

(3-methyl-3-phenyl-N-((1S,2R)-2-phenylcyclopropyl)p...)Show SMILES CC1(CCCN(C1)C(=O)N[C@H]1C[C@@H]1c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C22H26N2O/c1-22(18-11-6-3-7-12-18)13-8-14-24(16-22)21(25)23-20-15-19(20)17-9-4-2-5-10-17/h2-7,9-12,19-20H,8,13-16H2,1H3,(H,23,25)/t19-,20+,22?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310799
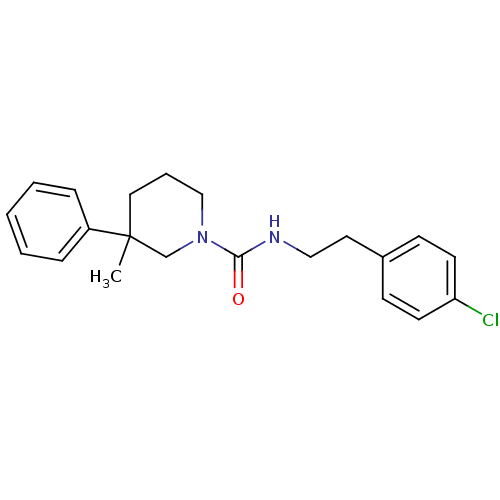
(CHEMBL1078265 | N-(4-chlorophenethyl)-3-methyl-3-p...)Show InChI InChI=1S/C21H25ClN2O/c1-21(18-6-3-2-4-7-18)13-5-15-24(16-21)20(25)23-14-12-17-8-10-19(22)11-9-17/h2-4,6-11H,5,12-16H2,1H3,(H,23,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310797

(3-methyl-3-phenyl-N-(4-(pyridin-2-yl)benzyl)piperi...)Show SMILES CC1(CCCN(C1)C(=O)NCc1ccc(cc1)-c1ccccn1)c1ccccc1 Show InChI InChI=1S/C25H27N3O/c1-25(22-8-3-2-4-9-22)15-7-17-28(19-25)24(29)27-18-20-11-13-21(14-12-20)23-10-5-6-16-26-23/h2-6,8-14,16H,7,15,17-19H2,1H3,(H,27,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310792

(CHEMBL1079290 | N-(4-chlorophenyl)-3-(2-hydroxyeth...)Show InChI InChI=1S/C20H23ClN2O2/c21-17-7-9-18(10-8-17)22-19(25)23-13-4-11-20(15-23,12-14-24)16-5-2-1-3-6-16/h1-3,5-10,24H,4,11-15H2,(H,22,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268384

(CHEMBL522211 | N-((trans)-2-phenylcyclopropyl)-3',...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ncccc1O2 |r| Show InChI InChI=1S/C22H25N3O2/c26-21(24-19-15-17(19)16-5-2-1-3-6-16)25-13-10-22(11-14-25)9-8-18-20(27-22)7-4-12-23-18/h1-7,12,17,19H,8-11,13-15H2,(H,24,26)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310793

(CHEMBL1079291 | N-(4-chlorophenyl)-3-(3-hydroxypro...)Show InChI InChI=1S/C21H25ClN2O2/c22-18-8-10-19(11-9-18)23-20(26)24-14-4-12-21(16-24,13-5-15-25)17-6-2-1-3-7-17/h1-3,6-11,25H,4-5,12-16H2,(H,23,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310804
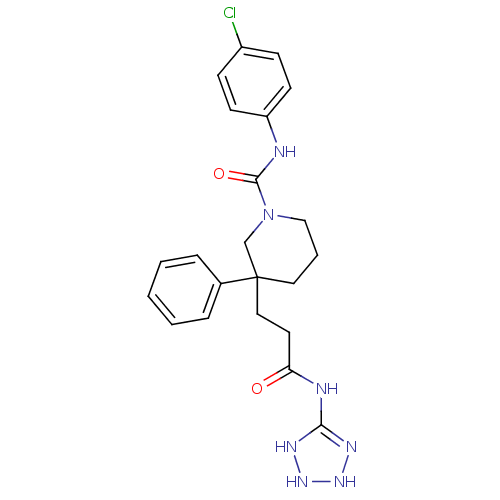
(CHEMBL1079245 | N-(4-chlorophenyl)-3-(3-(2,3-dihyd...)Show SMILES Clc1ccc(NC(=O)N2CCCC(CCC(=O)NC3=NNNN3)(C2)c2ccccc2)cc1 |t:18| Show InChI InChI=1S/C22H26ClN7O2/c23-17-7-9-18(10-8-17)24-21(32)30-14-4-12-22(15-30,16-5-2-1-3-6-16)13-11-19(31)25-20-26-28-29-27-20/h1-3,5-10,28-29H,4,11-15H2,(H,24,32)(H2,25,26,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268439

