 Found 306 hits with Last Name = 'angiolini' and Initial = 'm'
Found 306 hits with Last Name = 'angiolini' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50314074
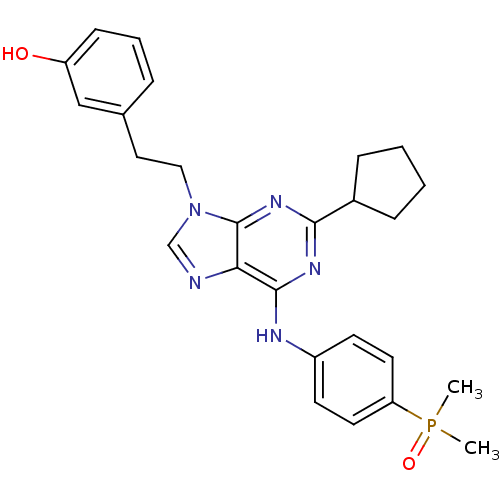
(2,6,9-Trisubstitute purine, 6 (AP23464) | 3-(2-(2-...)Show SMILES CP(C)(=O)c1ccc(Nc2nc(nc3n(CCc4cccc(O)c4)cnc23)C2CCCC2)cc1 Show InChI InChI=1S/C26H30N5O2P/c1-34(2,33)22-12-10-20(11-13-22)28-25-23-26(30-24(29-25)19-7-3-4-8-19)31(17-27-23)15-14-18-6-5-9-21(32)16-18/h5-6,9-13,16-17,19,32H,3-4,7-8,14-15H2,1-2H3,(H,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of SRC |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50236362

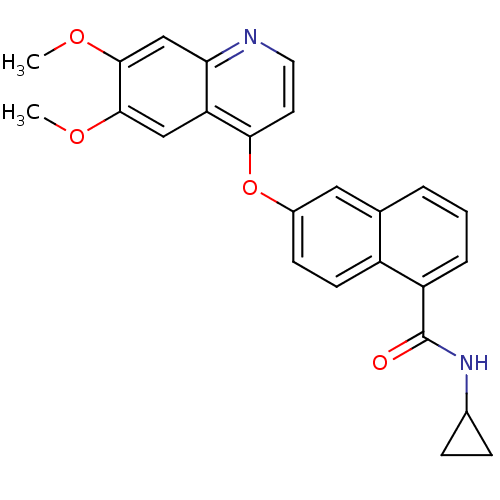
(CHEMBL429743 | N-(4-chlorophenyl)-6-(6,7-dimethoxy...)Show SMILES COc1cc2nccc(Oc3ccc4c(cccc4c3)C(=O)Nc3ccc(Cl)cc3)c2cc1OC Show InChI InChI=1S/C28H21ClN2O4/c1-33-26-15-23-24(16-27(26)34-2)30-13-12-25(23)35-20-10-11-21-17(14-20)4-3-5-22(21)28(32)31-19-8-6-18(29)7-9-19/h3-16H,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50236856
(CHEMBL272198 | N-cyclopropyl-6-(6,7-dimethoxyquino...)Show SMILES COc1cc2nccc(Oc3ccc4c(cccc4c3)C(=O)NC3CC3)c2cc1OC Show InChI InChI=1S/C25H22N2O4/c1-29-23-13-20-21(14-24(23)30-2)26-11-10-22(20)31-17-8-9-18-15(12-17)4-3-5-19(18)25(28)27-16-6-7-16/h3-5,8-14,16H,6-7H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149692

(US8975267, 34)Show SMILES CSc1ncc2ccc3c(cn([C@@H]4CC[C@H](N)CC4)c3c2n1)C(N)=O |wU:15.15,12.11,(6.59,1.87,;5.05,1.92,;4.24,.61,;4.97,-.75,;4.16,-2.06,;2.62,-2.01,;1.81,-3.32,;.27,-3.27,;-.46,-1.92,;-1.96,-1.55,;-2.07,-.01,;-.64,.57,;-.28,2.07,;1.2,2.49,;1.57,3.99,;.46,5.06,;.82,6.55,;-1.02,4.63,;-1.39,3.13,;.35,-.61,;1.89,-.65,;2.7,.65,;-3.13,-2.54,;-2.86,-4.06,;-4.58,-2.02,)| Show InChI InChI=1S/C18H21N5OS/c1-25-18-21-8-10-2-7-13-14(17(20)24)9-23(16(13)15(10)22-18)12-5-3-11(19)4-6-12/h2,7-9,11-12H,3-6,19H2,1H3,(H2,20,24)/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149692

