 Found 2801 hits with Last Name = 'hall' and Initial = 'c'
Found 2801 hits with Last Name = 'hall' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50007522
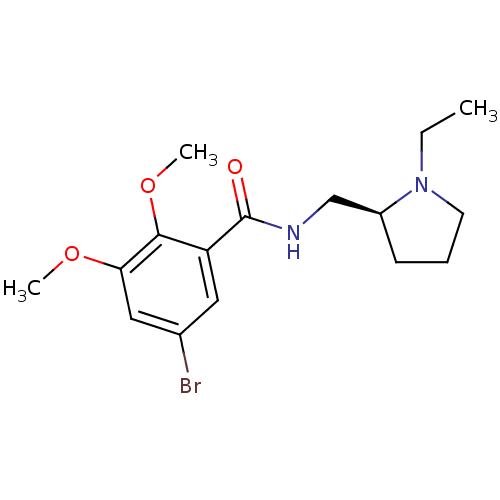
(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor (unknown origin) |
Bioorg Med Chem 16: 6467-73 (2008)
Article DOI: 10.1016/j.bmc.2008.05.039
BindingDB Entry DOI: 10.7270/Q2QN66JM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM82247

(8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...)Show InChI InChI=1S/C17H18ClNO/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12/h2-6,9-10,15,20H,7-8,11H2,1H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 113: 149-56 (1993)
Article DOI: 10.1007/BF02245691
BindingDB Entry DOI: 10.7270/Q29P3047 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM81778

(CAS_132421 | NNC-756 | NSC_132421)Show InChI InChI=1S/C19H20ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 113: 149-56 (1993)
Article DOI: 10.1007/BF02245691
BindingDB Entry DOI: 10.7270/Q29P3047 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50007522

