

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318372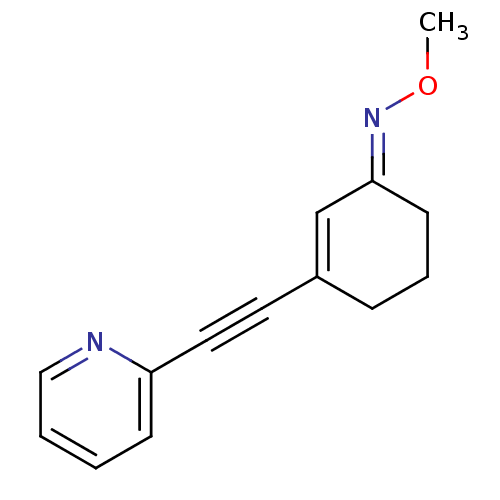 ((E)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11123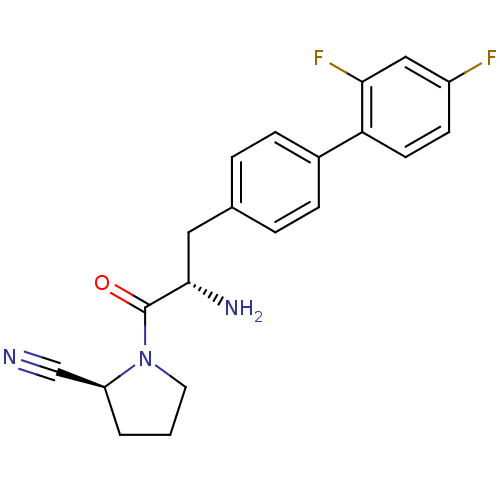 ((2S)-1-[(2S)-2-amino-3-[4-(2,4-difluorophenyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11121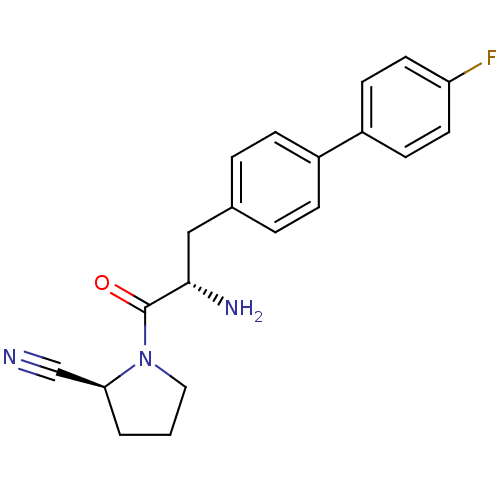 ((2S)-1-[(2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.10 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318382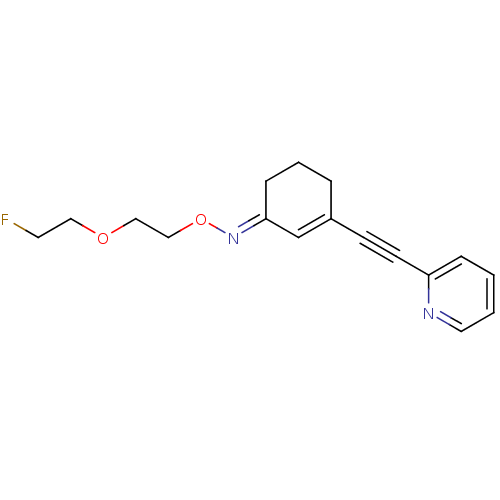 (3-[pyridine-2-yl)ethynyl]cyclohex-2-enone-O-fluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318375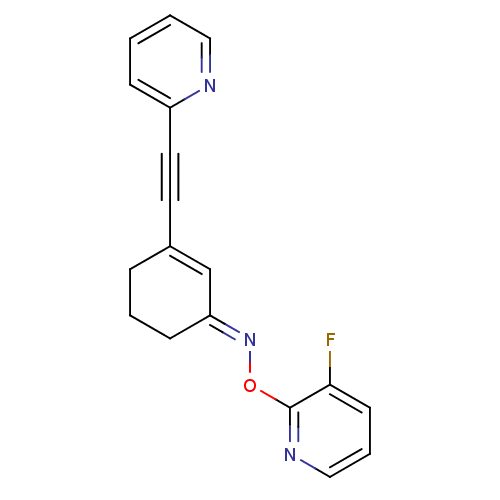 ((E)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-3-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50198702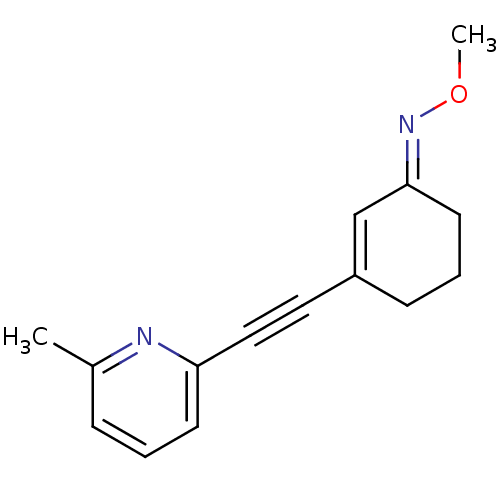 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11122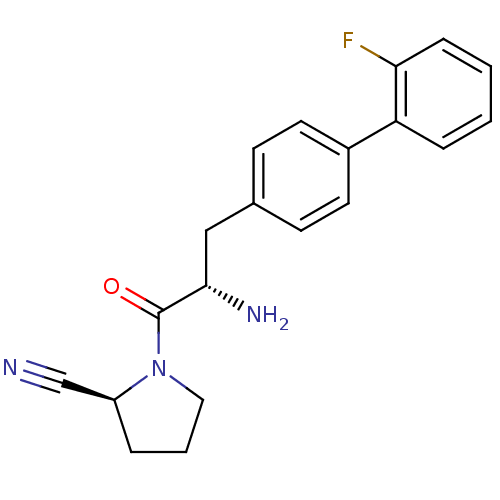 ((2S)-1-[(2S)-2-amino-3-[4-(2-fluorophenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318381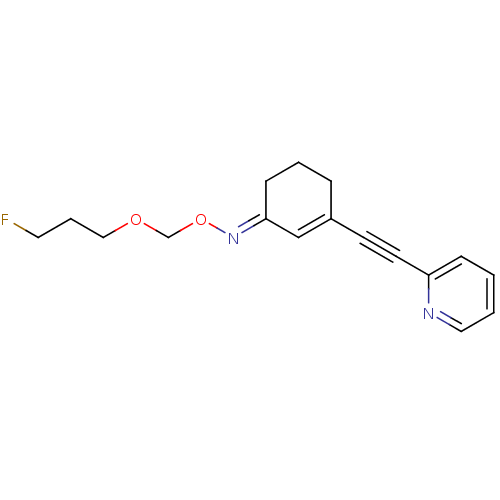 ((E)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-(3-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323923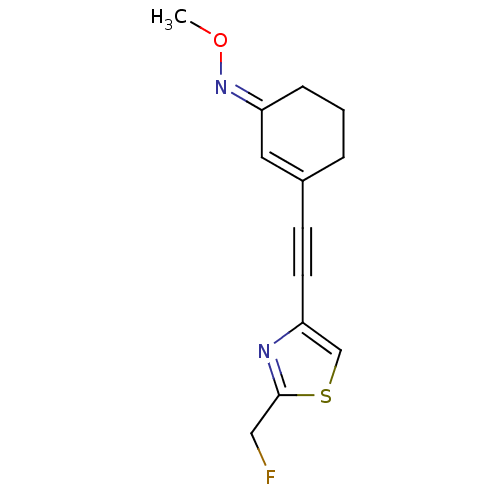 ((E)-3-((2-(Fluoromethyl)thiazol-4-yl)ethynyl)cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebellum membranes