(US8975267, 34)Show SMILES CSc1ncc2ccc3c(cn([C@@H]4CC[C@H](N)CC4)c3c2n1)C(N)=O |wU:15.15,12.11,(6.59,1.87,;5.05,1.92,;4.24,.61,;4.97,-.75,;4.16,-2.06,;2.62,-2.01,;1.81,-3.32,;.27,-3.27,;-.46,-1.92,;-1.96,-1.55,;-2.07,-.01,;-.64,.57,;-.28,2.07,;1.2,2.49,;1.57,3.99,;.46,5.06,;.82,6.55,;-1.02,4.63,;-1.39,3.13,;.35,-.61,;1.89,-.65,;2.7,.65,;-3.13,-2.54,;-2.86,-4.06,;-4.58,-2.02,)| Show InChI InChI=1S/C18H21N5OS/c1-25-18-21-8-10-2-7-13-14(17(20)24)9-23(16(13)15(10)22-18)12-5-3-11(19)4-6-12/h2,7-9,11-12H,3-6,19H2,1H3,(H2,20,24)/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50321580
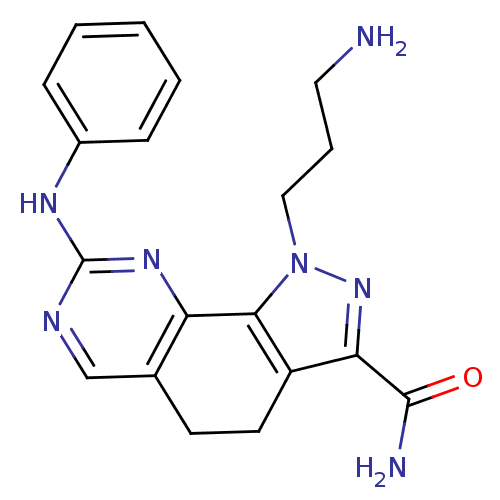
(1-(3-aminopropyl)-8-(phenylamino)-4,5-dihydro-1H-p...)Show SMILES NCCCn1nc(C(N)=O)c2CCc3cnc(Nc4ccccc4)nc3-c12 Show InChI InChI=1S/C19H21N7O/c20-9-4-10-26-17-14(16(25-26)18(21)27)8-7-12-11-22-19(24-15(12)17)23-13-5-2-1-3-6-13/h1-3,5-6,11H,4,7-10,20H2,(H2,21,27)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 4095-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.070
BindingDB Entry DOI: 10.7270/Q2NP25C5 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31532
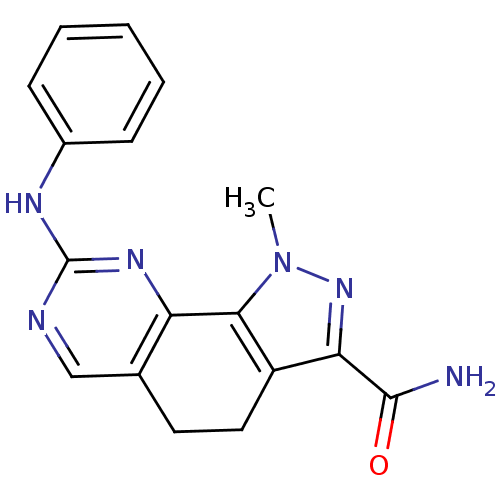
(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50318085
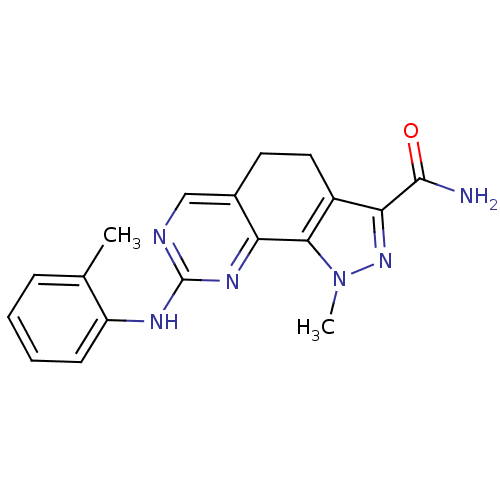
(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149689
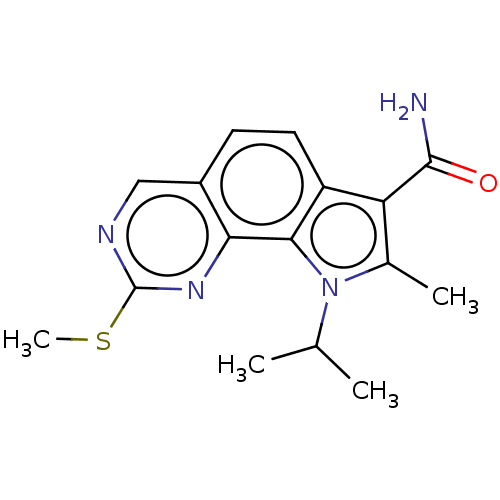
(US8975267, 30)Show InChI InChI=1S/C16H18N4OS/c1-8(2)20-9(3)12(15(17)21)11-6-5-10-7-18-16(22-4)19-13(10)14(11)20/h5-8H,1-4H3,(H2,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149691
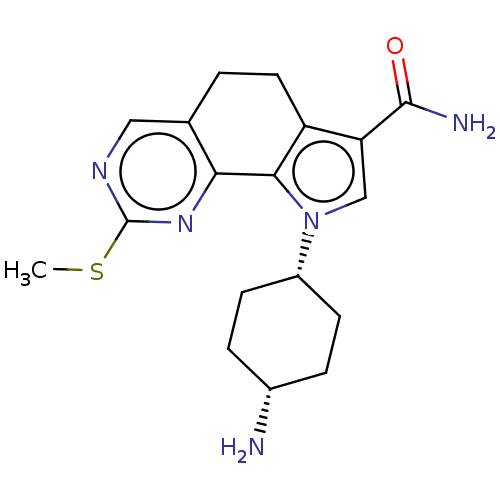
(US8975267, 33)Show SMILES CSc1ncc2CCc3c(cn([C@@H]4CC[C@H](N)CC4)c3-c2n1)C(N)=O |wU:15.15,12.11,(6.59,1.87,;5.05,1.92,;4.24,.61,;4.97,-.75,;4.16,-2.06,;2.62,-2.01,;1.81,-3.32,;.27,-3.27,;-.46,-1.92,;-1.96,-1.55,;-2.07,-.01,;-.64,.57,;-.28,2.07,;1.2,2.49,;1.57,3.99,;.46,5.06,;.82,6.55,;-1.02,4.63,;-1.39,3.13,;.35,-.61,;1.89,-.65,;2.7,.65,;-3.13,-2.54,;-2.86,-4.06,;-4.58,-2.02,)| Show InChI InChI=1S/C18H23N5OS/c1-25-18-21-8-10-2-7-13-14(17(20)24)9-23(16(13)15(10)22-18)12-5-3-11(19)4-6-12/h8-9,11-12H,2-7,19H2,1H3,(H2,20,24)/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM50204694
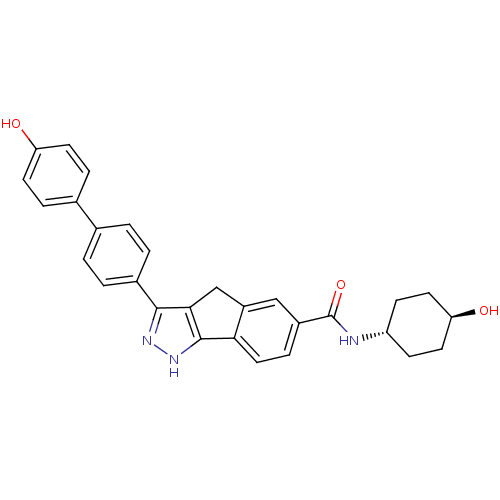
(3-(4'-HYDROXYBIPHENYL-4-YL)-N-(4-HYDROXYCYCLOHEXYL...)Show SMILES O[C@H]1CC[C@@H](CC1)NC(=O)c1ccc-2c(Cc3c(n[nH]c-23)-c2ccc(cc2)-c2ccc(O)cc2)c1 |wU:4.7,wD:1.0,(19.77,-1.9,;21.02,-1.01,;22.42,-1.66,;23.69,-.77,;23.53,.76,;22.14,1.41,;20.89,.52,;24.79,1.65,;26.19,1.01,;26.33,-.53,;27.44,1.9,;27.3,3.44,;28.55,4.33,;29.95,3.7,;30.1,2.15,;31.61,1.81,;32.4,3.14,;33.83,3.77,;33.68,5.31,;32.16,5.65,;31.37,4.31,;35.16,2.99,;35.15,1.44,;36.46,.66,;37.81,1.42,;37.82,2.96,;36.49,3.74,;39.13,.64,;39.12,-.9,;40.44,-1.67,;41.78,-.91,;43.11,-1.69,;41.78,.63,;40.46,1.4,;28.85,1.26,)| Show InChI InChI=1S/C29H27N3O3/c33-23-10-5-18(6-11-23)17-1-3-19(4-2-17)27-26-16-21-15-20(7-14-25(21)28(26)32-31-27)29(35)30-22-8-12-24(34)13-9-22/h1-7,10-11,14-15,22,24,33-34H,8-9,12-13,16H2,(H,30,35)(H,31,32)/t22-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149693
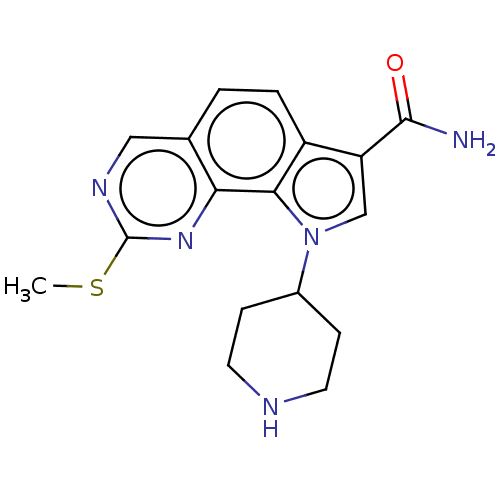
(US8975267, 35)Show InChI InChI=1S/C17H19N5OS/c1-24-17-20-8-10-2-3-12-13(16(18)23)9-22(15(12)14(10)21-17)11-4-6-19-7-5-11/h2-3,8-9,11,19H,4-7H2,1H3,(H2,18,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149691