((6-(methylsulfonyl)spiro[chroman-2,4'-piperidine]-...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)c3ccc(cc3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C22H22F3NO4S/c1-31(28,29)18-6-7-19-16(14-18)8-9-21(30-19)10-12-26(13-11-21)20(27)15-2-4-17(5-3-15)22(23,24)25/h2-7,14H,8-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268441
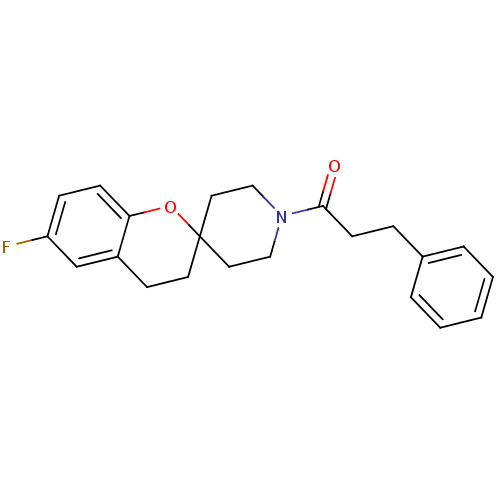
(1-(6-fluorospiro[chroman-2,4'-piperidine]-1'-yl)-3...)Show InChI InChI=1S/C22H24FNO2/c23-19-7-8-20-18(16-19)10-11-22(26-20)12-14-24(15-13-22)21(25)9-6-17-4-2-1-3-5-17/h1-5,7-8,16H,6,9-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268321

