 Found 516 hits with Last Name = 'danley' and Initial = 'de'
Found 516 hits with Last Name = 'danley' and Initial = 'de' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123058
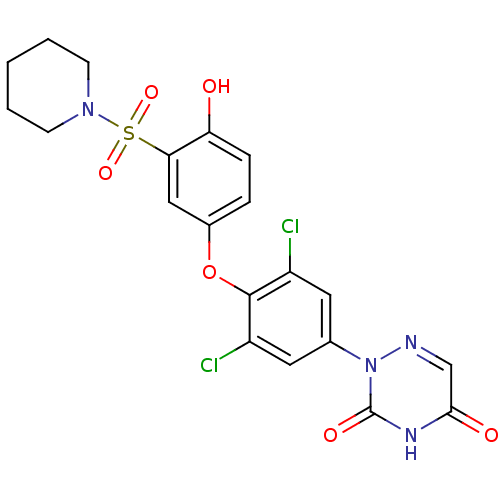
(2-(3,5-dichloro-4-(4-hydroxy-3-(piperidin-1-ylsulf...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1CCCCC1 Show InChI InChI=1S/C20H18Cl2N4O6S/c21-14-8-12(26-20(29)24-18(28)11-23-26)9-15(22)19(14)32-13-4-5-16(27)17(10-13)33(30,31)25-6-2-1-3-7-25/h4-5,8-11,27H,1-3,6-7H2,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123046
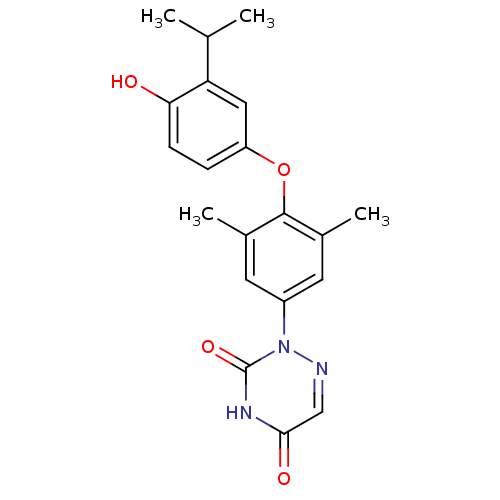
(2-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...)Show SMILES CC(C)c1cc(Oc2c(C)cc(cc2C)-n2ncc(=O)[nH]c2=O)ccc1O Show InChI InChI=1S/C20H21N3O4/c1-11(2)16-9-15(5-6-17(16)24)27-19-12(3)7-14(8-13(19)4)23-20(26)22-18(25)10-21-23/h5-11,24H,1-4H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044
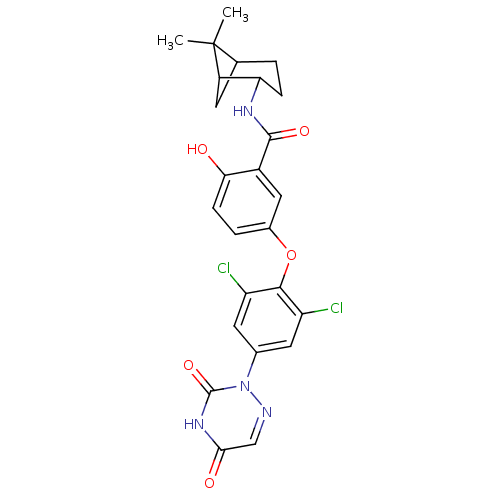
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123044

(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES CC1(C)C2CC1C(CC2)NC(=O)c1cc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O |THB:9:6:1:4| Show InChI InChI=1S/C25H24Cl2N4O5/c1-25(2)12-3-5-19(16(25)7-12)29-23(34)15-10-14(4-6-20(15)32)36-22-17(26)8-13(9-18(22)27)31-24(35)30-21(33)11-28-31/h4,6,8-12,16,19,32H,3,5,7H2,1-2H3,(H,29,34)(H,30,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123045
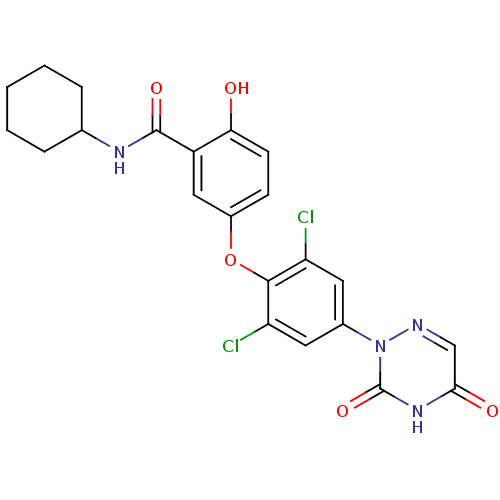
(CHEMBL413699 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)NC1CCCCC1 Show InChI InChI=1S/C22H20Cl2N4O5/c23-16-8-13(28-22(32)27-19(30)11-25-28)9-17(24)20(16)33-14-6-7-18(29)15(10-14)21(31)26-12-4-2-1-3-5-12/h6-12,29H,1-5H2,(H,26,31)(H,27,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123054
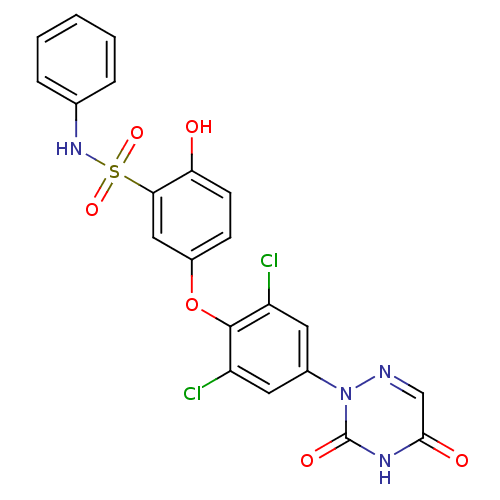
(5-(2,6-dichloro-4-(3,5-dioxo-4,5-dihydro-1,2,4-tri...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)Nc1ccccc1 Show InChI InChI=1S/C21H14Cl2N4O6S/c22-15-8-13(27-21(30)25-19(29)11-24-27)9-16(23)20(15)33-14-6-7-17(28)18(10-14)34(31,32)26-12-4-2-1-3-5-12/h1-11,26,28H,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123064
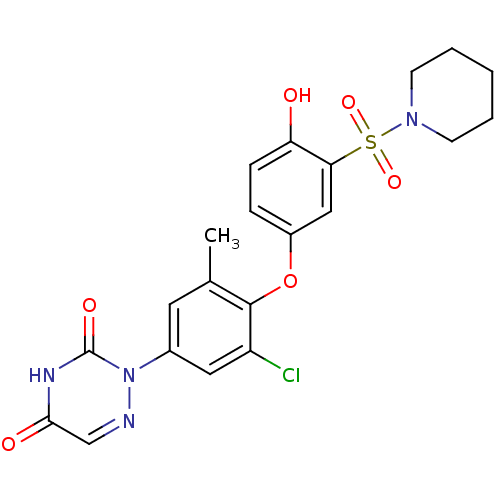
(2-(3-chloro-4-(4-hydroxy-3-(piperidin-1-ylsulfonyl...)Show SMILES Cc1cc(cc(Cl)c1Oc1ccc(O)c(c1)S(=O)(=O)N1CCCCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C21H21ClN4O6S/c1-13-9-14(26-21(29)24-19(28)12-23-26)10-16(22)20(13)32-15-5-6-17(27)18(11-15)33(30,31)25-7-3-2-4-8-25/h5-6,9-12,27H,2-4,7-8H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123063