after 45 mins by liquid scintillation counting | Bioorg Med Chem 18: 6044-54 (2010) Article DOI: 10.1016/j.bmc.2010.06.070 BindingDB Entry DOI: 10.7270/Q2862GMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318380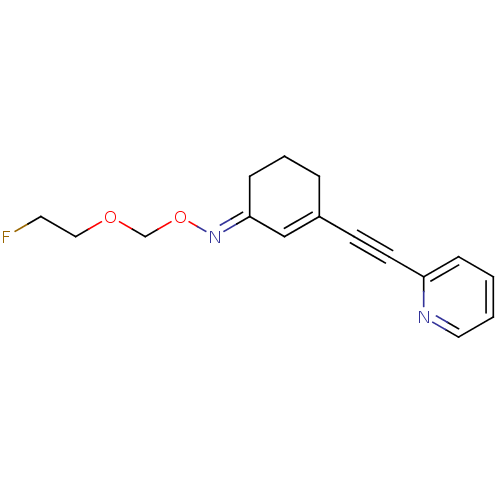 ((E)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-(2-f...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318374 ((E)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-6-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11118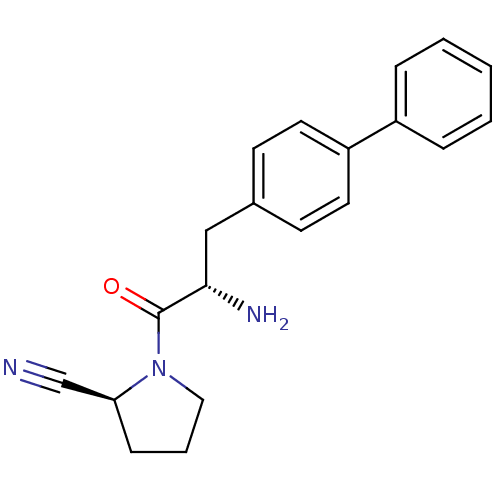 ((2S)-1-[(2S)-2-amino-3-(4-phenylphenyl)propanoyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11119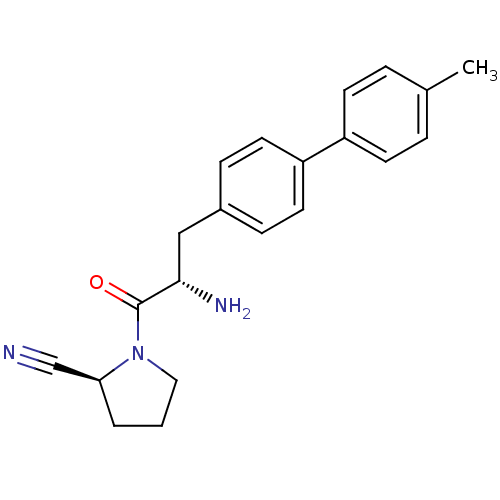 ((2S)-1-[(2S)-2-amino-3-[4-(4-methylphenyl)phenyl]p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323924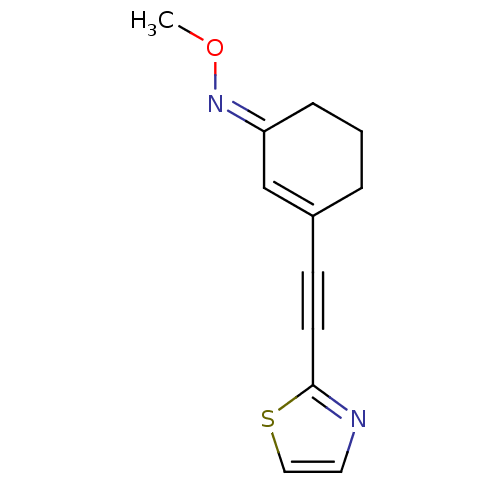 ((E)-3-(Thiazol-2-ylethynyl)cyclohex-2-enone O-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebellum membranes after 45 mins by liquid scintillation counting | Bioorg Med Chem 18: 6044-54 (2010) Article DOI: 10.1016/j.bmc.2010.06.070 BindingDB Entry DOI: 10.7270/Q2862GMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323926 ((E)-3-((2-Methylthiazol-4-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebellum membranes after 45 mins by liquid scintillation counting | Bioorg Med Chem 18: 