(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor (unknown origin) |
Bioorg Med Chem 16: 6467-73 (2008)
Article DOI: 10.1016/j.bmc.2008.05.039
BindingDB Entry DOI: 10.7270/Q2QN66JM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253862
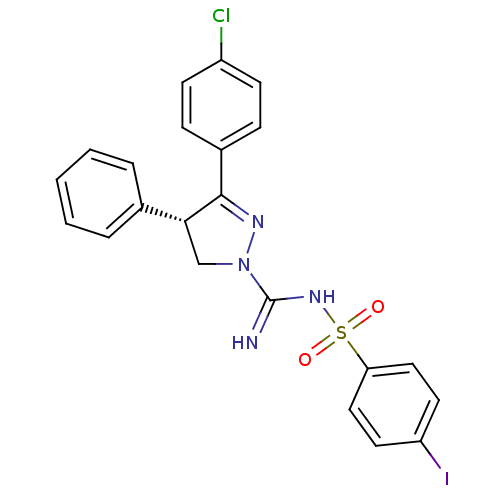
((-)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110577
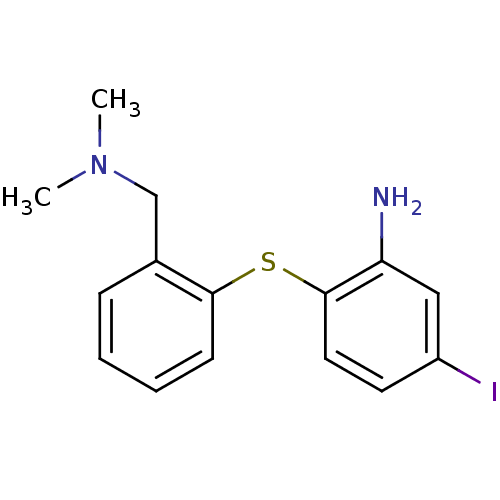
(2-(2-((dimethylamino)methyl)phenylthio)-5-iodoanil...)Show InChI InChI=1S/C15H17IN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253826
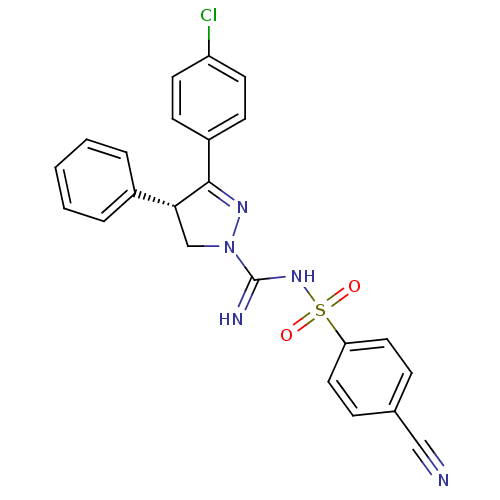
((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426188
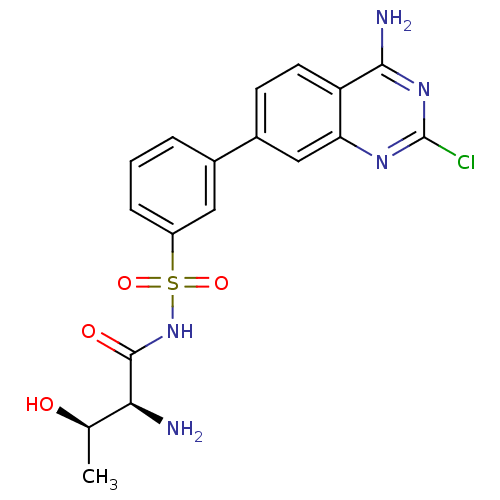
(CHEMBL2311920)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)nc(Cl)nc2c1 |r| Show InChI InChI=1S/C18H18ClN5O4S/c1-9(25)15(20)17(26)24-29(27,28)12-4-2-3-10(7-12)11-5-6-13-14(8-11)22-18(19)23-16(13)21/h2-9,15,25H,20H2,1H3,(H,24,26)(H2,21,22,23)/t9-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50433561
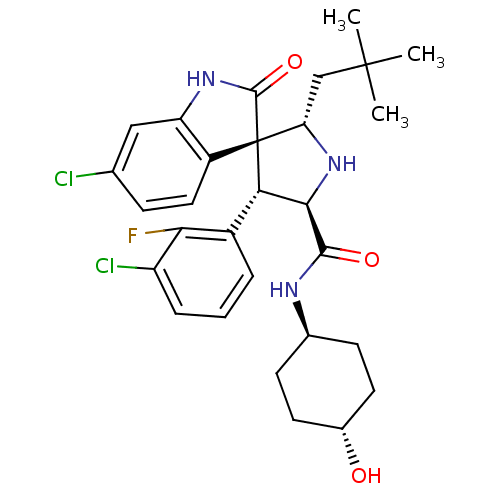
(CHEMBL2381408)Show SMILES CC(C)(C)C[C@@H]1N[C@H]([C@H](c2cccc(Cl)c2F)[C@]11C(=O)Nc2cc(Cl)ccc12)C(=O)N[C@H]1CC[C@H](O)CC1 |r,wU:8.8,5.4,31.34,wD:17.29,7.31,34.38,(40.88,-57.18,;39.34,-57.18,;38.57,-58.51,;40.11,-58.5,;38.57,-55.84,;37.04,-55.84,;37.52,-54.37,;36.27,-53.47,;35.03,-54.37,;33.51,-54.09,;32.52,-55.27,;31.01,-54.99,;30.49,-53.53,;31.49,-52.36,;30.98,-50.9,;33,-52.64,;34.01,-51.48,;35.49,-55.84,;36.38,-57.1,;37.93,-57.11,;35.48,-58.34,;34.01,-57.86,;32.69,-58.61,;31.36,-57.84,;30.03,-58.6,;31.37,-56.3,;32.71,-55.54,;34.03,-56.32,;36.26,-51.93,;34.93,-51.16,;37.6,-51.15,;37.6,-49.61,;36.26,-48.86,;36.26,-47.31,;37.6,-46.54,;37.6,-45,;38.93,-47.32,;38.93,-48.85,)| Show InChI InChI=1S/C29H34Cl2FN3O3/c1-28(2,3)14-22-29(19-12-7-15(30)13-21(19)34-27(29)38)23(18-5-4-6-20(31)24(18)32)25(35-22)26(37)33-16-8-10-17(36)11-9-16/h4-7,12-13,16-17,22-23,25,35-36H,8-11,14H2,1-3H3,(H,33,37)(H,34,38)/t16-,17-,22-,23-,25+,29+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to recombinant human His6-tagged HDM2 (1 to 118 residues) assessed as reduction in PMDM6-F binding incubated for 15 to 30 mins by fl... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01524
BindingDB Entry DOI: 10.7270/Q2Z03D1V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110782