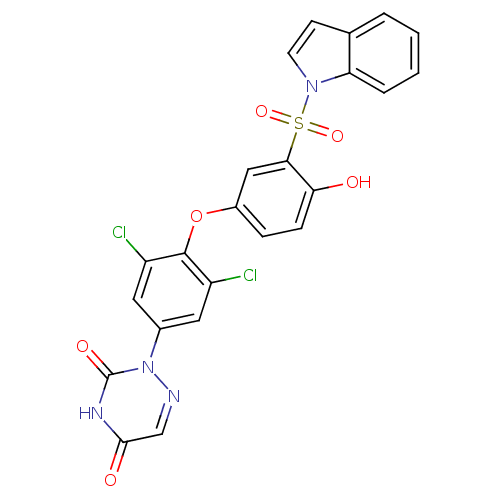
(2-{3,5-Dichloro-4-[4-hydroxy-3-(indole-1-sulfonyl)...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)n1ccc2ccccc12 Show InChI InChI=1S/C23H14Cl2N4O6S/c24-16-9-14(29-23(32)27-21(31)12-26-29)10-17(25)22(16)35-15-5-6-19(30)20(11-15)36(33,34)28-8-7-13-3-1-2-4-18(13)28/h1-12,30H,(H,27,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123052
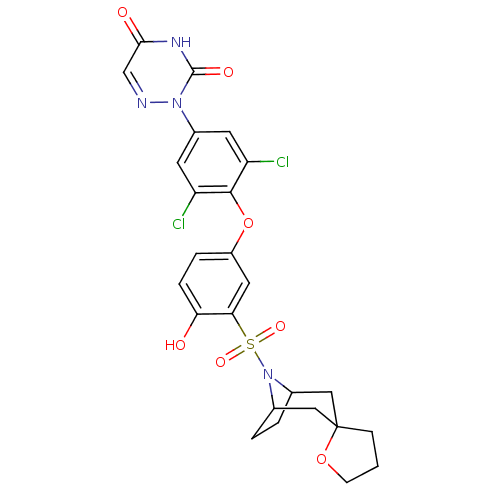
(2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-oxol...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1C2CCC1CC1(CCCO1)C2 |TLB:34:33:27:29.30,THB:24:27:33.38.32:29.30,37:33:27:29.30| Show InChI InChI=1S/C25H24Cl2N4O7S/c26-18-8-16(30-24(34)29-22(33)13-28-30)9-19(27)23(18)38-17-4-5-20(32)21(10-17)39(35,36)31-14-2-3-15(31)12-25(11-14)6-1-7-37-25/h4-5,8-10,13-15,32H,1-3,6-7,11-12H2,(H,29,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123061
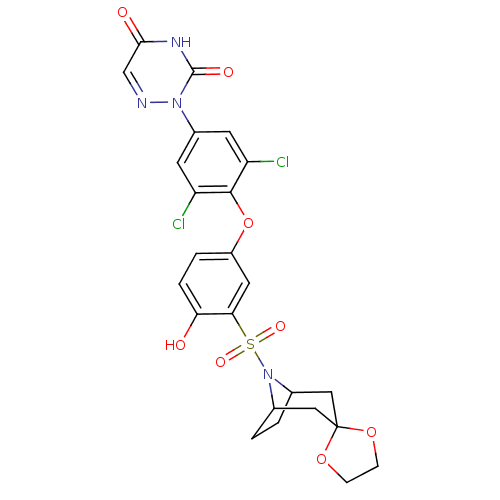
(2-[4-(3-{8-azaspiro[bicyclo[3.2.1]octane-3,2'-[1,3...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)N1C2CCC1CC1(C2)OCCO1 |THB:24:27:33.32.34:29.30| Show InChI InChI=1S/C24H22Cl2N4O8S/c25-17-7-15(29-23(33)28-21(32)12-27-29)8-18(26)22(17)38-16-3-4-19(31)20(9-16)39(34,35)30-13-1-2-14(30)11-24(10-13)36-5-6-37-24/h3-4,7-9,12-14,31H,1-2,5-6,10-11H2,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059
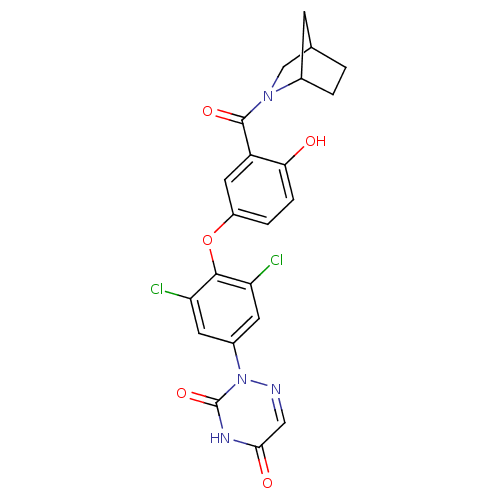
(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059

(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123057
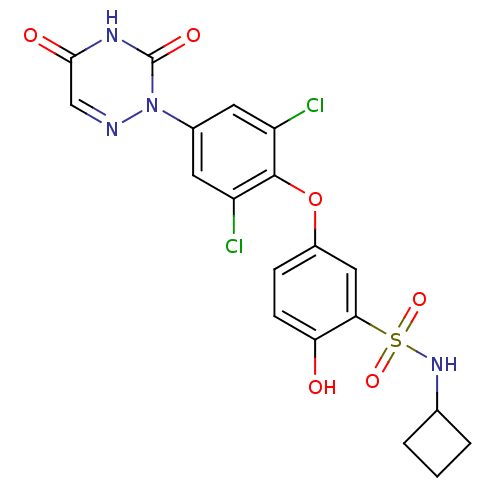
(CHEMBL124039 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)NC1CCC1 Show InChI InChI=1S/C19H16Cl2N4O6S/c20-13-6-11(25-19(28)23-17(27)9-22-25)7-14(21)18(13)31-12-4-5-15(26)16(8-12)32(29,30)24-10-2-1-3-10/h4-10,24,26H,1-3H2,(H,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123062
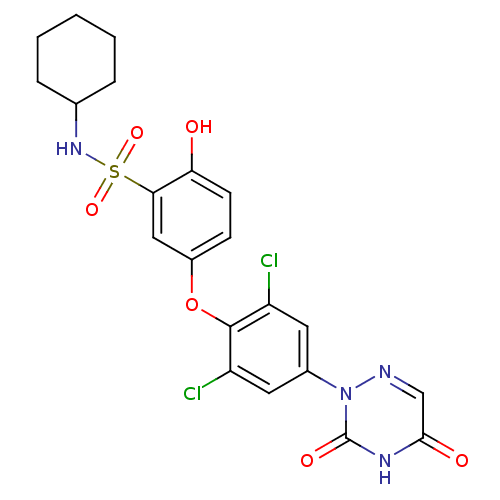
(CHEMBL340158 | N-Cyclohexyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1S(=O)(=O)NC1CCCCC1 Show InChI InChI=1S/C21H20Cl2N4O6S/c22-15-8-13(27-21(30)25-19(29)11-24-27)9-16(23)20(15)33-14-6-7-17(28)18(10-14)34(31,32)26-12-4-2-1-3-5-12/h6-12,26,28H,1-5H2,(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123056
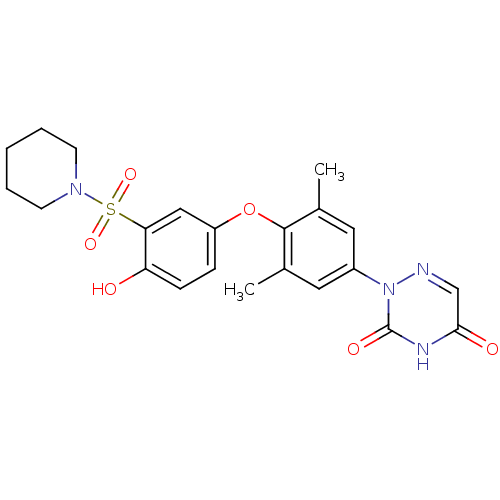