6044-54 (2010) Article DOI: 10.1016/j.bmc.2010.06.070 BindingDB Entry DOI: 10.7270/Q2862GMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11120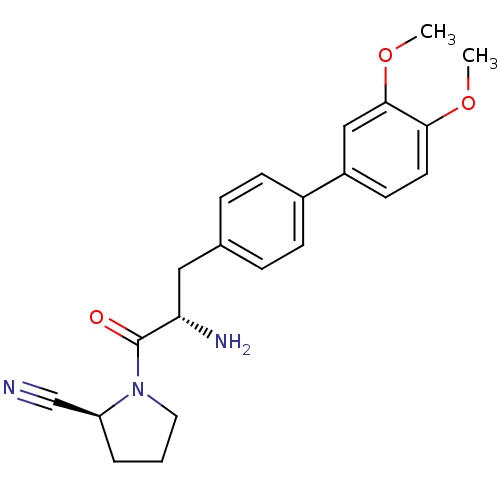 ((2S)-1-[(2S)-2-amino-3-[4-(3,4-dimethoxyphenyl)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318383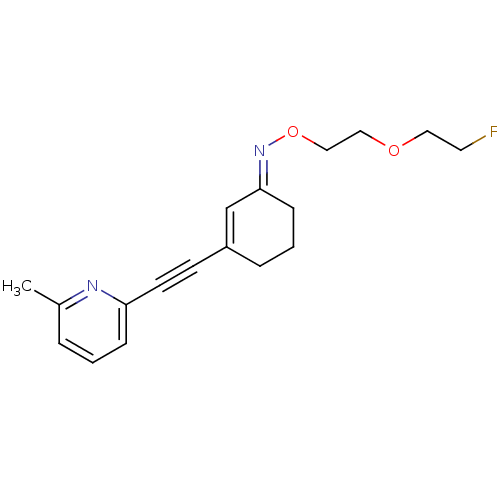 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11113 (6-{[2-({2-[(2S)-2-cyanopyrrolidin-1-yl]-2-oxoethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11116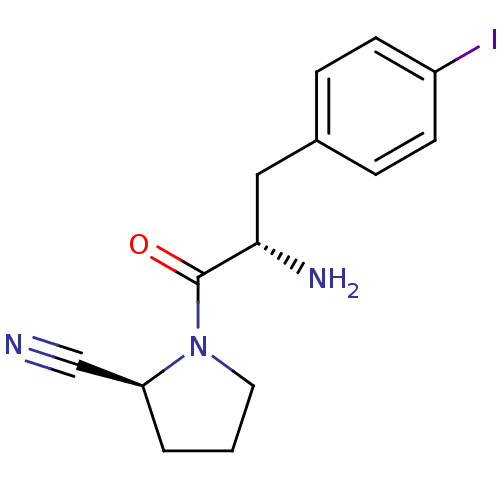 ((2S)-1-[(2S)-2-amino-3-(4-iodophenyl)propanoyl]pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -42.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50323925 ((E)-3-(Thiazol-4-ylethynyl)cyclohex-2-enone O-2-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich (Swiss Federal Institute of Technology) Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat cerebellum membranes after 45 mins by liquid scintillation counting | Bioorg Med Chem 18: 6044-54 (2010) Article DOI: 10.1016/j.bmc.2010.06.070 BindingDB Entry DOI: 10.7270/Q2862GMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11124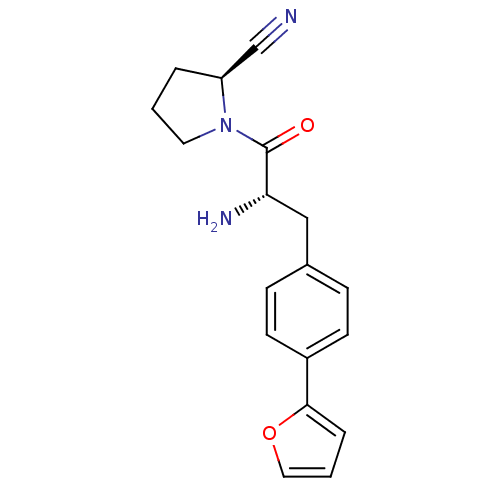 ((2S)-1-[(2S)-2-amino-3-[4-(furan-2-yl)phenyl]propa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | -42.