(6-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl)...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C24H28N2O4S/c1-31(28,29)19-7-8-22-18(15-19)9-10-24(30-22)11-13-26(14-12-24)23(27)25-21-16-20(21)17-5-3-2-4-6-17/h2-8,15,20-21H,9-14,16H2,1H3,(H,25,27)/t20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268322
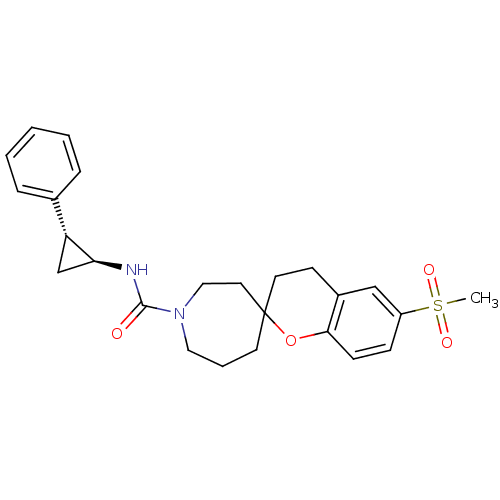
(6'-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCc2c1)CCCN(CC3)C(=O)N[C@H]1C[C@@H]1c1ccccc1 |r| Show InChI InChI=1S/C25H30N2O4S/c1-32(29,30)20-8-9-23-19(16-20)10-12-25(31-23)11-5-14-27(15-13-25)24(28)26-22-17-21(22)18-6-3-2-4-7-18/h2-4,6-9,16,21-22H,5,10-15,17H2,1H3,(H,26,28)/t21-,22+,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310794
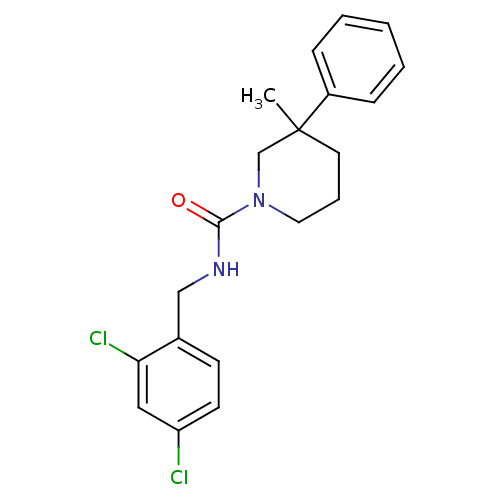
(CHEMBL1080973 | N-(2,4-dichlorobenzyl)-3-methyl-3-...)Show InChI InChI=1S/C20H22Cl2N2O/c1-20(16-6-3-2-4-7-16)10-5-11-24(14-20)19(25)23-13-15-8-9-17(21)12-18(15)22/h2-4,6-9,12H,5,10-11,13-14H2,1H3,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380
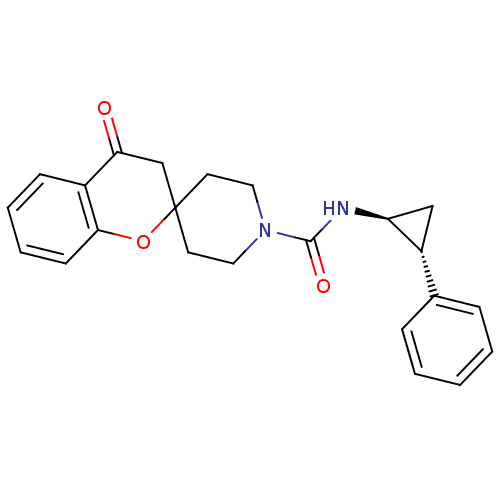
((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310797

(3-methyl-3-phenyl-N-(4-(pyridin-2-yl)benzyl)piperi...)Show SMILES CC1(CCCN(C1)C(=O)NCc1ccc(cc1)-c1ccccn1)c1ccccc1 Show InChI InChI=1S/C25H27N3O/c1-25(22-8-3-2-4-9-22)15-7-17-28(19-25)24(29)27-18-20-11-13-21(14-12-20)23-10-5-6-16-26-23/h2-6,8-14,16H,7,15,17-19H2,1H3,(H,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310791
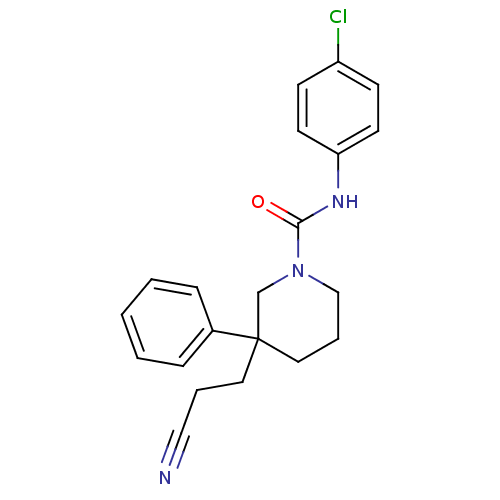
(CHEMBL1078755 | N-(4-chlorophenyl)-3-(2-cyanoethyl...)Show SMILES Clc1ccc(NC(=O)N2CCCC(CCC#N)(C2)c2ccccc2)cc1 Show InChI InChI=1S/C21H22ClN3O/c22-18-8-10-19(11-9-18)24-20(26)25-15-5-13-21(16-25,12-4-14-23)17-6-2-1-3-7-17/h1-3,6-11H,4-5,12-13,15-16H2,(H,24,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268435

