

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044868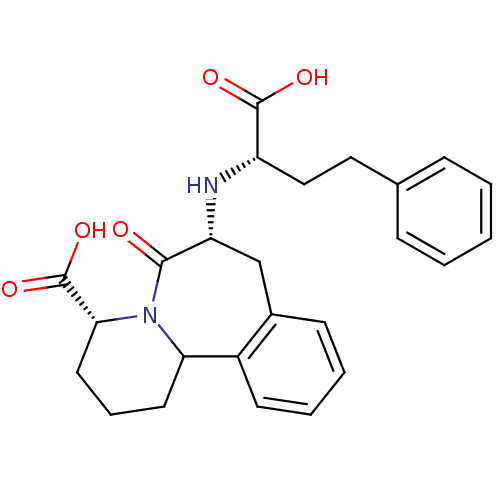 (7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044866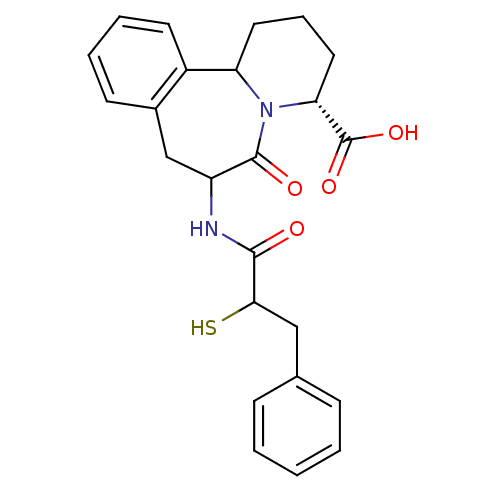 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044866 (7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287694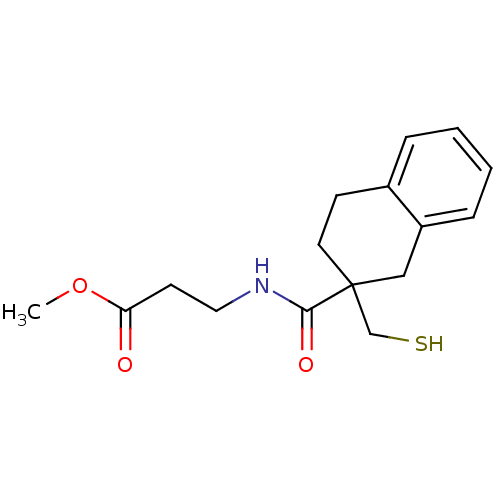 (3-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287702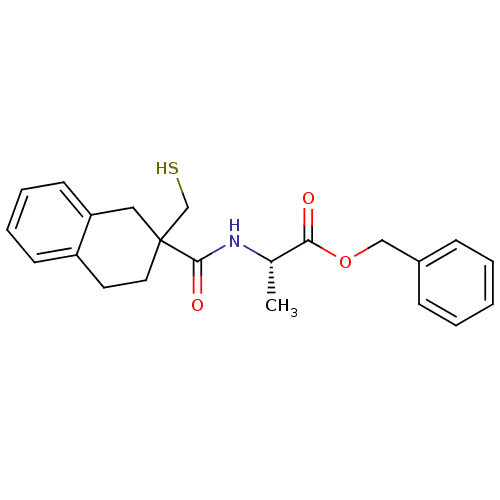 ((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287689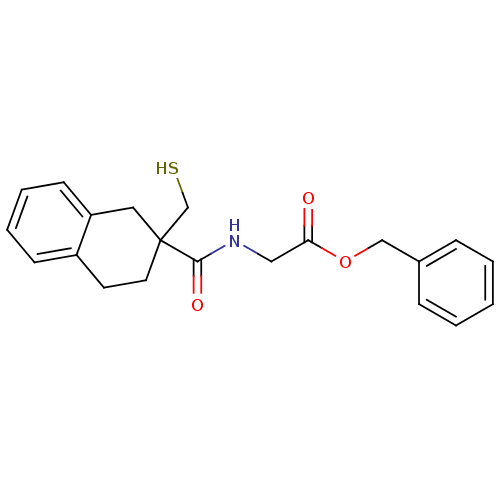 (CHEMBL304233 | [(2-Mercaptomethyl-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287700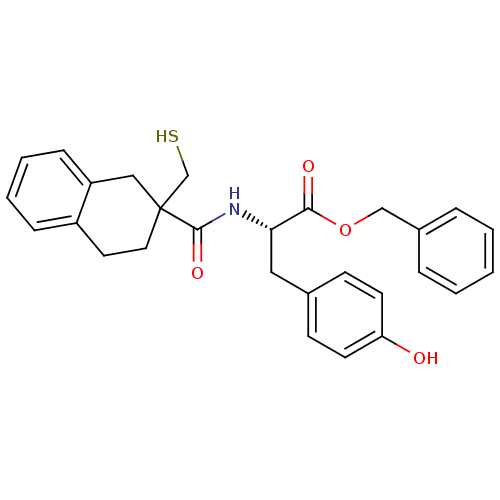 ((S)-3-(4-Hydroxy-phenyl)-2-[(2-mercaptomethyl-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287699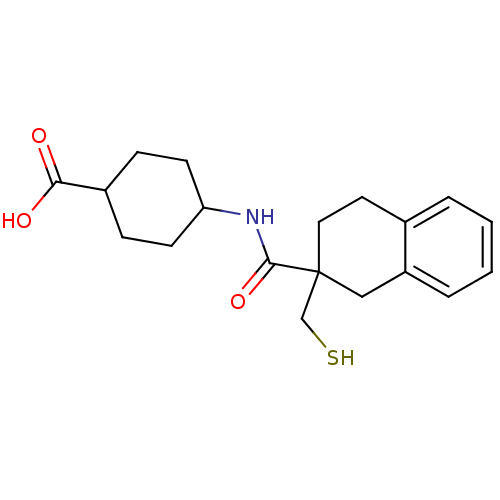 (4-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287690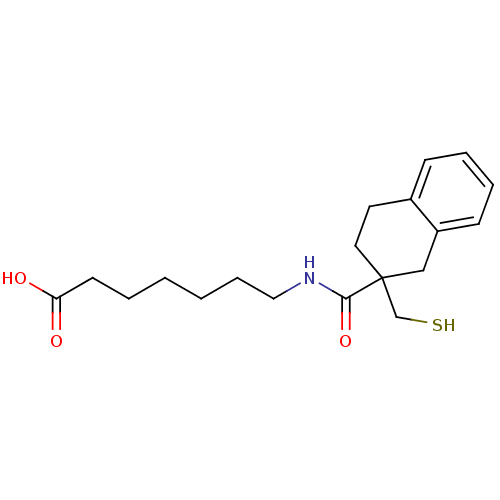 (7-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50024096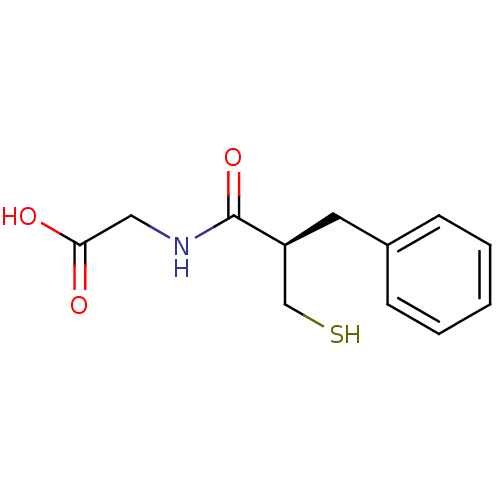 (((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50024102 (((S)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287696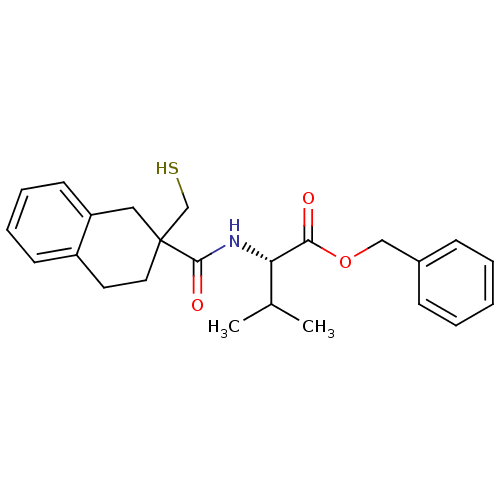 ((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044867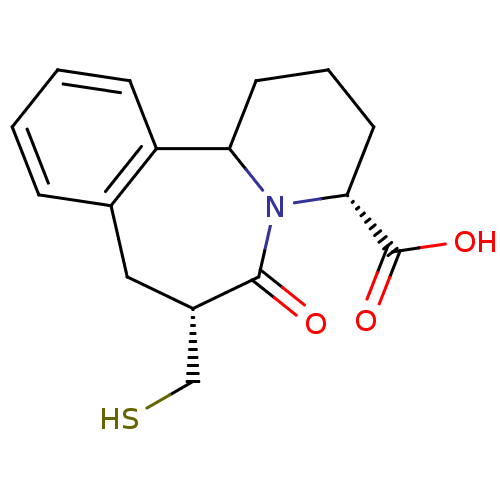 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50024096 (((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044869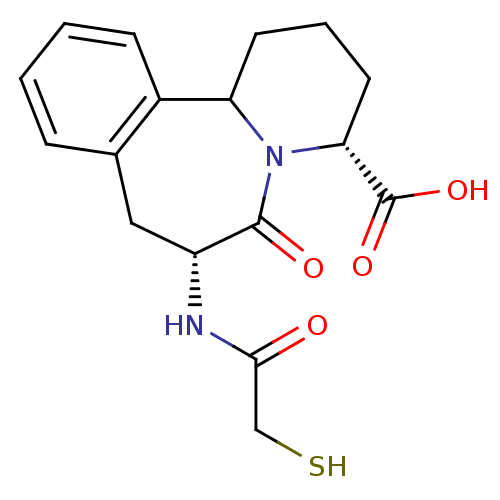 (7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287695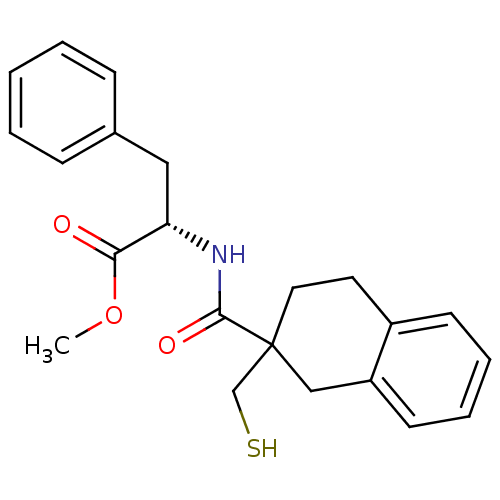 ((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044869 (7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6,7,8,12b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287701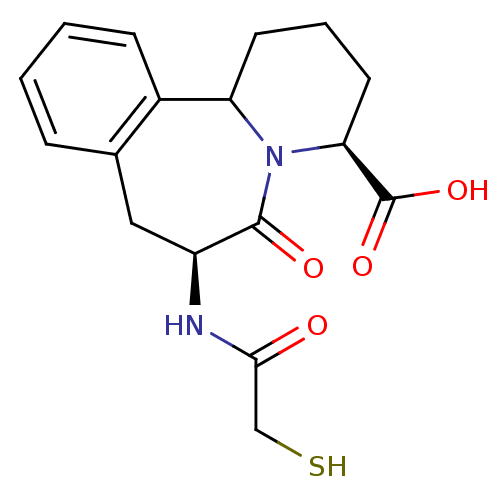 ((4S,7S)-7-(2-Mercapto-acetylamino)-6-oxo-1,2,3,4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50044867 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287693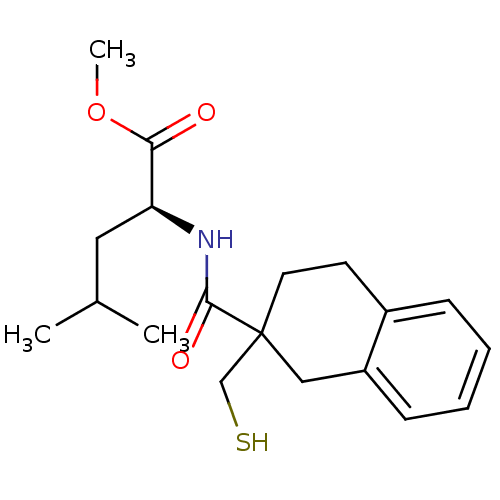 ((S)-2-[(2-Mercaptomethyl-1,2,3,4-tetrahydro-naphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287692 (CHEMBL431957 | [(2-Mercaptomethyl-indane-2-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287691 (2-Mercaptomethyl-1,2,3,4-tetrahydro-naphthalene-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287698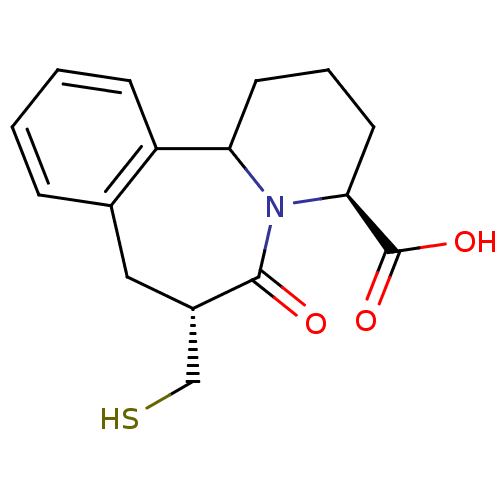 ((4S,7R)-7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044867 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Oryctolagus cuniculus) | BDBM50024096 (((R)-2-Mercaptomethyl-3-phenyl-propionylamino)-ace...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50287697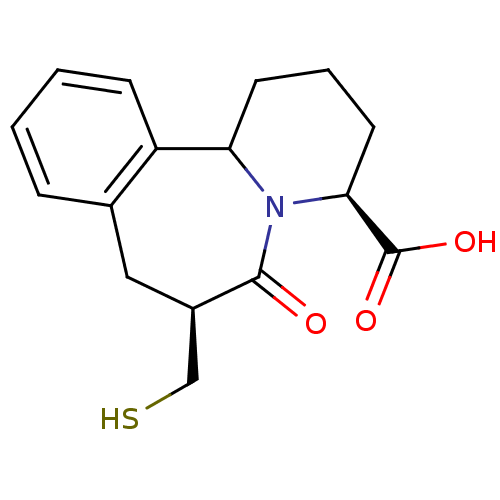 ((4S,7S)-7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase(NEP). | Bioorg Med Chem Lett 6: 2053-2058 (1996) Article DOI: 10.1016/0960-894X(96)00367-8 BindingDB Entry DOI: 10.7270/Q29G5MSW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044867 (7-Mercaptomethyl-6-oxo-1,2,3,4,6,7,8,12b-octahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50044868 (7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute Curated by ChEMBL | Assay Description Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined | J Med Chem 36: 2420-3 (1993) BindingDB Entry DOI: 10.7270/Q22N51C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988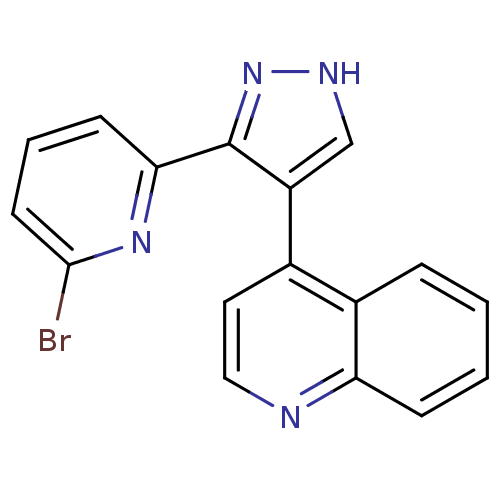 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132988 (4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase production in mink lung cells (p3TP Lux) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161301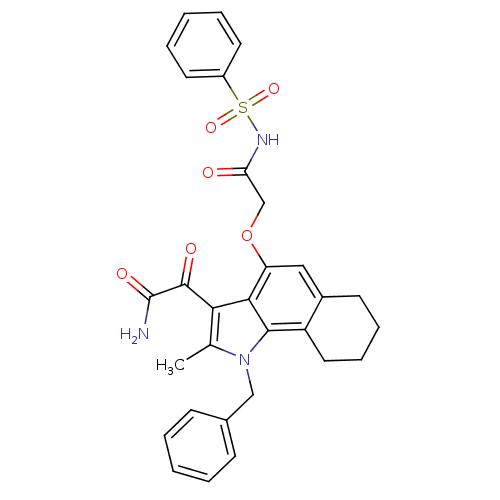 (2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161305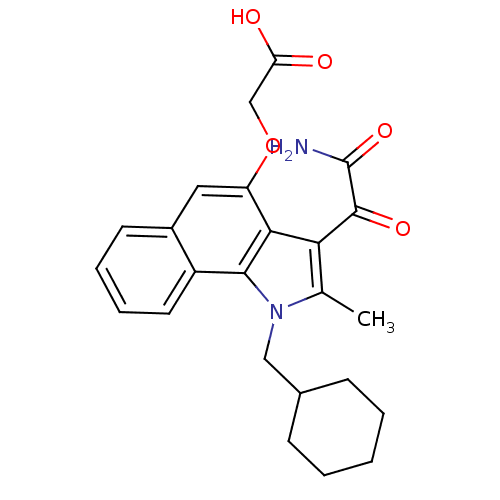 ((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161293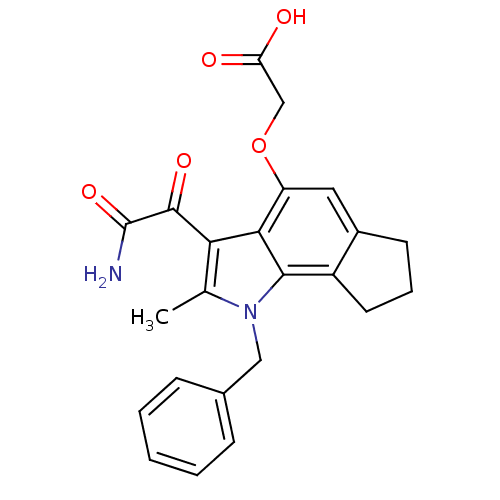 ((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132986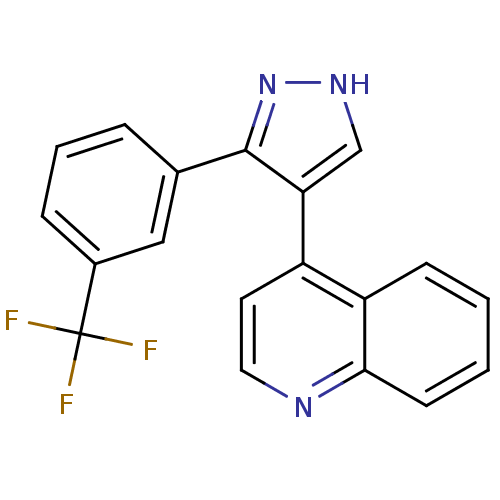 (4-(3-(3-(trifluoromethyl)phenyl)-1H-pyrazol-4-yl)q...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Mitogen-activated protein kinase p38 | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161299 ((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161301 (2-[4-(2-Benzenesulfonylamino-2-oxo-ethoxy)-1-benzy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50132989 (4-[3-(6-Methyl-pyridin-2-yl)-1H-pyrazol-4-yl]-quin...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) | J Med Chem 46: 3953-6 (2003) Article DOI: 10.1021/jm0205705 BindingDB Entry DOI: 10.7270/Q2RV0N38 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161308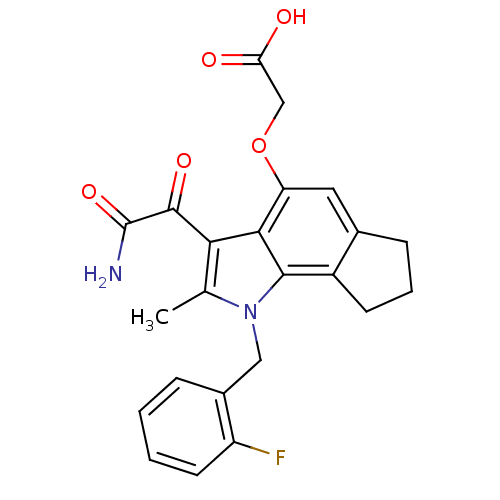 (CHEMBL179118 | [3-Aminooxalyl-1-(2-fluoro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161298 ((3-Aminooxalyl-1-benzyl-2-methyl-2,3,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161294 (CHEMBL179966 | [3-Aminooxalyl-1-(3-fluoro-benzyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161293 ((3-Aminooxalyl-1-benzyl-2-methyl-1,6,7,8-tetrahydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161296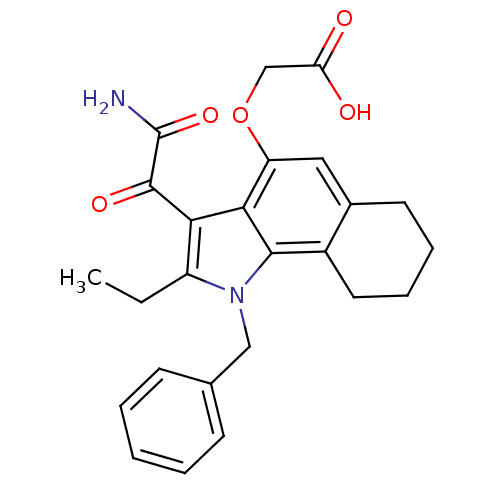 ((3-Aminooxalyl-1-benzyl-2-ethyl-2,3,6,7,8,9-hexahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161305 ((3-Aminooxalyl-1-cyclohexylmethyl-2-methyl-2,3-dih...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161299 ((3-Aminooxalyl-1-benzyl-2-ethyl-1,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50161298 ((3-Aminooxalyl-1-benzyl-2-methyl-2,3,6,7,8,9-hexah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against human nonpancreatic secretory phospholipase A2 | J Med Chem 48: 893-6 (2005) Article DOI: 10.1021/jm0401309 BindingDB Entry DOI: 10.7270/Q20001M3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 135 total ) | Next | Last >> |