(US8975267, 33)Show SMILES CSc1ncc2CCc3c(cn([C@@H]4CC[C@H](N)CC4)c3-c2n1)C(N)=O |wU:15.15,12.11,(6.59,1.87,;5.05,1.92,;4.24,.61,;4.97,-.75,;4.16,-2.06,;2.62,-2.01,;1.81,-3.32,;.27,-3.27,;-.46,-1.92,;-1.96,-1.55,;-2.07,-.01,;-.64,.57,;-.28,2.07,;1.2,2.49,;1.57,3.99,;.46,5.06,;.82,6.55,;-1.02,4.63,;-1.39,3.13,;.35,-.61,;1.89,-.65,;2.7,.65,;-3.13,-2.54,;-2.86,-4.06,;-4.58,-2.02,)| Show InChI InChI=1S/C18H23N5OS/c1-25-18-21-8-10-2-7-13-14(17(20)24)9-23(16(13)15(10)22-18)12-5-3-11(19)4-6-12/h8-9,11-12H,2-7,19H2,1H3,(H2,20,24)/t11-,12+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50321578
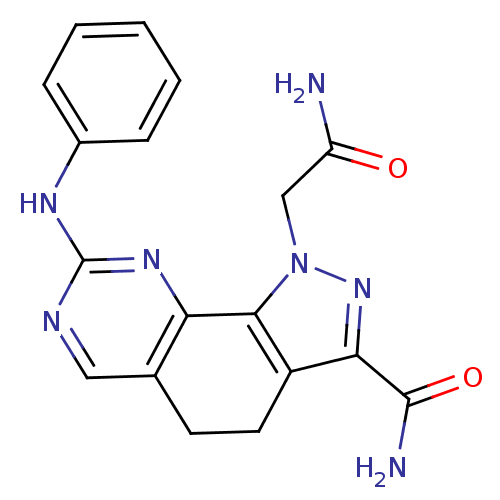
(1-(2-amino-2-oxoethyl)-8-(phenylamino)-4,5-dihydro...)Show SMILES NC(=O)Cn1nc(C(N)=O)c2CCc3cnc(Nc4ccccc4)nc3-c12 Show InChI InChI=1S/C18H17N7O2/c19-13(26)9-25-16-12(15(24-25)17(20)27)7-6-10-8-21-18(23-14(10)16)22-11-4-2-1-3-5-11/h1-5,8H,6-7,9H2,(H2,19,26)(H2,20,27)(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 4095-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.070
BindingDB Entry DOI: 10.7270/Q2NP25C5 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50321579
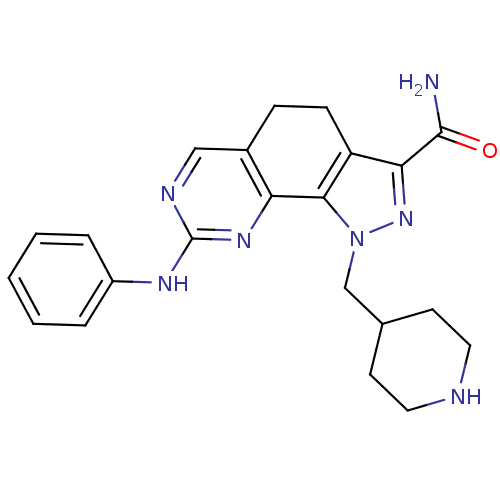
(8-(phenylamino)-1-(piperidin-4-ylmethyl)-4,5-dihyd...)Show SMILES NC(=O)c1nn(CC2CCNCC2)c-2c1CCc1cnc(Nc3ccccc3)nc-21 Show InChI InChI=1S/C22H25N7O/c23-21(30)19-17-7-6-15-12-25-22(26-16-4-2-1-3-5-16)27-18(15)20(17)29(28-19)13-14-8-10-24-11-9-14/h1-5,12,14,24H,6-11,13H2,(H2,23,30)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2 |
Bioorg Med Chem Lett 20: 4095-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.070
BindingDB Entry DOI: 10.7270/Q2NP25C5 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149689