(2-(4-(4-hydroxy-3-(piperidin-1-ylsulfonyl)phenoxy)...)Show SMILES Cc1cc(cc(C)c1Oc1ccc(O)c(c1)S(=O)(=O)N1CCCCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C22H24N4O6S/c1-14-10-16(26-22(29)24-20(28)13-23-26)11-15(2)21(14)32-17-6-7-18(27)19(12-17)33(30,31)25-8-4-3-5-9-25/h6-7,10-13,27H,3-5,8-9H2,1-2H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123059

(2-{4-[3-(2-Aza-bicyclo[2.2.1]heptane-2-carbonyl)-4...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CC2CCC1C2 Show InChI InChI=1S/C22H18Cl2N4O5/c23-16-6-13(28-22(32)26-19(30)9-25-28)7-17(24)20(16)33-14-3-4-18(29)15(8-14)21(31)27-10-11-1-2-12(27)5-11/h3-4,6-9,11-12,29H,1-2,5,10H2,(H,26,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123049
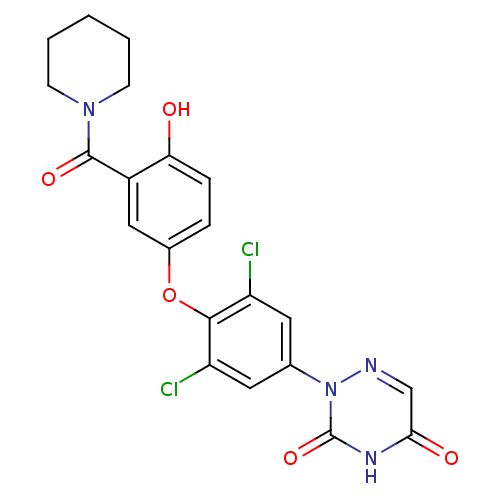
(2-(3,5-dichloro-4-(4-hydroxy-3-(piperidine-1-carbo...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CCCCC1 Show InChI InChI=1S/C21H18Cl2N4O5/c22-15-8-12(27-21(31)25-18(29)11-24-27)9-16(23)19(15)32-13-4-5-17(28)14(10-13)20(30)26-6-2-1-3-7-26/h4-5,8-11,28H,1-3,6-7H2,(H,25,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123050
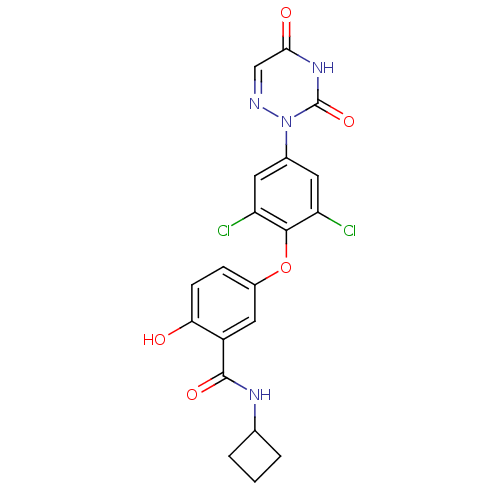
(CHEMBL124318 | N-Cyclobutyl-5-[2,6-dichloro-4-(3,5...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)NC1CCC1 Show InChI InChI=1S/C20H16Cl2N4O5/c21-14-6-11(26-20(30)25-17(28)9-23-26)7-15(22)18(14)31-12-4-5-16(27)13(8-12)19(29)24-10-2-1-3-10/h4-10,27H,1-3H2,(H,24,29)(H,25,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123055

(2-(3,5-dichloro-4-(4-hydroxy-3-(morpholine-4-carbo...)Show SMILES Oc1ccc(Oc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)cc1C(=O)N1CCOCC1 Show InChI InChI=1S/C20H16Cl2N4O6/c21-14-7-11(26-20(30)24-17(28)10-23-26)8-15(22)18(14)32-12-1-2-16(27)13(9-12)19(29)25-3-5-31-6-4-25/h1-2,7-10,27H,3-6H2,(H,24,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073839
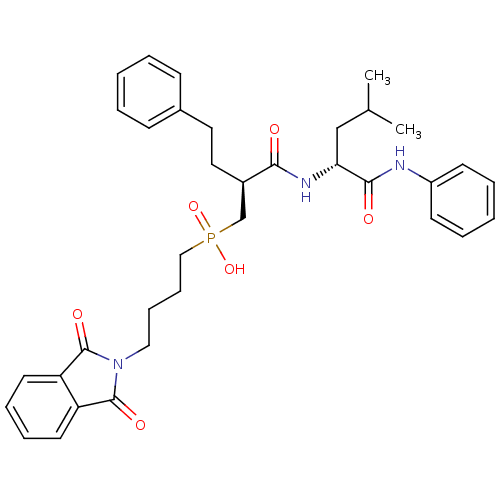
(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123060
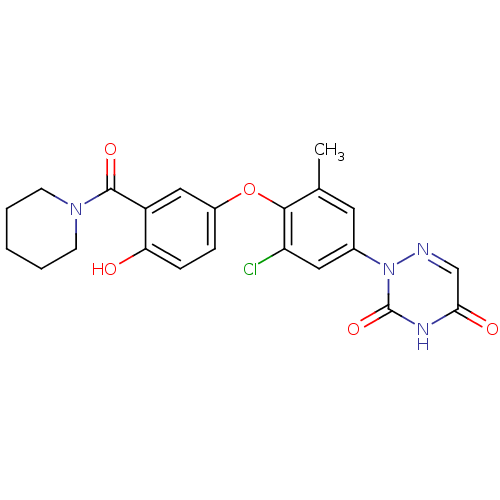
(2-{3-Chloro-4-[4-hydroxy-3-(piperidine-1-carbonyl)...)Show SMILES Cc1cc(cc(Cl)c1Oc1ccc(O)c(c1)C(=O)N1CCCCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C22H21ClN4O5/c1-13-9-14(27-22(31)25-19(29)12-24-27)10-17(23)20(13)32-15-5-6-18(28)16(11-15)21(30)26-7-3-2-4-8-26/h5-6,9-12,28H,2-4,7-8H2,1H3,(H,25,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123053
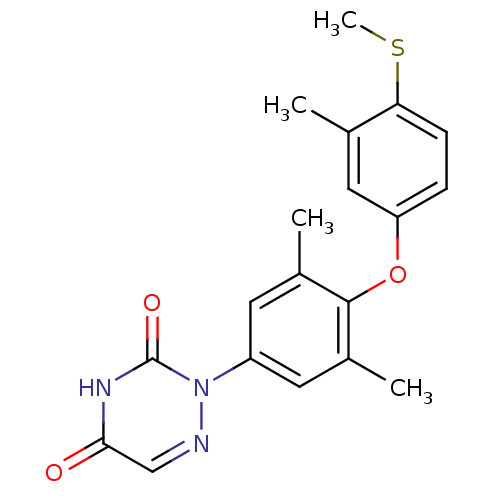