(CHEMBL429065 | [5-Iodo-2-(2-methylaminomethyl-phen...)Show InChI InChI=1S/C15H16INOS/c1-17-9-11-4-2-3-5-14(11)19-15-7-6-13(16)8-12(15)10-18/h2-8,17-18H,9-10H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Translocator protein
(Rattus norvegicus (rat)) | BDBM22032
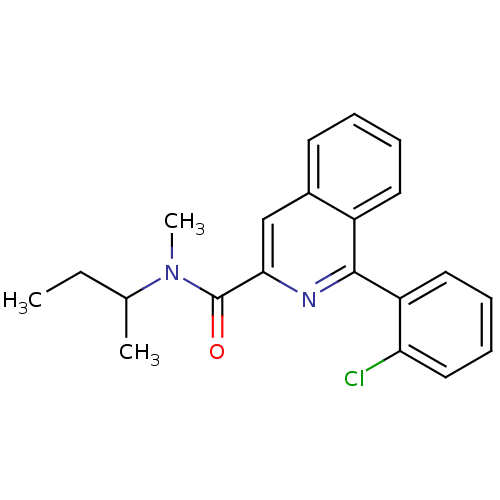
(1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)isoq...)Show InChI InChI=1S/C21H21ClN2O/c1-4-14(2)24(3)21(25)19-13-15-9-5-6-10-16(15)20(23-19)17-11-7-8-12-18(17)22/h5-14H,4H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO receptor in rat kidney membranes |
J Med Chem 54: 2961-70 (2011)
Article DOI: 10.1021/jm2000536
BindingDB Entry DOI: 10.7270/Q2KS6SJS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426188

(CHEMBL2311920)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)nc(Cl)nc2c1 |r| Show InChI InChI=1S/C18H18ClN5O4S/c1-9(25)15(20)17(26)24-29(27,28)12-4-2-3-10(7-12)11-5-6-13-14(8-11)22-18(19)23-16(13)21/h2-9,15,25H,20H2,1H3,(H,24,26)(H2,21,22,23)/t9-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110780
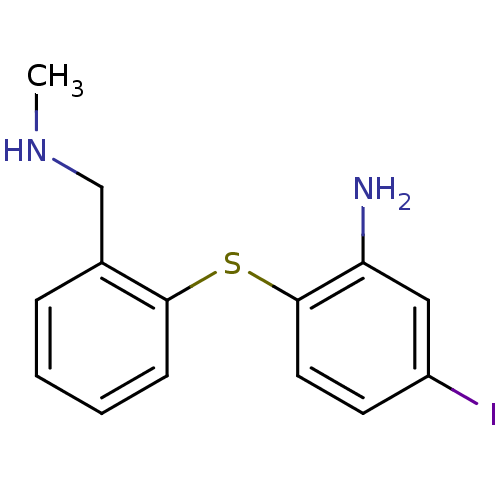
(5-Iodo-2-(2-methylaminomethyl-phenylsulfanyl)-phen...)Show InChI InChI=1S/C14H15IN2S/c1-17-9-10-4-2-3-5-13(10)18-14-7-6-11(15)8-12(14)16/h2-8,17H,9,16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from CB1 receptor |
J Med Chem 54: 2961-70 (2011)
Article DOI: 10.1021/jm2000536
BindingDB Entry DOI: 10.7270/Q2KS6SJS |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110777