((+/-)-6-(1-methyl-1H-pyrazol-5-ylamino)-4-oxo-N-((...)Show SMILES Cn1nccc1Nc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H29N5O3/c1-31-25(9-12-28-31)29-19-7-8-24-21(15-19)23(33)17-27(35-24)10-13-32(14-11-27)26(34)30-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22,29H,10-11,13-14,16-17H2,1H3,(H,30,34)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310822
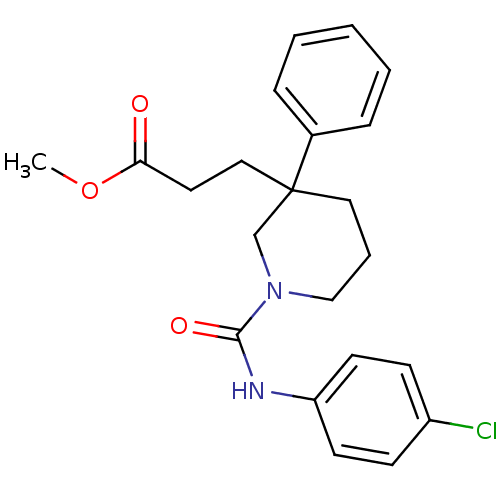
(CHEMBL1078754 | methyl 3-(1-(4-chlorophenylcarbamo...)Show SMILES COC(=O)CCC1(CCCN(C1)C(=O)Nc1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C22H25ClN2O3/c1-28-20(26)12-14-22(17-6-3-2-4-7-17)13-5-15-25(16-22)21(27)24-19-10-8-18(23)9-11-19/h2-4,6-11H,5,12-16H2,1H3,(H,24,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268320

((+/-)-6-fluoro-N-((trans)-2-phenylcyclopropyl)spir...)Show SMILES Fc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C23H25FN2O2/c24-18-6-7-21-17(14-18)8-9-23(28-21)10-12-26(13-11-23)22(27)25-20-15-19(20)16-4-2-1-3-5-16/h1-7,14,19-20H,8-13,15H2,(H,25,27)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50310806
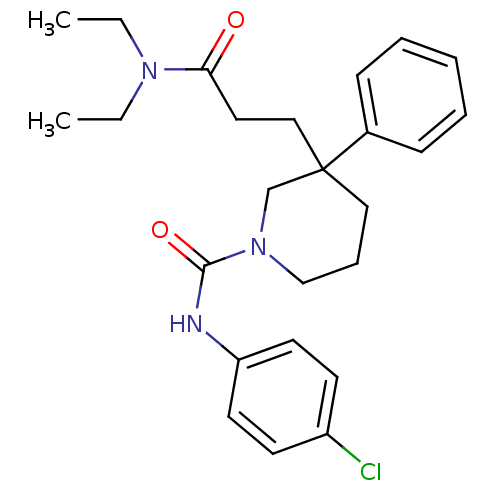
(CHEMBL1078628 | N-(4-chlorophenyl)-3-(3-(diethylam...)Show SMILES CCN(CC)C(=O)CCC1(CCCN(C1)C(=O)Nc1ccc(Cl)cc1)c1ccccc1 Show InChI InChI=1S/C25H32ClN3O2/c1-3-28(4-2)23(30)15-17-25(20-9-6-5-7-10-20)16-8-18-29(19-25)24(31)27-22-13-11-21(26)12-14-22/h5-7,9-14H,3-4,8,15-19H2,1-2H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50391764