(2-[3,5-Dimethyl-4-(3-methyl-4-methylsulfanyl-pheno...)Show SMILES CSc1ccc(Oc2c(C)cc(cc2C)-n2ncc(=O)[nH]c2=O)cc1C Show InChI InChI=1S/C19H19N3O3S/c1-11-9-15(5-6-16(11)26-4)25-18-12(2)7-14(8-13(18)3)22-19(24)21-17(23)10-20-22/h5-10H,1-4H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123047

(2-{3-Chloro-4-[4-hydroxy-3-(4-methyl-piperazine-1-...)Show SMILES CN1CCN(CC1)S(=O)(=O)c1cc(Oc2c(C)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)ccc1O Show InChI InChI=1S/C21H22ClN5O6S/c1-13-9-14(27-21(30)24-19(29)12-23-27)10-16(22)20(13)33-15-3-4-17(28)18(11-15)34(31,32)26-7-5-25(2)6-8-26/h3-4,9-12,28H,5-8H2,1-2H3,(H,24,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50073839

(CHEMBL283066 | [4-(1,3-Dioxo-1,3-dihydro-isoindol-...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCN1C(=O)c2ccccc2C1=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C35H42N3O6P/c1-25(2)23-31(33(40)36-28-15-7-4-8-16-28)37-32(39)27(20-19-26-13-5-3-6-14-26)24-45(43,44)22-12-11-21-38-34(41)29-17-9-10-18-30(29)35(38)42/h3-10,13-18,25,27,31H,11-12,19-24H2,1-2H3,(H,36,40)(H,37,39)(H,43,44)/t27-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-2 (MMP-2) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123048
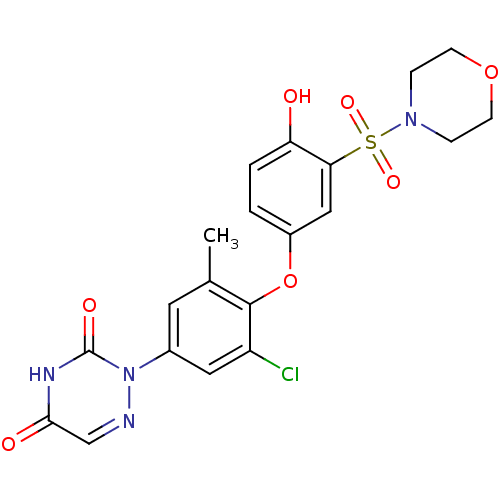
(2-(3-chloro-4-(4-hydroxy-3-(morpholinosulfonyl)phe...)Show SMILES Cc1cc(cc(Cl)c1Oc1ccc(O)c(c1)S(=O)(=O)N1CCOCC1)-n1ncc(=O)[nH]c1=O Show InChI InChI=1S/C20H19ClN4O7S/c1-12-8-13(25-20(28)23-18(27)11-22-25)9-15(21)19(12)32-14-2-3-16(26)17(10-14)33(29,30)24-4-6-31-7-5-24/h2-3,8-11,26H,4-7H2,1H3,(H,23,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50073823

(CHEMBL24871 | {4-[((S)-1-Acetyl-pyrrolidine-2-carb...)Show SMILES CC(C)C[C@@H](NC(=O)[C@H](CCc1ccccc1)CP(O)(=O)CCCCNC(=O)[C@@H]1CCCN1C(C)=O)C(=O)Nc1ccccc1 Show InChI InChI=1S/C34H49N4O6P/c1-25(2)23-30(33(41)36-29-15-8-5-9-16-29)37-32(40)28(19-18-27-13-6-4-7-14-27)24-45(43,44)22-11-10-20-35-34(42)31-17-12-21-38(31)26(3)39/h4-9,13-16,25,28,30-31H,10-12,17-24H2,1-3H3,(H,35,42)(H,36,41)(H,37,40)(H,43,44)/t28-,30-,31+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc
Curated by ChEMBL
| Assay Description
Inhibition of recombinant matrix metalloprotease-3 (MMP-3) |
Bioorg Med Chem Lett 9: 127-32 (1999)
BindingDB Entry DOI: 10.7270/Q2JD4VZ4 |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50123051
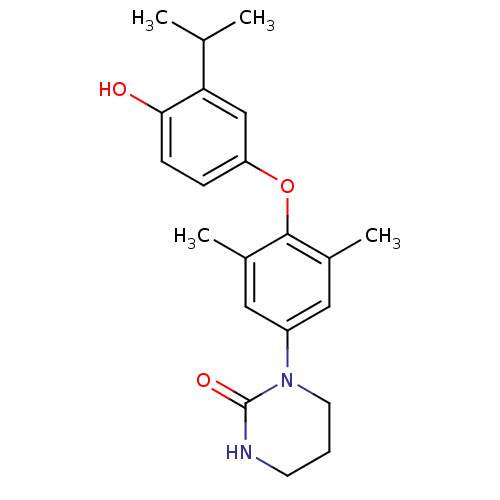