(US8975267, 30)Show InChI InChI=1S/C16H18N4OS/c1-8(2)20-9(3)12(15(17)21)11-6-5-10-7-18-16(22-4)19-13(10)14(11)20/h5-8H,1-4H3,(H2,17,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149694
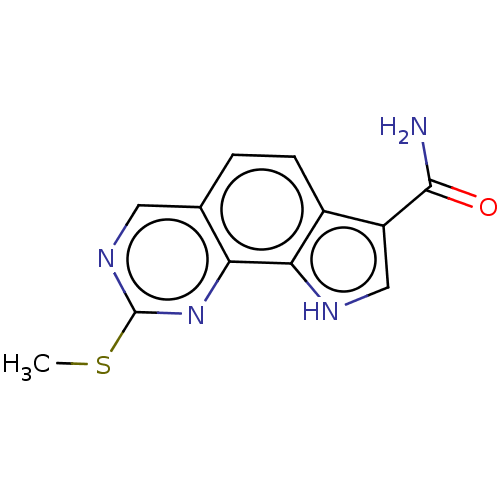
(US8975267, 36)Show InChI InChI=1S/C12H10N4OS/c1-18-12-15-4-6-2-3-7-8(11(13)17)5-14-10(7)9(6)16-12/h2-5,14H,1H3,(H2,13,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50318085

(1-Methyl-8-[(2-methylphenyl)amino]-4,5-dihydro-1H-...)Show InChI InChI=1S/C18H18N6O/c1-10-5-3-4-6-13(10)21-18-20-9-11-7-8-12-15(17(19)25)23-24(2)16(12)14(11)22-18/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149686

(US8975267, 22)Show InChI InChI=1S/C15H18N4OS/c1-8(2)19-7-11(14(16)20)10-5-4-9-6-17-15(21-3)18-12(9)13(10)19/h6-8H,4-5H2,1-3H3,(H2,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50290019
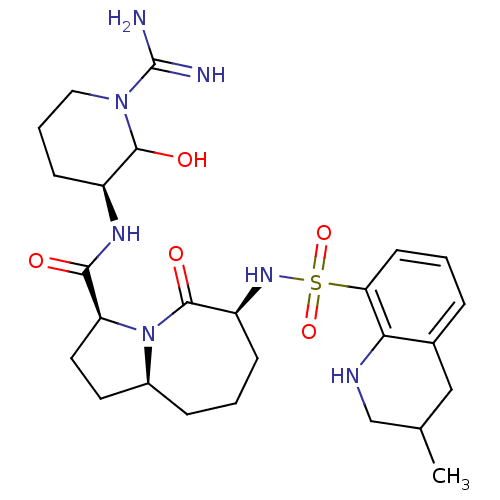
((3S,6S,9aS)-6-(3-Methyl-1,2,3,4-tetrahydro-quinoli...)Show SMILES CC1CNc2c(C1)cccc2S(=O)(=O)N[C@H]1CCC[C@H]2CC[C@H](N2C1=O)C(=O)N[C@H]1CCCN(C1O)C(N)=N |r| Show InChI InChI=1S/C26H39N7O5S/c1-15-13-16-5-2-9-21(22(16)29-14-15)39(37,38)31-19-7-3-6-17-10-11-20(33(17)25(19)36)23(34)30-18-8-4-12-32(24(18)35)26(27)28/h2,5,9,15,17-20,24,29,31,35H,3-4,6-8,10-14H2,1H3,(H3,27,28)(H,30,34)/t15?,17-,18-,19-,20-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit the activity of human alpha Thrombin |
Bioorg Med Chem Lett 7: 2205-2210 (1997)
Article DOI: 10.1016/S0960-894X(97)00403-4
BindingDB Entry DOI: 10.7270/Q21G0MR2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349092
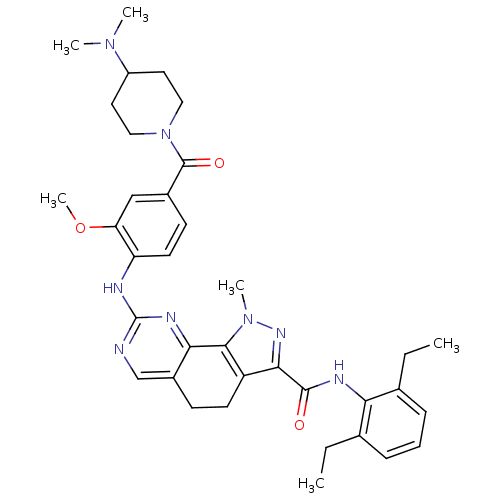
(CHEMBL1807303)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCC(CC3)N(C)C)nc-21 Show InChI InChI=1S/C36H44N8O3/c1-7-22-10-9-11-23(8-2)30(22)39-34(45)32-27-14-12-25-21-37-36(40-31(25)33(27)43(5)41-32)38-28-15-13-24(20-29(28)47-6)35(46)44-18-16-26(17-19-44)42(3)4/h9-11,13,15,20-21,26H,7-8,12,14,16-19H2,1-6H3,(H,39,45)(H,37,38,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM31533

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 16)Show InChI InChI=1S/C18H18N6O/c1-19-17(25)15-13-9-8-11-10-20-18(21-12-6-4-3-5-7-12)22-14(11)16(13)24(2)23-15/h3-7,10H,8-9H2,1-2H3,(H,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Serine protease 1
(Bos taurus (bovine)) | BDBM50060000