(CHEMBL2146865)Show SMILES CC(CN1CCN(CCc2ccc(cc2)[N+]([O-])=O)CC1)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C21H26N4O4/c1-17(19-4-8-21(9-5-19)25(28)29)16-23-14-12-22(13-15-23)11-10-18-2-6-20(7-3-18)24(26)27/h2-9,17H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG |
ACS Med Chem Lett 3: 367-372 (2012)
Article DOI: 10.1021/ml3000066
BindingDB Entry DOI: 10.7270/Q2MK6DZV |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50391760
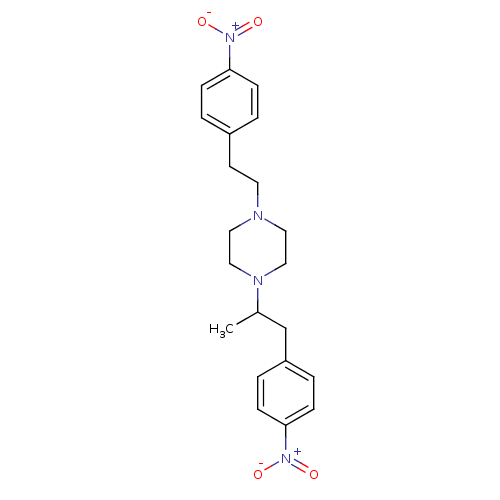
(CHEMBL2146866)Show SMILES CC(Cc1ccc(cc1)[N+]([O-])=O)N1CCN(CCc2ccc(cc2)[N+]([O-])=O)CC1 Show InChI InChI=1S/C21H26N4O4/c1-17(16-19-4-8-21(9-5-19)25(28)29)23-14-12-22(13-15-23)11-10-18-2-6-20(7-3-18)24(26)27/h2-9,17H,10-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG |
ACS Med Chem Lett 3: 367-372 (2012)
Article DOI: 10.1021/ml3000066
BindingDB Entry DOI: 10.7270/Q2MK6DZV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268437

((+/-)-N-((trans)-2-phenylcyclopropyl)spiro[chroman...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ccccc1O2 |r| Show InChI InChI=1S/C23H26N2O2/c26-22(24-20-16-19(20)17-6-2-1-3-7-17)25-14-12-23(13-15-25)11-10-18-8-4-5-9-21(18)27-23/h1-9,19-20H,10-16H2,(H,24,26)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268438

(CHEMBL524992 | spiro[chroman-2,4'-piperidine]-1'-y...)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCC2(CC1)CCc1ccccc1O2 Show InChI InChI=1S/C21H20F3NO2/c22-21(23,24)17-7-5-16(6-8-17)19(26)25-13-11-20(12-14-25)10-9-15-3-1-2-4-18(15)27-20/h1-8H,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310818

(3-methyl-3-phenyl-N-((1S,2R)-2-phenylcyclopropyl)p...)Show SMILES CC1(CCCN(C1)C(=O)N[C@H]1C[C@@H]1c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C22H26N2O/c1-22(18-11-6-3-7-12-18)13-8-14-24(16-22)21(25)23-20-15-19(20)17-9-4-2-5-10-17/h2-7,9-12,19-20H,8,13-16H2,1H3,(H,23,25)/t19-,20+,22?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50301869