(1-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-...)Show InChI InChI=1S/C21H26N2O3/c1-13(2)18-12-17(6-7-19(18)24)26-20-14(3)10-16(11-15(20)4)23-9-5-8-22-21(23)25/h6-7,10-13,24H,5,8-9H2,1-4H3,(H,22,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against [125I]-T3 binding to human TRbeta1 receptor |
Bioorg Med Chem Lett 13: 379-82 (2003)
BindingDB Entry DOI: 10.7270/Q26T0KZB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224846
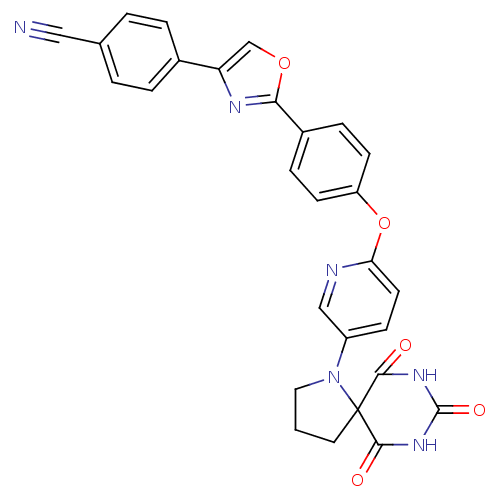
(4-(2-{4-[5-(6,8,10-trioxo-1,7,9-triaza-spiro[4.5]d...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc(co3)-c3ccc(cc3)C#N)nc2)C(=O)N1 Show InChI InChI=1S/C28H20N6O5/c29-14-17-2-4-18(5-3-17)22-16-38-24(31-22)19-6-9-21(10-7-19)39-23-11-8-20(15-30-23)34-13-1-12-28(34)25(35)32-27(37)33-26(28)36/h2-11,15-16H,1,12-13H2,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224838
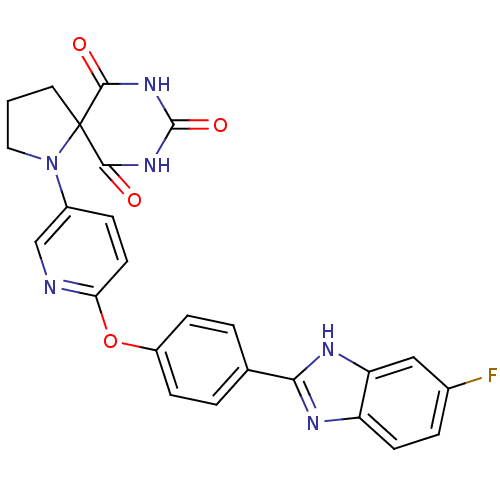
(1-{6-[4-(6-fluoro-1H-benzoimidazol-2-yl)-phenoxy]-...)Show SMILES Fc1ccc2nc([nH]c2c1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C25H19FN6O4/c26-15-4-8-18-19(12-15)29-21(28-18)14-2-6-17(7-3-14)36-20-9-5-16(13-27-20)32-11-1-10-25(32)22(33)30-24(35)31-23(25)34/h2-9,12-13H,1,10-11H2,(H,28,29)(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224842

(1-(6-{4-[4-(3-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1cccc(c1)-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-18-4-1-3-17(13-18)21-15-37-23(30-21)16-5-8-20(9-6-16)38-22-10-7-19(14-29-22)33-12-2-11-27(33)24(34)31-26(36)32-25(27)35/h1,3-10,13-15H,2,11-12H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224852
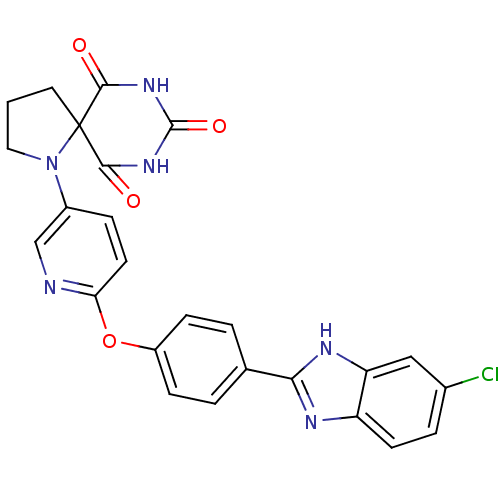
(1-{6-[4-(6-chloro-1H-benzoimidazol-2-yl)-phenoxy]-...)Show SMILES Clc1ccc2nc([nH]c2c1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C25H19ClN6O4/c26-15-4-8-18-19(12-15)29-21(28-18)14-2-6-17(7-3-14)36-20-9-5-16(13-27-20)32-11-1-10-25(32)22(33)30-24(35)31-23(25)34/h2-9,12-13H,1,10-11H2,(H,28,29)(H2,30,31,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224856

(1-{6-[4-(1H-benzoimidazol-2-yl)-phenoxy]-pyridin-3...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc4ccccc4[nH]3)nc2)C(=O)N1 Show InChI InChI=1S/C25H20N6O4/c32-22-25(23(33)30-24(34)29-22)12-3-13-31(25)16-8-11-20(26-14-16)35-17-9-6-15(7-10-17)21-27-18-4-1-2-5-19(18)28-21/h1-2,4-11,14H,3,12-13H2,(H,27,28)(H2,29,30,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224847
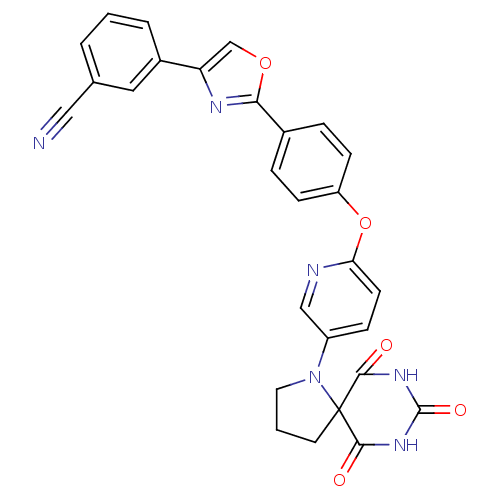