(5-Bromo-2-(2-methylaminomethyl-phenylsulfanyl)-phe...)Show InChI InChI=1S/C14H15BrN2S/c1-17-9-10-4-2-3-5-13(10)18-14-7-6-11(15)8-12(14)16/h2-8,17H,9,16H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110788
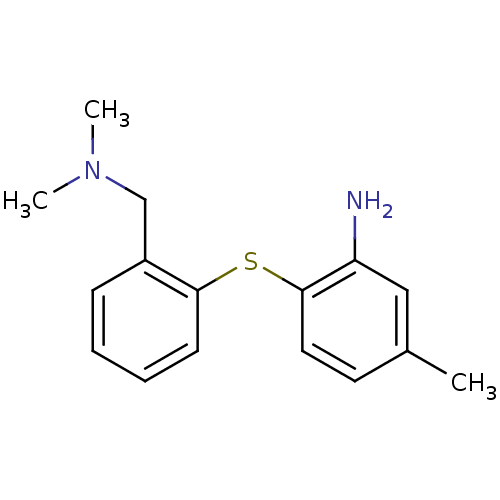
(2-(2-((dimethylamino)methyl)phenylthio)-5-methylan...)Show InChI InChI=1S/C16H20N2S/c1-12-8-9-16(14(17)10-12)19-15-7-5-4-6-13(15)11-18(2)3/h4-10H,11,17H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426182
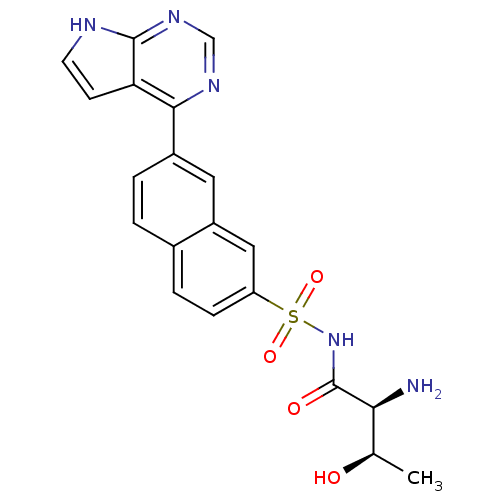
(CHEMBL2311926)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1ccc2ccc(cc2c1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H19N5O4S/c1-11(26)17(21)20(27)25-30(28,29)15-5-4-12-2-3-13(8-14(12)9-15)18-16-6-7-22-19(16)24-10-23-18/h2-11,17,26H,21H2,1H3,(H,25,27)(H,22,23,24)/t11-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426183
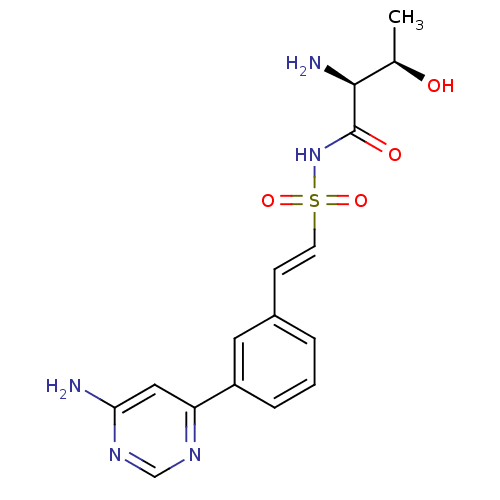
(CHEMBL2311925)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)\C=C\c1cccc(c1)-c1cc(N)ncn1 |r| Show InChI InChI=1S/C16H19N5O4S/c1-10(22)15(18)16(23)21-26(24,25)6-5-11-3-2-4-12(7-11)13-8-14(17)20-9-19-13/h2-10,15,22H,18H2,1H3,(H,21,23)(H2,17,19,20)/b6-5+/t10-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426182