((S)-1-((R)-2-Methylamino-2-phenyl-acetyl)-pyrrolid...)Show SMILES CN[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C=O)c1ccccc1 Show InChI InChI=1S/C20H30N6O3/c1-23-17(14-7-3-2-4-8-14)19(29)26-12-6-10-16(26)18(28)25-15(13-27)9-5-11-24-20(21)22/h2-4,7-8,13,15-17,23H,5-6,9-12H2,1H3,(H,25,28)(H4,21,22,24)/t15-,16-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit the activity of Trypsin |
Bioorg Med Chem Lett 7: 2205-2210 (1997)
Article DOI: 10.1016/S0960-894X(97)00403-4
BindingDB Entry DOI: 10.7270/Q21G0MR2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349094

(CHEMBL1807305)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)N3CCCN(C)CC3)nc-21 Show InChI InChI=1S/C35H42N8O3/c1-6-22-10-8-11-23(7-2)29(22)38-33(44)31-26-14-12-25-21-36-35(39-30(25)32(26)42(4)40-31)37-27-15-13-24(20-28(27)46-5)34(45)43-17-9-16-41(3)18-19-43/h8,10-11,13,15,20-21H,6-7,9,12,14,16-19H2,1-5H3,(H,38,44)(H,36,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149693

(US8975267, 35)Show InChI InChI=1S/C17H19N5OS/c1-24-17-20-8-10-2-3-12-13(16(18)23)9-22(15(12)14(10)21-17)11-4-6-19-7-5-11/h2-3,8-9,11,19H,4-7H2,1H3,(H2,18,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM149685
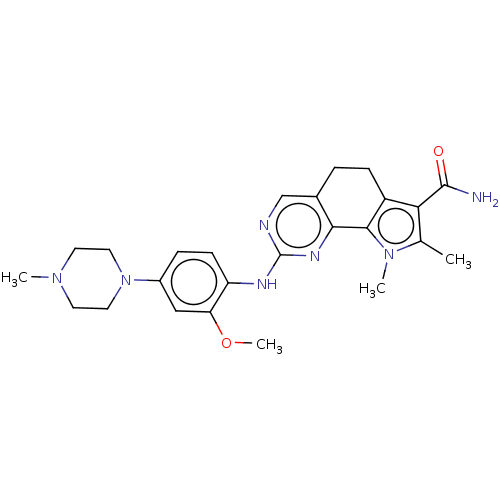
(US8975267, 7)Show SMILES COc1cc(ccc1Nc1ncc2CCc3c(C(N)=O)c(C)n(C)c3-c2n1)N1CCN(C)CC1 Show InChI InChI=1S/C25H31N7O2/c1-15-21(24(26)33)18-7-5-16-14-27-25(29-22(16)23(18)31(15)3)28-19-8-6-17(13-20(19)34-4)32-11-9-30(2)10-12-32/h6,8,13-14H,5,7,9-12H2,1-4H3,(H2,26,33)(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149686

(US8975267, 22)Show InChI InChI=1S/C15H18N4OS/c1-8(2)19-7-11(14(16)20)10-5-4-9-6-17-15(21-3)18-12(9)13(10)19/h6-8H,4-5H2,1-3H3,(H2,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM149695
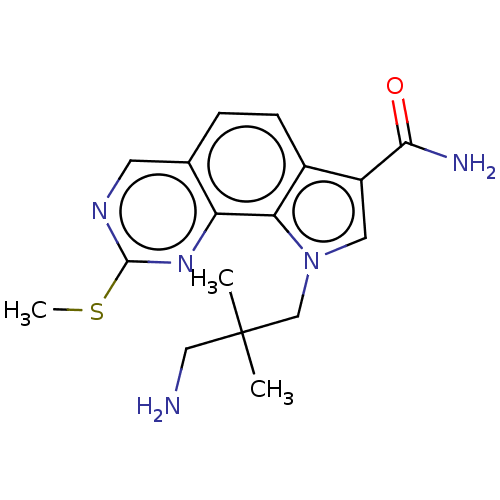
(US8975267, 38)Show InChI InChI=1S/C17H21N5OS/c1-17(2,8-18)9-22-7-12(15(19)23)11-5-4-10-6-20-16(24-3)21-13(10)14(11)22/h4-7H,8-9,18H2,1-3H3,(H2,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | 3.0 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
Specific peptide or protein substrates are trans-phosphorylated by their specific ser-thr or tyr kinase in the presence of ATP traced with 33P-gamma-... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424917

(CHEMBL2311578)Show SMILES Cc1cc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)ccc1-c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C27H28N4O3S2/c1-19-16-22(11-14-25(19)20-9-12-24(13-10-20)36(2,32)33)34-18-26-29-30-27(35-23-7-3-4-8-23)31(26)21-6-5-15-28-17-21/h5-6,9-17,23H,3-4,7-8,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424919
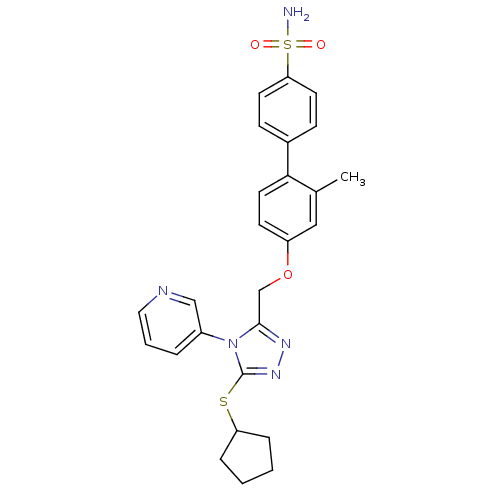
(CHEMBL2315422)Show SMILES Cc1cc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)ccc1-c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C26H27N5O3S2/c1-18-15-21(10-13-24(18)19-8-11-23(12-9-19)36(27,32)33)34-17-25-29-30-26(35-22-6-2-3-7-22)31(25)20-5-4-14-28-16-20/h4-5,8-16,22H,2-3,6-7,17H2,1H3,(H2,27,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060000