(3-(2-{4-[5-(6,8,10-trioxo-1,7,9-triaza-spiro[4.5]d...)Show SMILES O=C1NC(=O)C2(CCCN2c2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(c3)C#N)nc2)C(=O)N1 Show InChI InChI=1S/C28H20N6O5/c29-14-17-3-1-4-19(13-17)22-16-38-24(31-22)18-5-8-21(9-6-18)39-23-10-7-20(15-30-23)34-12-2-11-28(34)25(35)32-27(37)33-26(28)36/h1,3-10,13,15-16H,2,11-12H2,(H2,32,33,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224837

(1-(6-{4-[4-(4-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1ccc(cc1)-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-18-6-2-16(3-7-18)21-15-37-23(30-21)17-4-9-20(10-5-17)38-22-11-8-19(14-29-22)33-13-1-12-27(33)24(34)31-26(36)32-25(27)35/h2-11,14-15H,1,12-13H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224857
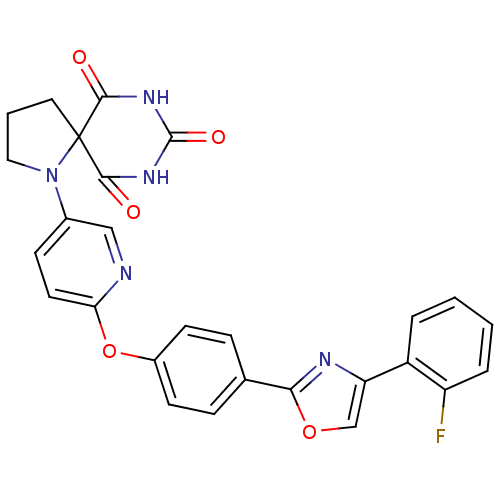
(1-(6-{4-[4-(2-fluoro-phenyl)-oxazol-2-yl]-phenoxy}...)Show SMILES Fc1ccccc1-c1coc(n1)-c1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C27H20FN5O5/c28-20-5-2-1-4-19(20)21-15-37-23(30-21)16-6-9-18(10-7-16)38-22-11-8-17(14-29-22)33-13-3-12-27(33)24(34)31-26(36)32-25(27)35/h1-2,4-11,14-15H,3,12-13H2,(H2,31,32,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50285792

(4-Oxo-piperidine-1-carboxylic acid {(S)-1-[(R)-1-(...)Show SMILES CSC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)N1CCC(=O)CC1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)CC(C)C Show InChI InChI=1S/C33H52N4O6S/c1-22(2)18-29(39)30(40)26(19-23-10-6-4-7-11-23)34-32(42)28(21-44-3)35-31(41)27(20-24-12-8-5-9-13-24)36-33(43)37-16-14-25(38)15-17-37/h5,8-9,12-13,22-23,26-30,39-40H,4,6-7,10-11,14-21H2,1-3H3,(H,34,42)(H,35,41)(H,36,43)/t26-,27-,28-,29-,30+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition against recombinant human renin (rHR) |
Bioorg Med Chem Lett 5: 2623-2626 (1995)
Article DOI: 10.1016/0960-894X(95)00456-4
BindingDB Entry DOI: 10.7270/Q2C24WDC |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194306

(5-(2-ethoxyethyl)-5-(4-(4-(4-(3-fluorophenyl)oxazo...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(F)c3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H24FN3O7/c1-2-37-15-14-29(26(34)32-28(36)33-27(29)35)40-23-12-10-22(11-13-23)39-21-8-6-18(7-9-21)25-31-24(17-38-25)19-4-3-5-20(30)16-19/h3-13,16-17H,2,14-15H2,1H3,(H2,32,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224845
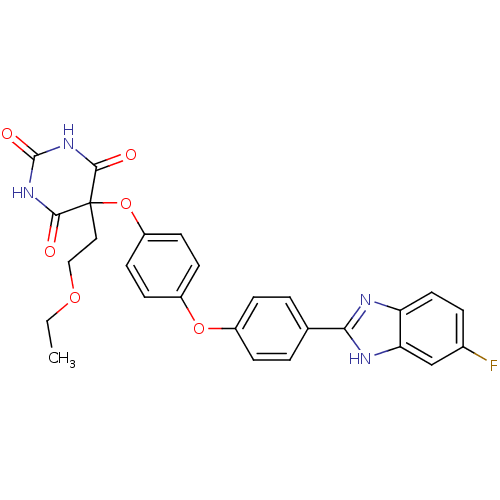