(7-(2-(trifluoromethyl)phenyl)-N-(4-(trifluoromethy...)Show SMILES FC(F)(F)c1ccc(Nc2noc3c(cccc23)-c2ccccc2C(F)(F)F)cc1 Show InChI InChI=1S/C21H12F6N2O/c22-20(23,24)12-8-10-13(11-9-12)28-19-16-6-3-5-15(18(16)30-29-19)14-4-1-2-7-17(14)21(25,26)27/h1-11H,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
Bioorg Med Chem Lett 19: 5716-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.006
BindingDB Entry DOI: 10.7270/Q2Z0388B |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50301856
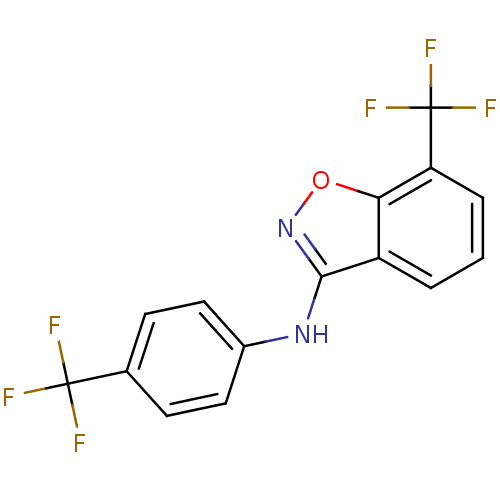
(7-(trifluoromethyl)-N-(4-(trifluoromethyl)phenyl)b...)Show InChI InChI=1S/C15H8F6N2O/c16-14(17,18)8-4-6-9(7-5-8)22-13-10-2-1-3-11(15(19,20)21)12(10)24-23-13/h1-7H,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
Bioorg Med Chem Lett 19: 5716-21 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.006
BindingDB Entry DOI: 10.7270/Q2Z0388B |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
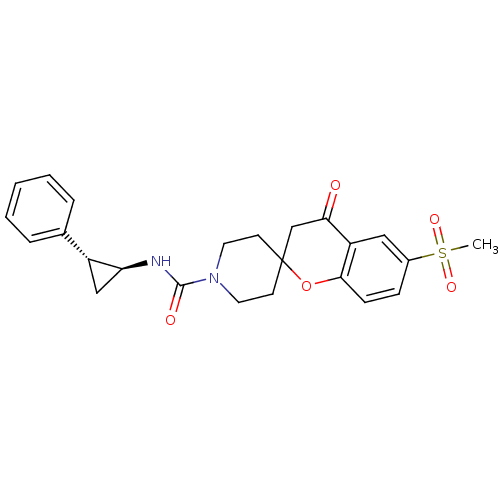
(Homo sapiens (Human)) | BDBM50268436
((+/-)-6-(methylsulfonyl)-4-oxo-N-((trans)-2-phenyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C24H26N2O5S/c1-32(29,30)17-7-8-22-19(13-17)21(27)15-24(31-22)9-11-26(12-10-24)23(28)25-20-14-18(20)16-5-3-2-4-6-16/h2-8,13,18,20H,9-12,14-15H2,1H3,(H,25,28)/t18-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268434