((S)-1-((R)-2-Methylamino-2-phenyl-acetyl)-pyrrolid...)Show SMILES CN[C@@H](C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C=O)c1ccccc1 Show InChI InChI=1S/C20H30N6O3/c1-23-17(14-7-3-2-4-8-14)19(29)26-12-6-10-16(26)18(28)25-15(13-27)9-5-11-24-20(21)22/h2-4,7-8,13,15-17,23H,5-6,9-12H2,1H3,(H,25,28)(H4,21,22,24)/t15-,16-,17+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit the activity of human alpha Thrombin |
Bioorg Med Chem Lett 7: 2205-2210 (1997)
Article DOI: 10.1016/S0960-894X(97)00403-4
BindingDB Entry DOI: 10.7270/Q21G0MR2 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349103
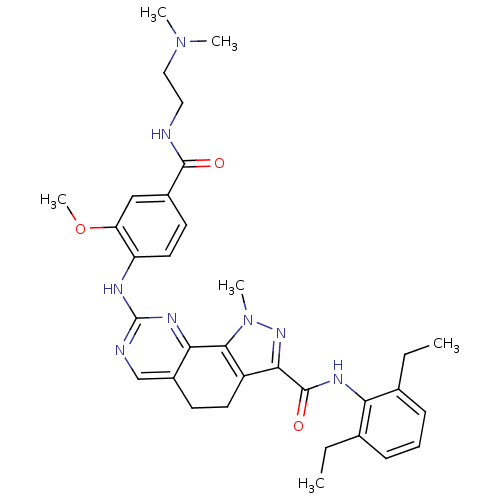
(CHEMBL1808341)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(=O)NCCN(C)C)nc-21 Show InChI InChI=1S/C33H40N8O3/c1-7-20-10-9-11-21(8-2)27(20)37-32(43)29-24-14-12-23-19-35-33(38-28(23)30(24)41(5)39-29)36-25-15-13-22(18-26(25)44-6)31(42)34-16-17-40(3)4/h9-11,13,15,18-19H,7-8,12,14,16-17H2,1-6H3,(H,34,42)(H,37,43)(H,35,36,38) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149695

(US8975267, 38)Show InChI InChI=1S/C17H21N5OS/c1-17(2,8-18)9-22-7-12(15(19)23)11-5-4-10-6-20-16(24-3)21-13(10)14(11)22/h4-7H,8-9,18H2,1-3H3,(H2,19,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50321580