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318376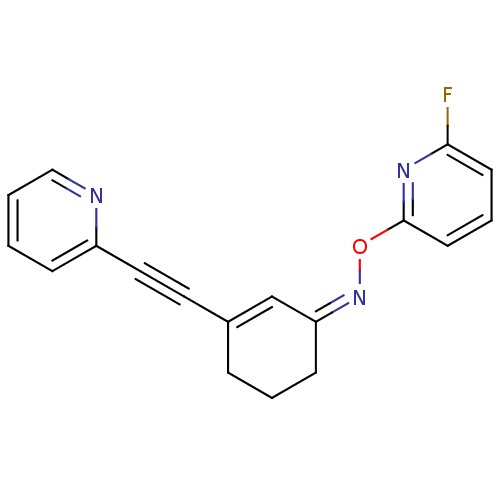 ((Z)-3-(pyridin-2-ylethynyl)cyclohex-2-enone O-6-fl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318373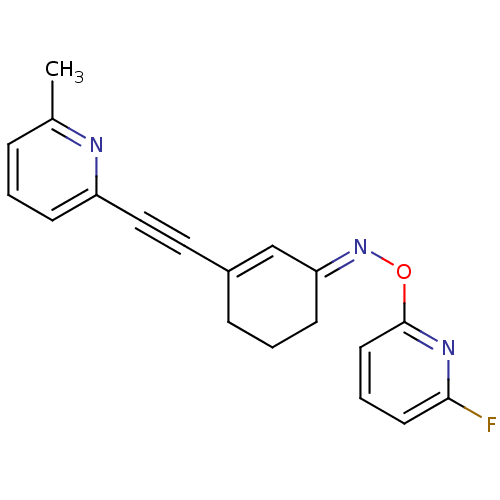 ((E)-3-((6-methylpyridin-2-yl)ethynyl)cyclohex-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318377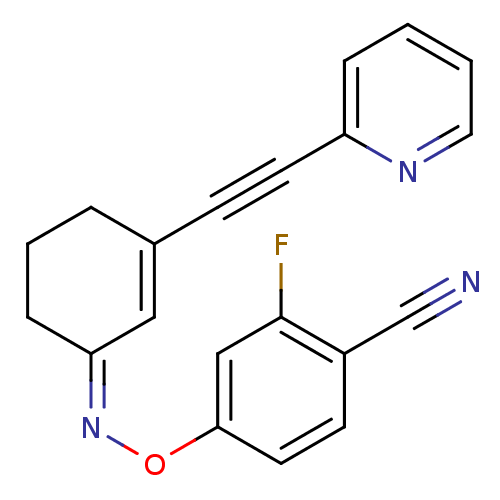 ((Z)-4-fluoro-2-(3-(pyridin-2-ylethynyl)cyclohex-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318371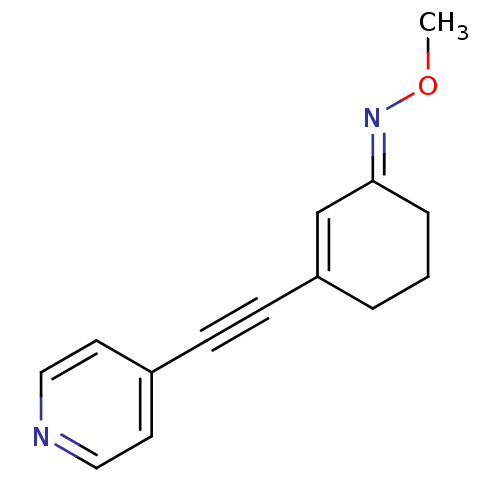 ((E)-3-(pyridin-4-ylethynyl)cyclohex-2-enone O-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 54.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318379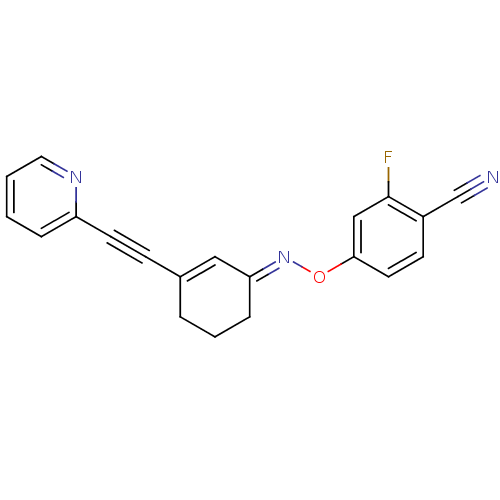 ((E)-4-fluoro-2-(3-(pyridin-2-ylethynyl)cyclohex-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11115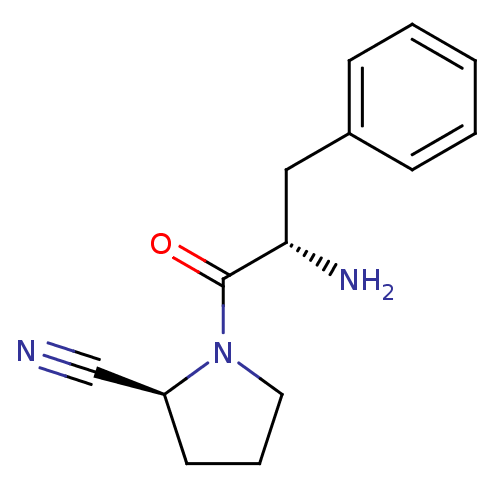 ((2S)-1-[(2S)-2-amino-3-phenylpropanoyl]pyrrolidine...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 63 | -41.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318378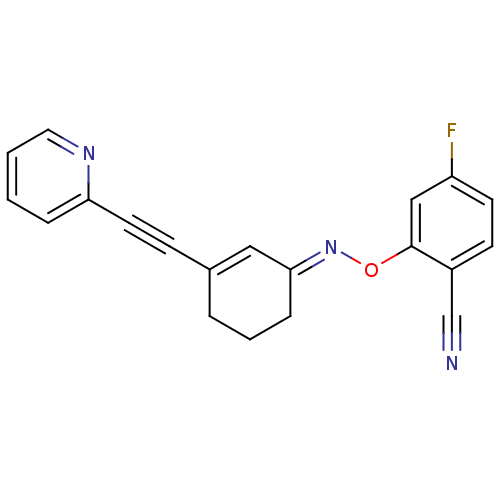 ((E)-2-fluoro-4-(3-(pyridin-2-ylethynyl)cyclohex-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 73.