(5-(2-ethoxyethyl)-5-(4-(4-(6-fluoro-1H-benzo[d]imi...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc4ccc(F)cc4[nH]3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C27H23FN4O6/c1-2-36-14-13-27(24(33)31-26(35)32-25(27)34)38-20-10-8-19(9-11-20)37-18-6-3-16(4-7-18)23-29-21-12-5-17(28)15-22(21)30-23/h3-12,15H,2,13-14H2,1H3,(H,29,30)(H2,31,32,33,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194301
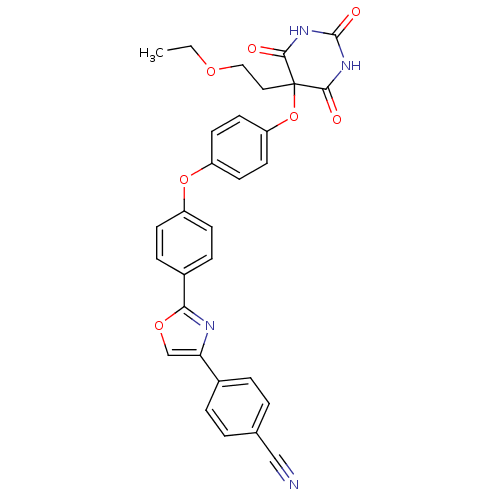
(4-(2-(4-(4-(5-(2-ethoxyethyl)-2,4,6-trioxo-hexahyd...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3ccc(cc3)C#N)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C30H24N4O7/c1-2-38-16-15-30(27(35)33-29(37)34-28(30)36)41-24-13-11-23(12-14-24)40-22-9-7-21(8-10-22)26-32-25(18-39-26)20-5-3-19(17-31)4-6-20/h3-14,18H,2,15-16H2,1H3,(H2,33,34,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224853
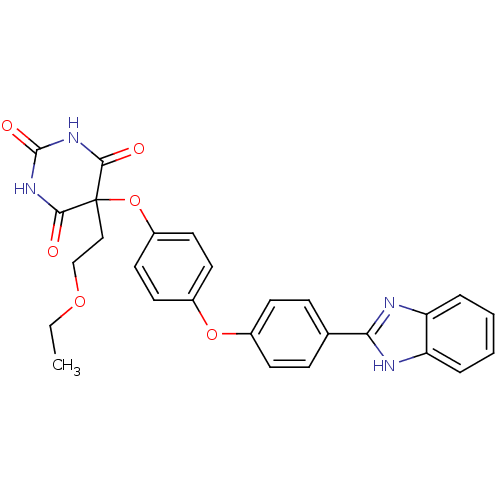
(5-(4-(4-(1H-benzo[d]imidazol-2-yl)phenoxy)phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc4ccccc4[nH]3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C27H24N4O6/c1-2-35-16-15-27(24(32)30-26(34)31-25(27)33)37-20-13-11-19(12-14-20)36-18-9-7-17(8-10-18)23-28-21-5-3-4-6-22(21)29-23/h3-14H,2,15-16H2,1H3,(H,28,29)(H2,30,31,32,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194304
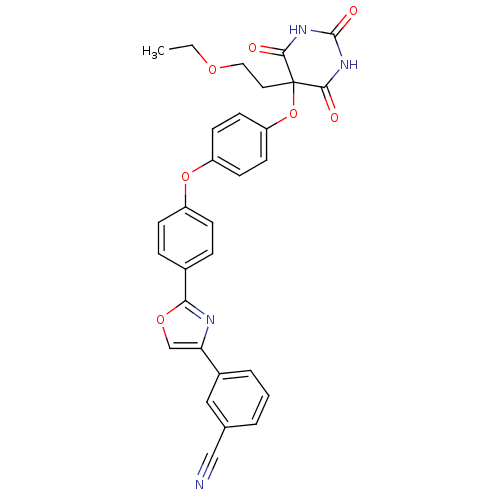
(3-(2-(4-(4-(5-(2-ethoxyethyl)-2,4,6-trioxo-hexahyd...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3cccc(c3)C#N)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C30H24N4O7/c1-2-38-15-14-30(27(35)33-29(37)34-28(30)36)41-24-12-10-23(11-13-24)40-22-8-6-20(7-9-22)26-32-25(18-39-26)21-5-3-4-19(16-21)17-31/h3-13,16,18H,2,14-15H2,1H3,(H2,33,34,35,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163809
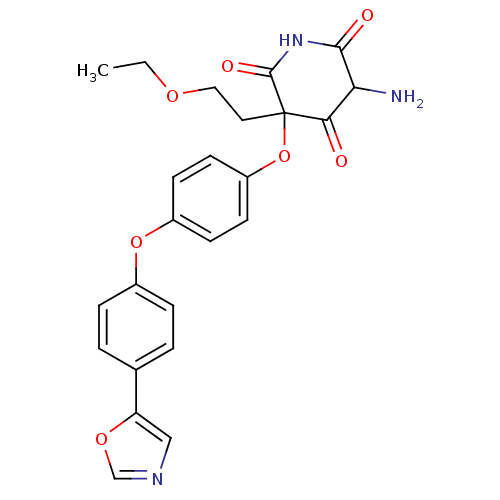
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-oxazol-5-yl-phe...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cnco3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H23N3O7/c1-2-31-12-11-24(21(28)20(25)22(29)27-23(24)30)34-18-9-7-17(8-10-18)33-16-5-3-15(4-6-16)19-13-26-14-32-19/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,27,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224849
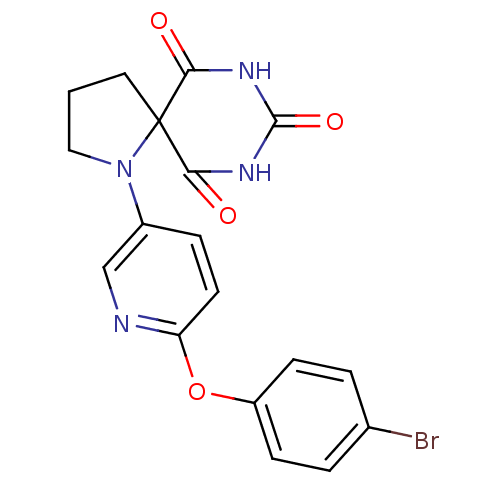
(1-[6-(4-bromo-phenoxy)-pyridin-3-yl]-1,7,9-triaza-...)Show SMILES Brc1ccc(Oc2ccc(cn2)N2CCCC22C(=O)NC(=O)NC2=O)cc1 Show InChI InChI=1S/C18H15BrN4O4/c19-11-2-5-13(6-3-11)27-14-7-4-12(10-20-14)23-9-1-8-18(23)15(24)21-17(26)22-16(18)25/h2-7,10H,1,8-9H2,(H2,21,22,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50224854