(1-(3-aminopropyl)-8-(phenylamino)-4,5-dihydro-1H-p...)Show SMILES NCCCn1nc(C(N)=O)c2CCc3cnc(Nc4ccccc4)nc3-c12 Show InChI InChI=1S/C19H21N7O/c20-9-4-10-26-17-14(16(25-26)18(21)27)8-7-12-11-22-19(24-15(12)17)23-13-5-2-1-3-6-13/h1-3,5-6,11H,4,7-10,20H2,(H2,21,27)(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl
Curated by ChEMBL
| Assay Description
Inhibition of PDK1 |
Bioorg Med Chem Lett 20: 4095-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.070
BindingDB Entry DOI: 10.7270/Q2NP25C5 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50349099
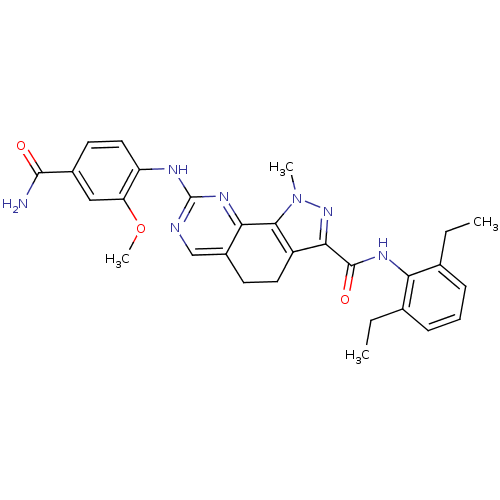
(CHEMBL1808338)Show SMILES CCc1cccc(CC)c1NC(=O)c1nn(C)c-2c1CCc1cnc(Nc3ccc(cc3OC)C(N)=O)nc-21 Show InChI InChI=1S/C29H31N7O3/c1-5-16-8-7-9-17(6-2)23(16)33-28(38)25-20-12-10-19-15-31-29(34-24(19)26(20)36(3)35-25)32-21-13-11-18(27(30)37)14-22(21)39-4/h7-9,11,13-15H,5-6,10,12H2,1-4H3,(H2,30,37)(H,33,38)(H,31,32,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50038001
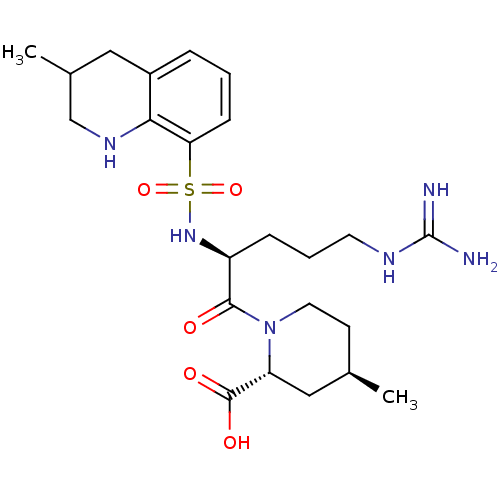
((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro ability to inhibit the activity of human alpha Thrombin |
Bioorg Med Chem Lett 7: 2205-2210 (1997)
Article DOI: 10.1016/S0960-894X(97)00403-4
BindingDB Entry DOI: 10.7270/Q21G0MR2 |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424939
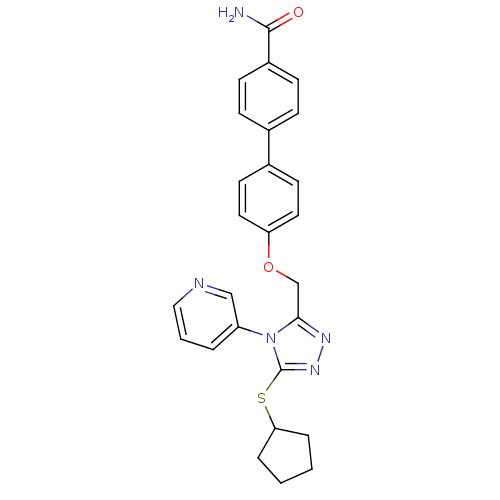
(CHEMBL2315430)Show SMILES NC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C26H25N5O2S/c27-25(32)20-9-7-18(8-10-20)19-11-13-22(14-12-19)33-17-24-29-30-26(34-23-5-1-2-6-23)31(24)21-4-3-15-28-16-21/h3-4,7-16,23H,1-2,5-6,17H2,(H2,27,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424938
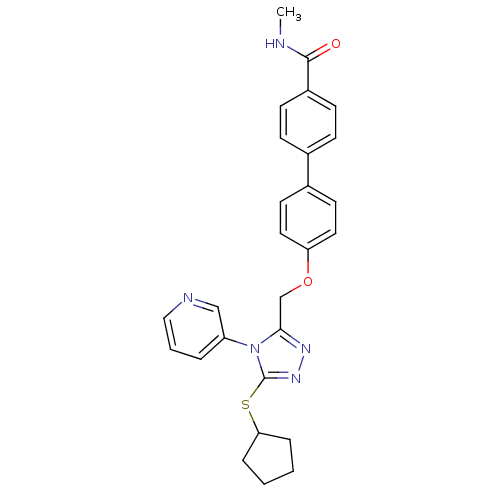
(CHEMBL2315431)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C27H27N5O2S/c1-28-26(33)21-10-8-19(9-11-21)20-12-14-23(15-13-20)34-18-25-30-31-27(35-24-6-2-3-7-24)32(25)22-5-4-16-29-17-22/h4-5,8-17,24H,2-3,6-7,18H2,1H3,(H,28,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50318081
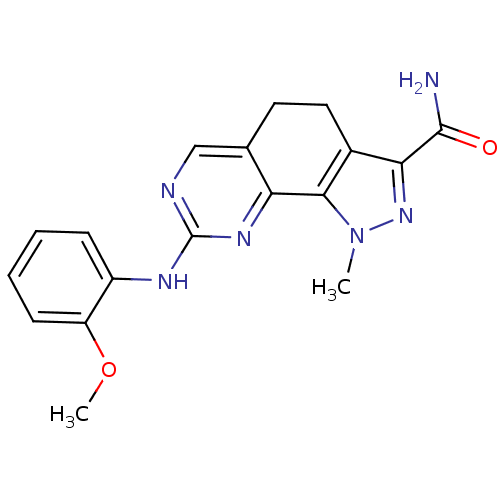
(8-[(2-Methoxyphenyl)amino]-1-methyl-4,5-dihydro-1H...)Show InChI InChI=1S/C18H18N6O2/c1-24-16-11(15(23-24)17(19)25)8-7-10-9-20-18(22-14(10)16)21-12-5-3-4-6-13(12)26-2/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of PLK1 assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424942
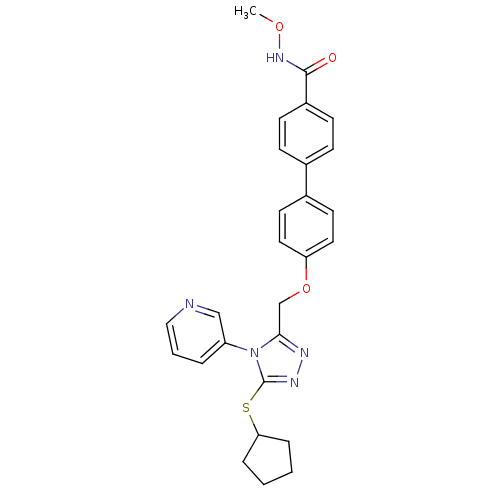
(CHEMBL2315427)Show SMILES CONC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C27H27N5O3S/c1-34-31-26(33)21-10-8-19(9-11-21)20-12-14-23(15-13-20)35-18-25-29-30-27(36-24-6-2-3-7-24)32(25)22-5-4-16-28-17-22/h4-5,8-17,24H,2-3,6-7,18H2,1H3,(H,31,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM149694

(US8975267, 36)Show InChI InChI=1S/C12H10N4OS/c1-18-12-15-4-6-2-3-7-8(11(13)17)5-14-10(7)9(6)16-12/h2-5,14H,1H3,(H2,13,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Nerviano Medical Sciences S.r.l.
US Patent
| Assay Description
The buffer for PIM-2 assay was composed of HEPES 50 mM, at pH 7.5, with 1 mM MgCl2, 1 mM DTT, 3 microM Na3VO4, and 0.2 mg/mL BSAFull-length human PIM... |
US Patent US8975267 (2015)
BindingDB Entry DOI: 10.7270/Q29C6W41 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM13336
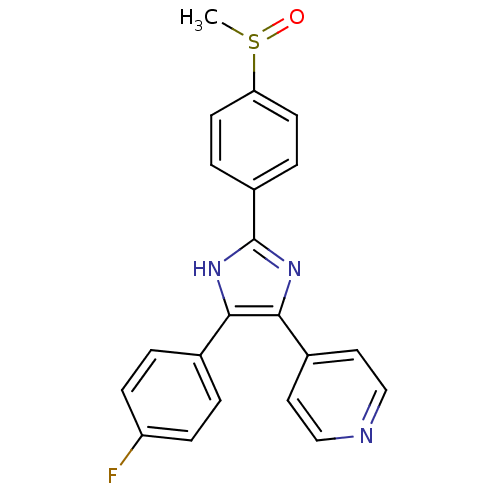
(4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-...)Show SMILES CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1 Show InChI InChI=1S/C21H16FN3OS/c1-27(26)18-8-4-16(5-9-18)21-24-19(14-2-6-17(22)7-3-14)20(25-21)15-10-12-23-13-11-15/h2-13H,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MAPK14 |
J Med Chem 53: 2681-94 (2010)
Article DOI: 10.1021/jm901443h
BindingDB Entry DOI: 10.7270/Q2XP75W8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424941