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11136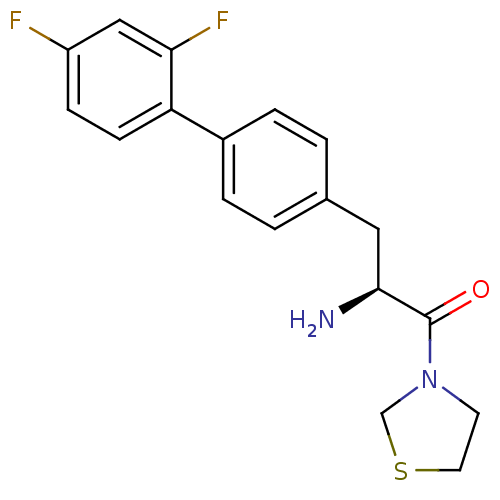 ((2S)-2-amino-3-[4-(2,4-difluorophenyl)phenyl]-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 96 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11134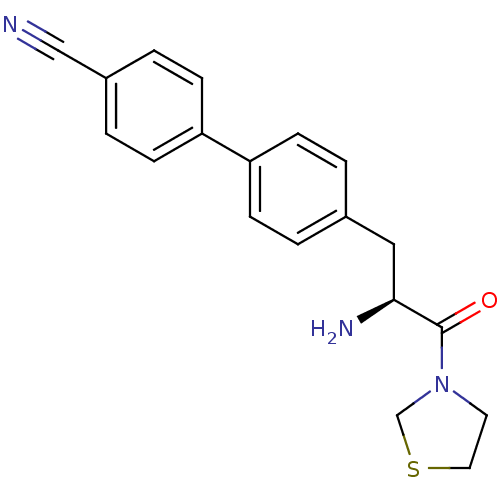 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11133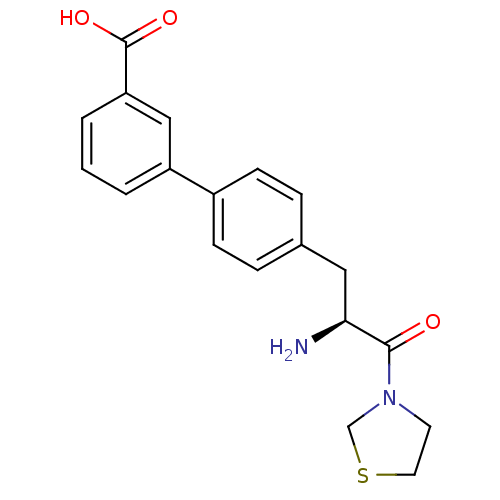 (3-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 166 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11135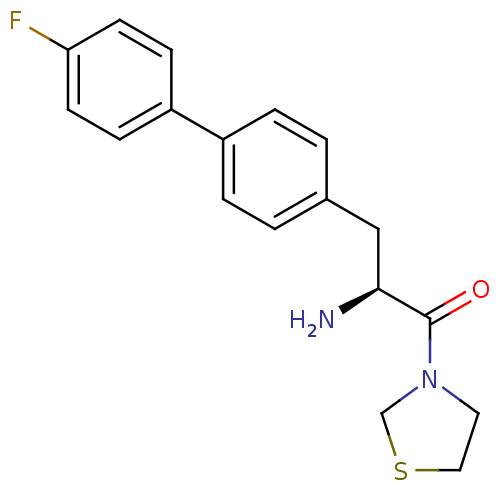 ((2S)-2-amino-3-[4-(4-fluorophenyl)phenyl]-1-(1,3-t...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 170 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50318370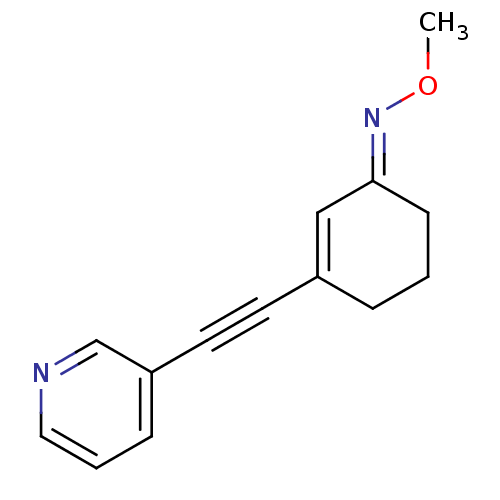 ((E)-3-(pyridin-3-ylethynyl)cyclohex-2-enone O-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 252 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ETH Zurich Curated by ChEMBL | Assay Description Displacement of [3H]M-MPEP from mGluR5 in Sprague-Dawley rat brain P2 membrane after 45 mins by beta counting | J Med Chem 53: 4009-17 (2010) Article DOI: 10.1021/jm901850k BindingDB Entry DOI: 10.7270/Q2T72HMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11132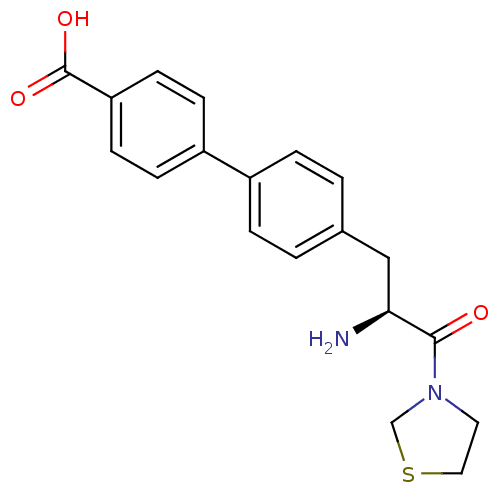 (4-{4-[(2S)-2-amino-3-oxo-3-(1,3-thiazolidin-3-yl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11131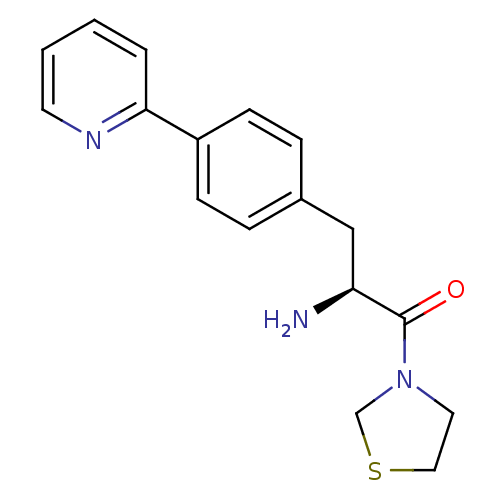 ((2S)-2-amino-3-[4-(pyridin-2-yl)phenyl]-1-(1,3-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 