(CHEMBL2311926)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1ccc2ccc(cc2c1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H19N5O4S/c1-11(26)17(21)20(27)25-30(28,29)15-5-4-12-2-3-13(8-14(12)9-15)18-16-6-7-22-19(16)24-10-23-18/h2-11,17,26H,21H2,1H3,(H,25,27)(H,22,23,24)/t11-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50073438
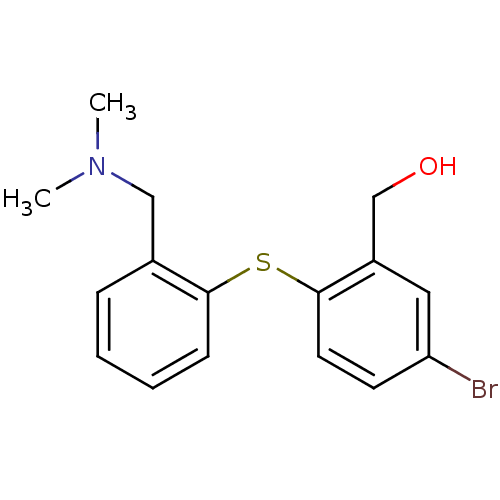
(CHEMBL278165 | [5-Bromo-2-(2-dimethylaminomethyl-p...)Show InChI InChI=1S/C16H18BrNOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426184
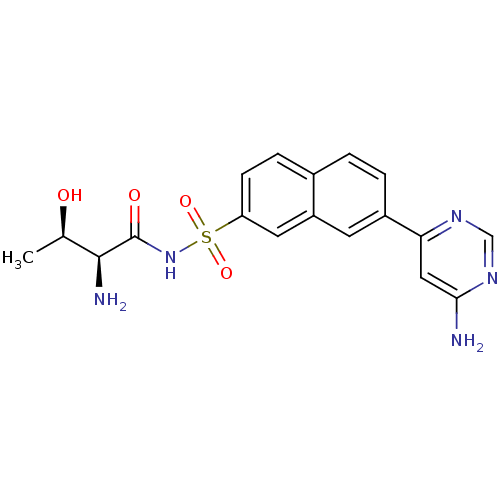
(CHEMBL2311924)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1ccc2ccc(cc2c1)-c1cc(N)ncn1 |r| Show InChI InChI=1S/C18H19N5O4S/c1-10(24)17(20)18(25)23-28(26,27)14-5-4-11-2-3-12(6-13(11)7-14)15-8-16(19)22-9-21-15/h2-10,17,24H,20H2,1H3,(H,23,25)(H2,19,21,22)/t10-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426190
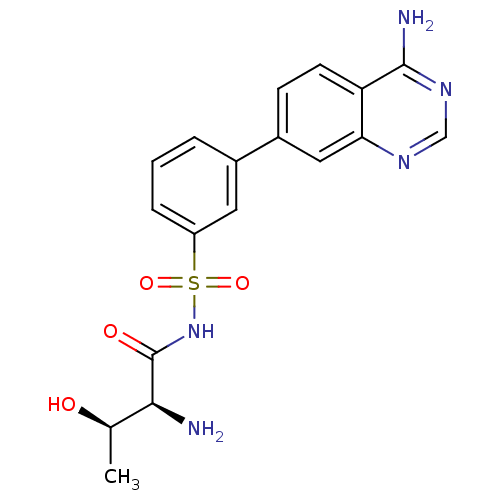
(CHEMBL2311918)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)ncnc2c1 |r| Show InChI InChI=1S/C18H19N5O4S/c1-10(24)16(19)18(25)23-28(26,27)13-4-2-3-11(7-13)12-5-6-14-15(8-12)21-9-22-17(14)20/h2-10,16,24H,19H2,1H3,(H,23,25)(H2,20,21,22)/t10-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110781