(CHEMBL2315428)Show SMILES OCC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C27H26N4O3S/c32-17-25(33)21-9-7-19(8-10-21)20-11-13-23(14-12-20)34-18-26-29-30-27(35-24-5-1-2-6-24)31(26)22-4-3-15-28-16-22/h3-4,7-16,24,32H,1-2,5-6,17-18H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31532

(pyrazolo[4,3-h]quinazoline-3-carboxamide, 1)Show InChI InChI=1S/C17H16N6O/c1-23-15-12(14(22-23)16(18)24)8-7-10-9-19-17(21-13(10)15)20-11-5-3-2-4-6-11/h2-6,9H,7-8H2,1H3,(H2,18,24)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of Aur-A assessed as [33P]-gamma-ATP incorporation into substrate by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424916
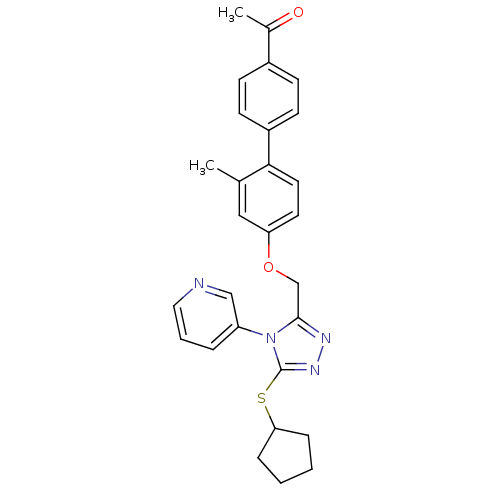
(CHEMBL2315424)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1C Show InChI InChI=1S/C28H28N4O2S/c1-19-16-24(13-14-26(19)22-11-9-21(10-12-22)20(2)33)34-18-27-30-31-28(35-25-7-3-4-8-25)32(27)23-6-5-15-29-17-23/h5-6,9-17,25H,3-4,7-8,18H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424918

(CHEMBL2315423)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1C Show InChI InChI=1S/C28H29N5O2S/c1-19-16-23(13-14-25(19)20-9-11-21(12-10-20)27(34)29-2)35-18-26-31-32-28(36-24-7-3-4-8-24)33(26)22-6-5-15-30-17-22/h5-6,9-17,24H,3-4,7-8,18H2,1-2H3,(H,29,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424920

(CHEMBL2315421)Show SMILES CONC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1C Show InChI InChI=1S/C28H29N5O3S/c1-19-16-23(13-14-25(19)20-9-11-21(12-10-20)27(34)32-35-2)36-18-26-30-31-28(37-24-7-3-4-8-24)33(26)22-6-5-15-29-17-22/h5-6,9-17,24H,3-4,7-8,18H2,1-2H3,(H,32,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424937
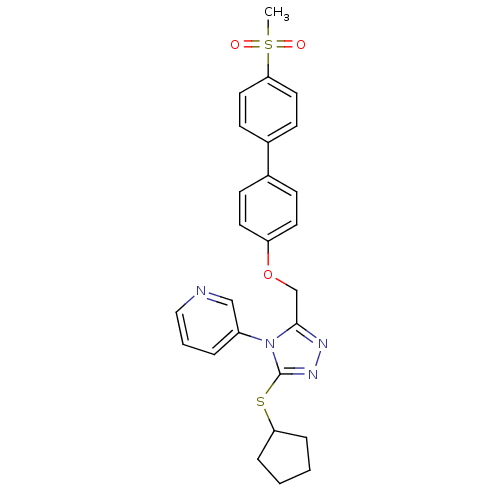
(CHEMBL2315432)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C26H26N4O3S2/c1-35(31,32)24-14-10-20(11-15-24)19-8-12-22(13-9-19)33-18-25-28-29-26(34-23-6-2-3-7-23)30(25)21-5-4-16-27-17-21/h4-5,8-17,23H,2-3,6-7,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Transitional endoplasmic reticulum ATPase
(Homo sapiens (Human)) | BDBM50424936
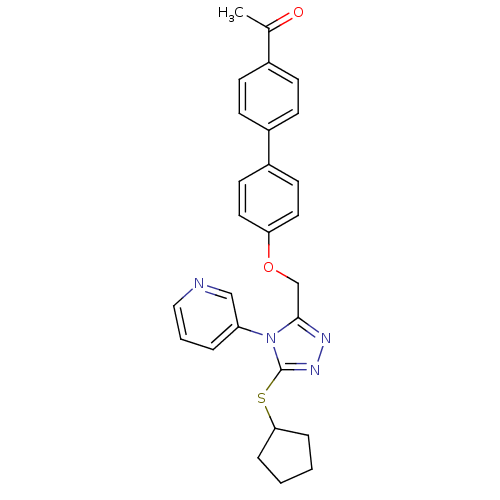
(CHEMBL2315433)Show SMILES CC(=O)c1ccc(cc1)-c1ccc(OCc2nnc(SC3CCCC3)n2-c2cccnc2)cc1 Show InChI InChI=1S/C27H26N4O2S/c1-19(32)20-8-10-21(11-9-20)22-12-14-24(15-13-22)33-18-26-29-30-27(34-25-6-2-3-7-25)31(26)23-5-4-16-28-17-23/h4-5,8-17,25H,2-3,6-7,18H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant His-GST tagged VCP expressed in baculovirus infected Hi5 cells assessed as ADP formation incubated for 20 mins proir ... |
J Med Chem 56: 437-50 (2013)
Article DOI: 10.1021/jm3013213
BindingDB Entry DOI: 10.7270/Q21Z45QK |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM50318081

(8-[(2-Methoxyphenyl)amino]-1-methyl-4,5-dihydro-1H...)Show InChI InChI=1S/C18H18N6O2/c1-24-16-11(15(23-24)17(19)25)8-7-10-9-20-18(22-14(10)16)21-12-5-3-4-6-13(12)26-2/h3-6,9H,7-8H2,1-2H3,(H2,19,25)(H,20,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences srl
Curated by ChEMBL
| Assay Description
Inhibition of MPS1 assessed as [33P]-gamma-ATP incorporation into substrate P38-betatide by gamma counting |
Bioorg Med Chem Lett 21: 4507-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.122
BindingDB Entry DOI: 10.7270/Q2R211RS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data