355 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11125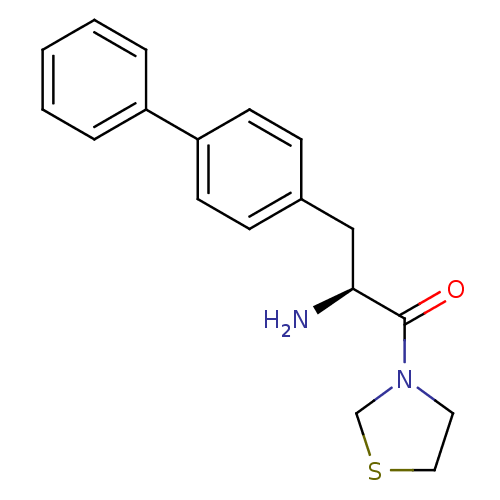 ((2S)-2-amino-3-(4-phenylphenyl)-1-(1,3-thiazolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -36.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11117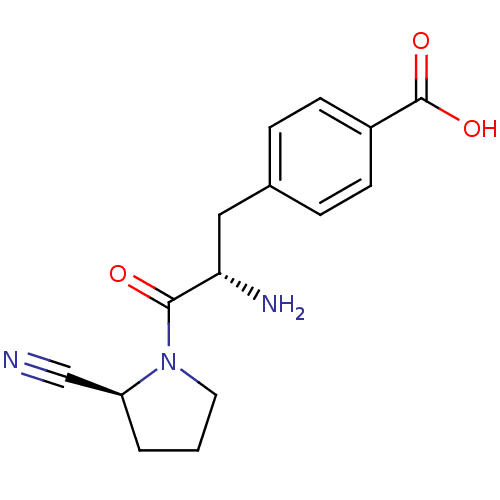 (4-[(2S)-2-amino-3-[(2S)-2-cyanopyrrolidin-1-yl]-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 470 | -36.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11130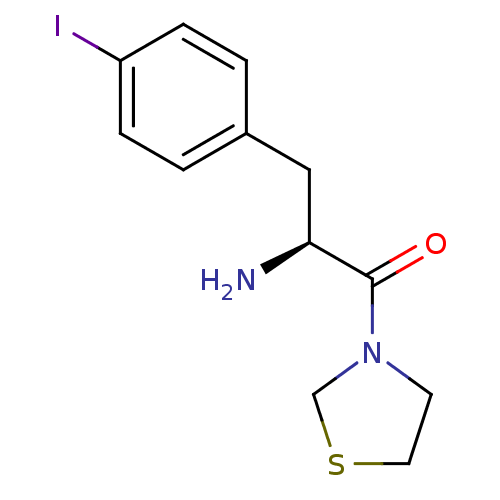 ((2S)-2-amino-3-(4-iodophenyl)-1-(1,3-thiazolidin-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 980 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11126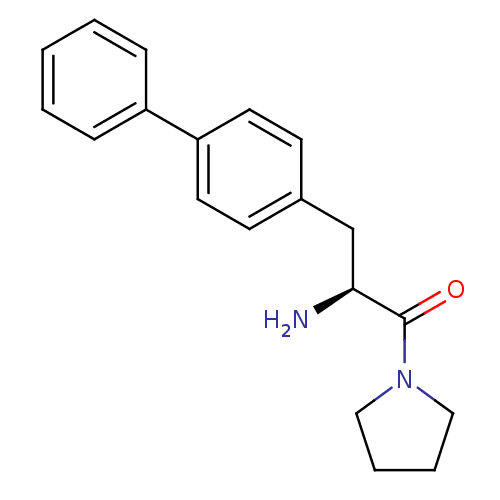 ((2S)-2-amino-3-(4-phenylphenyl)-1-(pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.16E+3 | -33.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11137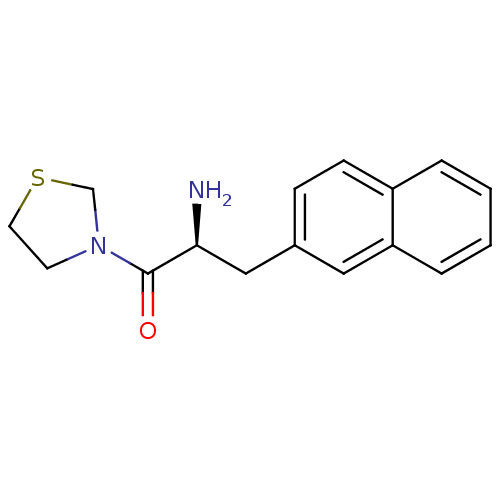 ((2S)-2-amino-3-(naphthalen-2-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | -28.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11129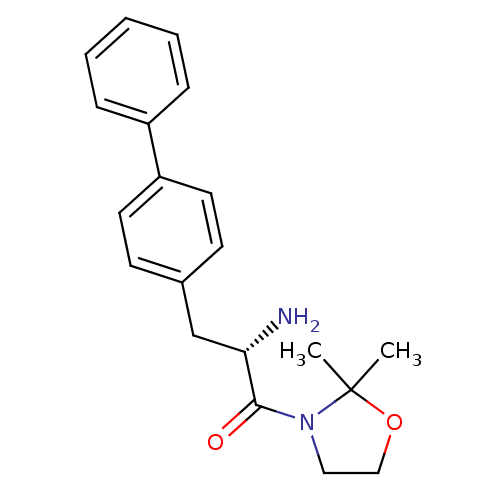 ((2S)-2-amino-1-(2,2-dimethyl-1,3-oxazolidin-3-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11128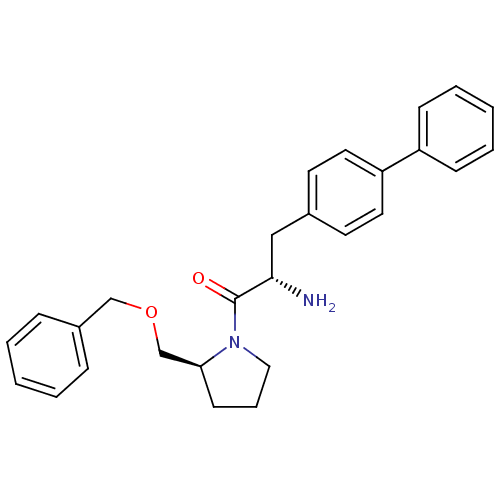 ((2S)-2-amino-1-[(2S)-2-[(benzyloxy)methyl]pyrrolid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11127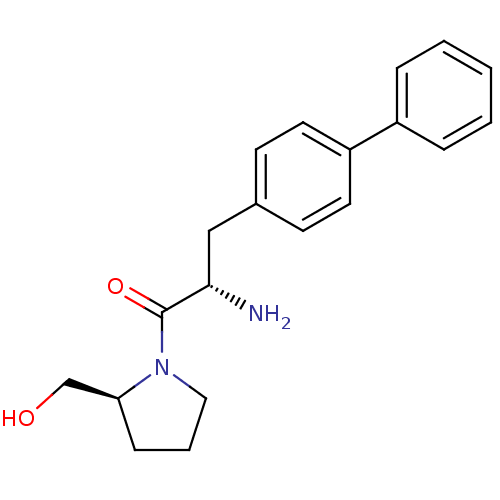 ((2S)-2-amino-1-[(2S)-2-(hydroxymethyl)pyrrolidin-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11138 ((2S)-2-amino-3-(naphthalen-1-yl)-1-(1,3-thiazolidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM11139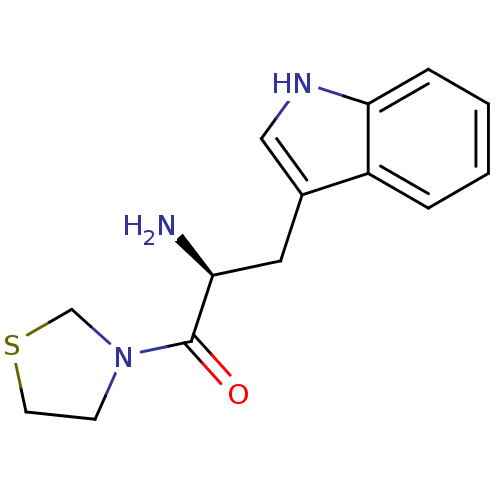 ((2S)-2-amino-3-(1H-indol-3-yl)-1-(1,3-thiazolidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.28E+4 | >-27.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Johnson & Johnson Pharmaceutical | Assay Description The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r... | Bioorg Med Chem Lett 16: 123-8 (2006) Article DOI: 10.1016/j.bmcl.2005.09.037 BindingDB Entry DOI: 10.7270/Q2S180QJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1/2 (Rattus norvegicus (Rat)) | BDBM24185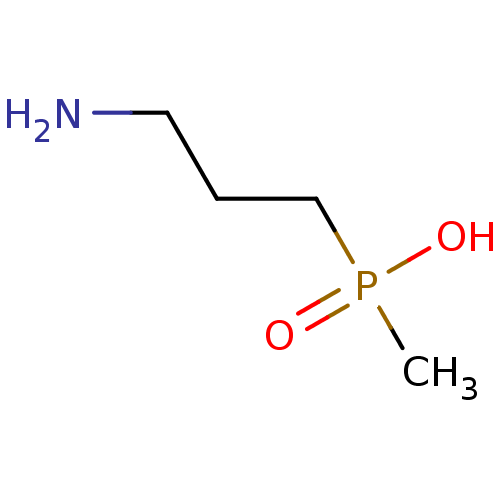 ((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG Curated by ChEMBL | Assay Description Inhibition of [3H]-CGP-27,492 binding to Gamma-aminobutyric acid type B receptor of rat cortex | J Med Chem 38: 3297-312 (1995) BindingDB Entry DOI: 10.7270/Q2N58KDP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50217358 (4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of human FLT3 | Bioorg Med Chem Lett 17: 4861-5 (2007) Article DOI: 10.1016/j.bmcl.2007.06.046 