(5-Bromo-2-(2-dimethylaminomethyl-phenylsulfanyl)-p...)Show InChI InChI=1S/C15H17BrN2S/c1-18(2)10-11-5-3-4-6-14(11)19-15-8-7-12(16)9-13(15)17/h3-9H,10,17H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM22167
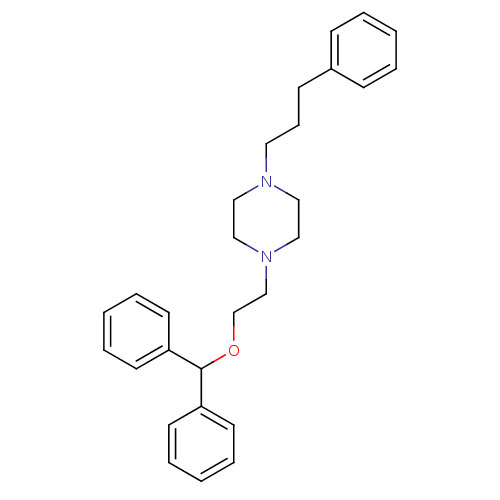
(1-[2-(diphenylmethoxy)ethyl]-4-(3-phenylpropyl)pip...)Show SMILES C(CN1CCN(CCOC(c2ccccc2)c2ccccc2)CC1)Cc1ccccc1 Show InChI InChI=1S/C28H34N2O/c1-4-11-25(12-5-1)13-10-18-29-19-21-30(22-20-29)23-24-31-28(26-14-6-2-7-15-26)27-16-8-3-9-17-27/h1-9,11-12,14-17,28H,10,13,18-24H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Univ. Tours
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 317: 147-52 (2006)
Article DOI: 10.1124/jpet.105.096792
BindingDB Entry DOI: 10.7270/Q2JW8CFZ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110784
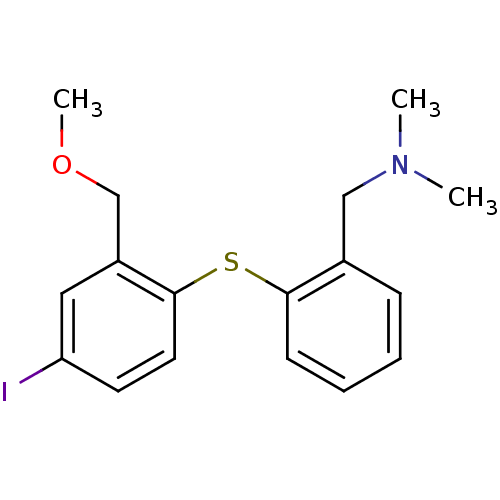
(CHEMBL284002 | [2-(4-Iodo-2-methoxymethyl-phenylsu...)Show InChI InChI=1S/C17H20INOS/c1-19(2)11-13-6-4-5-7-16(13)21-17-9-8-15(18)10-14(17)12-20-3/h4-10H,11-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426194
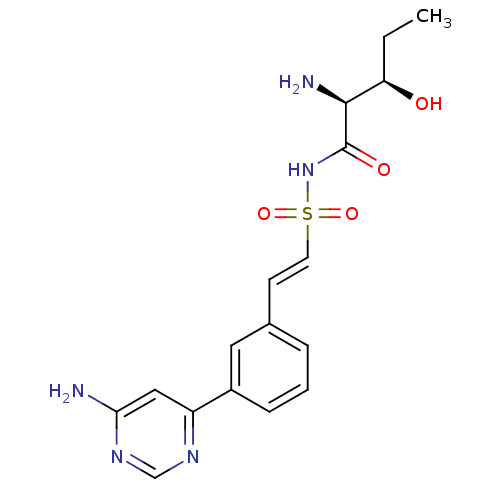
(CHEMBL2316966)Show SMILES CC[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)\C=C\c1cccc(c1)-c1cc(N)ncn1 |r| Show InChI InChI=1S/C17H21N5O4S/c1-2-14(23)16(19)17(24)22-27(25,26)7-6-11-4-3-5-12(8-11)13-9-15(18)21-10-20-13/h3-10,14,16,23H,2,19H2,1H3,(H,22,24)(H2,18,20,21)/b7-6+/t14-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110786

(CHEMBL22259 | [2-(4-Bromo-2-methoxymethyl-phenylsu...)Show InChI InChI=1S/C17H20BrNOS/c1-19(2)11-13-6-4-5-7-16(13)21-17-9-8-15(18)10-14(17)12-20-3/h4-10H,11-12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426184

(CHEMBL2311924)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1ccc2ccc(cc2c1)-c1cc(N)ncn1 |r| Show InChI InChI=1S/C18H19N5O4S/c1-10(24)17(20)18(25)23-28(26,27)14-5-4-11-2-3-12(6-13(11)7-14)15-8-16(19)22-9-21-15/h2-10,17,24H,20H2,1H3,(H,23,25)(H2,19,21,22)/t10-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426190