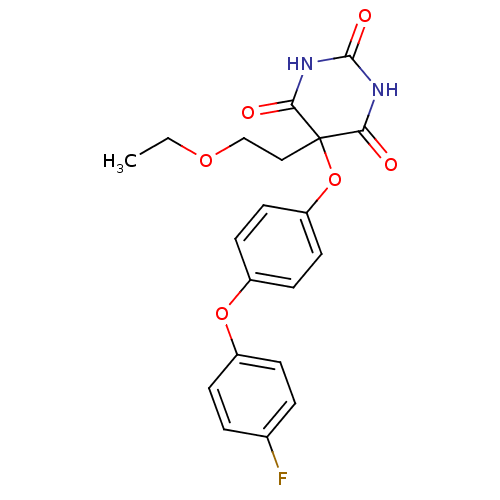
(5-(2-ethoxyethyl)-5-[4-(4-fluorophenoxy)phenoxy]ba...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(F)cc3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C20H19FN2O6/c1-2-27-12-11-20(17(24)22-19(26)23-18(20)25)29-16-9-7-15(8-10-16)28-14-5-3-13(21)4-6-14/h3-10H,2,11-12H2,1H3,(H2,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163808

(5-Amino-3-(2-ethoxy-ethyl)-3-{4-[4-(1H-pyrazol-4-y...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3cn[nH]c3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C24H24N4O6/c1-2-32-12-11-24(21(29)20(25)22(30)28-23(24)31)34-19-9-7-18(8-10-19)33-17-5-3-15(4-6-17)16-13-26-27-14-16/h3-10,13-14,20H,2,11-12,25H2,1H3,(H,26,27)(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50194289
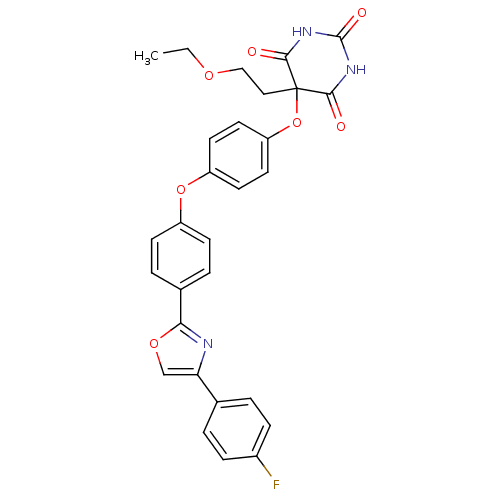
(5-(2-ethoxy-ethyl)-5-(4-{4-[4-(4-fluoro-phenyl)-ox...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(cc3)-c3nc(co3)-c3ccc(F)cc3)cc2)C(=O)NC(=O)NC1=O Show InChI InChI=1S/C29H24FN3O7/c1-2-37-16-15-29(26(34)32-28(36)33-27(29)35)40-23-13-11-22(12-14-23)39-21-9-5-19(6-10-21)25-31-24(17-38-25)18-3-7-20(30)8-4-18/h3-14,17H,2,15-16H2,1H3,(H2,32,33,34,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development
Curated by ChEMBL
| Assay Description
Inhibition of MMP13 |
Bioorg Med Chem Lett 17: 6529-34 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.085
BindingDB Entry DOI: 10.7270/Q2ZW1KMR |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163812
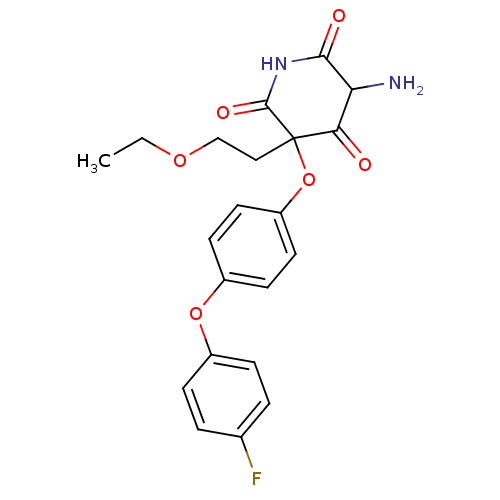
(5-Amino-3-(2-ethoxy-ethyl)-3-[4-(4-fluoro-phenoxy)...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(F)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21FN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-16-9-7-15(8-10-16)29-14-5-3-13(22)4-6-14/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50163807
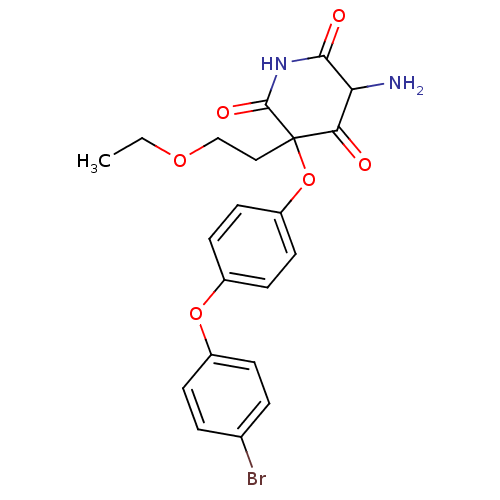
(5-Amino-3-[4-(4-bromo-phenoxy)-phenoxy]-3-(2-ethox...)Show SMILES CCOCCC1(Oc2ccc(Oc3ccc(Br)cc3)cc2)C(=O)NC(=O)C(N)C1=O Show InChI InChI=1S/C21H21BrN2O6/c1-2-28-12-11-21(18(25)17(23)19(26)24-20(21)27)30-16-9-7-15(8-10-16)29-14-5-3-13(22)4-6-14/h3-10,17H,2,11-12,23H2,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against matrix metalloprotease 13 |
Bioorg Med Chem Lett 15: 1807-10 (2005)
Article DOI: 10.1016/j.bmcl.2005.02.038
BindingDB Entry DOI: 10.7270/Q2JH3KPW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data