BindingDB Entry DOI: 10.7270/Q2T72H47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246060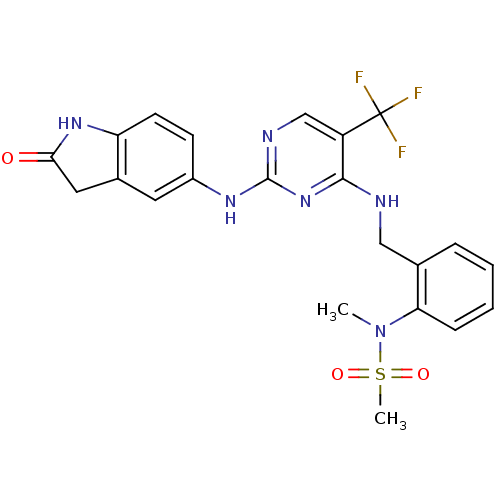 (CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of purified activated FAK kinase domain (410-689) using ATP and Glu and Tyr random peptide polymer substrate by fluorescence polarization ... | Bioorg Med Chem Lett 19: 3253-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.093 BindingDB Entry DOI: 10.7270/Q2GH9J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1/2 (Homo sapiens (Human)) | BDBM24184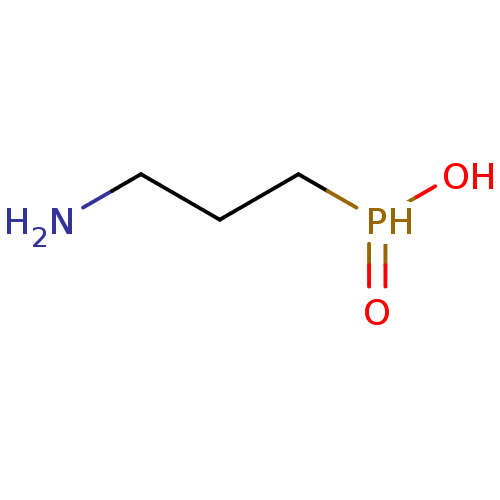 ((3-aminopropyl)phosphinic acid | 3-aminopropylphos...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CIBA-GEIGY AG Curated by ChEMBL | Assay Description Inhibition of [3H]-baclofen binding to Gamma-aminobutyric acid type B receptor of cat cerebellum | J Med Chem 38: 3297-312 (1995) BindingDB Entry DOI: 10.7270/Q2N58KDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50217367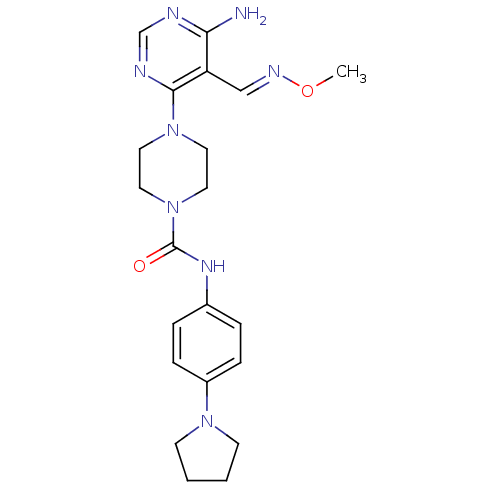 (4-(6-amino-5-((methoxyimino)methyl)pyrimidin-4-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibition of human FLT3 | Bioorg Med Chem Lett 17: 4861-5 (2007) Article DOI: 10.1016/j.bmcl.2007.06.046 BindingDB Entry DOI: 10.7270/Q2T72H47 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 291 total ) | Next | Last >> |