((+/-)-6-(1-methyl-1H-pyrazol-5-yl)-4-oxo-N-((tans)...)Show SMILES Cn1nccc1-c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H28N4O3/c1-30-23(9-12-28-30)19-7-8-25-21(15-19)24(32)17-27(34-25)10-13-31(14-11-27)26(33)29-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22H,10-11,13-14,16-17H2,1H3,(H,29,33)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
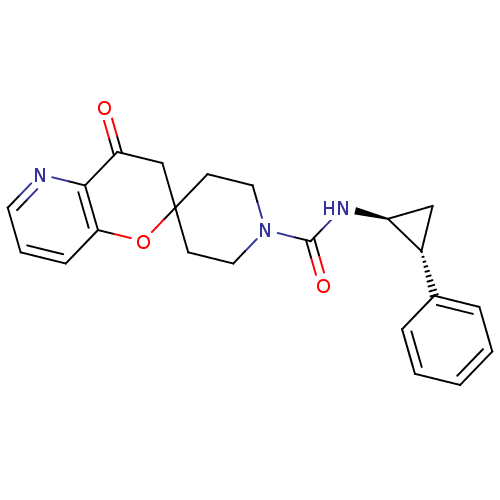
(Homo sapiens (Human)) | BDBM50268382
(4'-oxo-N-((trans)-2-phenylcyclopropyl)-3',4'-dihyd...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ncccc1O2 |r| Show InChI InChI=1S/C22H23N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-17H,8-9,11-14H2,(H,24,27)/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
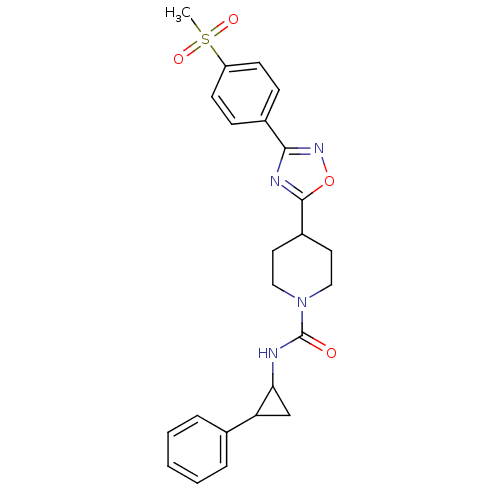
(Homo sapiens (Human)) | BDBM50295541
(4-(3-(4-(methylsulfonyl)phenyl)-1,2,4-oxadiazol-5-...)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1noc(n1)C1CCN(CC1)C(=O)NC1CC1c1ccccc1 Show InChI InChI=1S/C24H26N4O4S/c1-33(30,31)19-9-7-17(8-10-19)22-26-23(32-27-22)18-11-13-28(14-12-18)24(29)25-21-15-20(21)16-5-3-2-4-6-16/h2-10,18,20-21H,11-15H2,1H3,(H,25,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
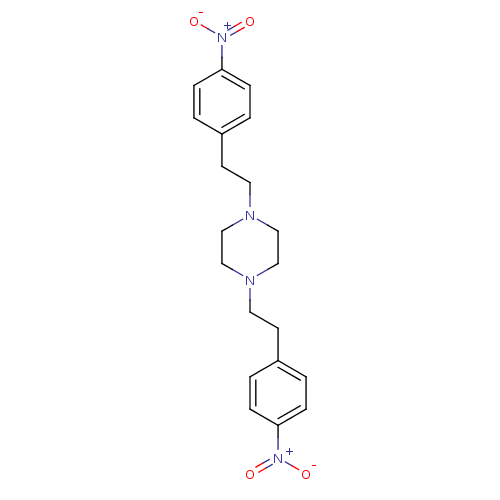
(Homo sapiens (Human)) | BDBM50391770
(CHEMBL2146755 | US9073882, 1)Show SMILES [O-][N+](=O)c1ccc(CCN2CCN(CCc3ccc(cc3)[N+]([O-])=O)CC2)cc1 Show InChI InChI=1S/C20H24N4O4/c25-23(26)19-5-1-17(2-6-19)9-11-21-13-15-22(16-14-21)12-10-18-3-7-20(8-4-18)24(27)28/h1-8H,9-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK499 from human ERG |
ACS Med Chem Lett 3: 367-372 (2012)
Article DOI: 10.1021/ml3000066
BindingDB Entry DOI: 10.7270/Q2MK6DZV |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM25737

(12-[(adamantan-1-ylcarbamoyl)amino]dodecanoic acid...)Show SMILES OC(=O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:25:20:27:24.23.26,25:24:20.21.19:27,THB:23:22:19:24.25.26,23:24:19:22.21.27| Show InChI InChI=1S/C23H40N2O3/c26-21(27)10-8-6-4-2-1-3-5-7-9-11-24-22(28)25-23-15-18-12-19(16-23)14-20(13-18)17-23/h18-20H,1-17H2,(H,26,27)(H2,24,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat sEH |
J Med Chem 52: 5009-12 (2010)
Article DOI: 10.1021/jm900725r
BindingDB Entry DOI: 10.7270/Q2TX3G8W |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310787

(CHEMBL1079360 | N-(4-chlorophenyl)-3-methyl-3-phen...)Show InChI InChI=1S/C19H21ClN2O/c1-19(15-6-3-2-4-7-15)12-5-13-22(14-19)18(23)21-17-10-8-16(20)9-11-17/h2-4,6-11H,5,12-14H2,1H3,(H,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50310799

(CHEMBL1078265 | N-(4-chlorophenethyl)-3-methyl-3-p...)Show InChI InChI=1S/C21H25ClN2O/c1-21(18-6-3-2-4-7-18)13-5-15-24(16-21)20(25)23-14-12-17-8-10-19(22)11-9-17/h2-4,6-11H,5,12-16H2,1H3,(H,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 5314-20 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.138
BindingDB Entry DOI: 10.7270/Q2S75GFP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data