(CHEMBL2311918)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)ncnc2c1 |r| Show InChI InChI=1S/C18H19N5O4S/c1-10(24)16(19)18(25)23-28(26,27)13-4-2-3-11(7-13)12-5-6-14-15(8-12)21-9-22-17(14)20/h2-10,16,24H,19H2,1H3,(H,23,25)(H2,20,21,22)/t10-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50311076
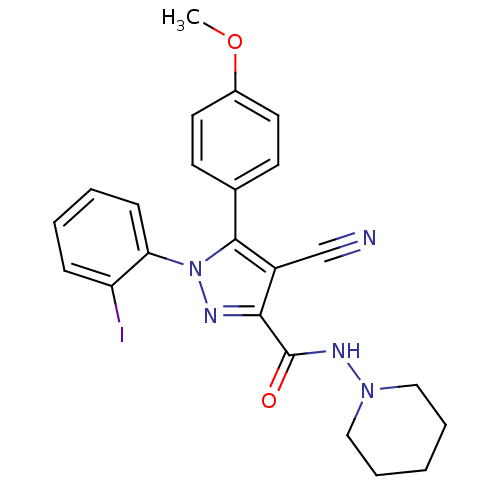
(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426180
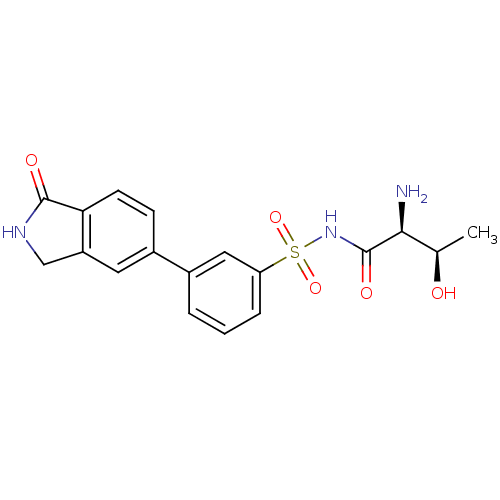
(CHEMBL2311928)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2C(=O)NCc2c1 |r| Show InChI InChI=1S/C18H19N3O5S/c1-10(22)16(19)18(24)21-27(25,26)14-4-2-3-11(8-14)12-5-6-15-13(7-12)9-20-17(15)23/h2-8,10,16,22H,9,19H2,1H3,(H,20,23)(H,21,24)/t10-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426183

(CHEMBL2311925)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)\C=C\c1cccc(c1)-c1cc(N)ncn1 |r| Show InChI InChI=1S/C16H19N5O4S/c1-10(22)15(18)16(23)21-26(24,25)6-5-11-3-2-4-12(7-11)13-8-14(17)20-9-19-13/h2-10,15,22H,18H2,1H3,(H,21,23)(H2,17,19,20)/b6-5+/t10-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM81778

(CAS_132421 | NNC-756 | NSC_132421)Show InChI InChI=1S/C19H20ClNO2/c1-21-7-5-13-9-17(20)18(22)10-15(13)16(11-21)14-4-2-3-12-6-8-23-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 113: 149-56 (1993)
Article DOI: 10.1007/BF02245691
BindingDB Entry DOI: 10.7270/Q29P3047 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426181

(CHEMBL2311927)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)N1CCc2ccc(cc2C1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C19H22N6O4S/c1-11(26)16(20)19(27)24-30(28,29)25-7-5-12-2-3-13(8-14(12)9-25)17-15-4-6-21-18(15)23-10-22-17/h2-4,6,8,10-11,16,26H,5,7,9,20H2,1H3,(H,24,27)(H,21,22,23)/t11-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426181

(CHEMBL2311927)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)N1CCc2ccc(cc2C1)-c1ncnc2[nH]ccc12 |r| Show InChI InChI=1S/C19H22N6O4S/c1-11(26)16(20)19(27)24-30(28,29)25-7-5-12-2-3-13(8-14(12)9-25)17-15-4-6-21-18(15)23-10-22-17/h2-4,6,8,10-11,16,26H,5,7,9,20H2,1H3,(H,24,27)(H,21,22,23)/t11-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM81779
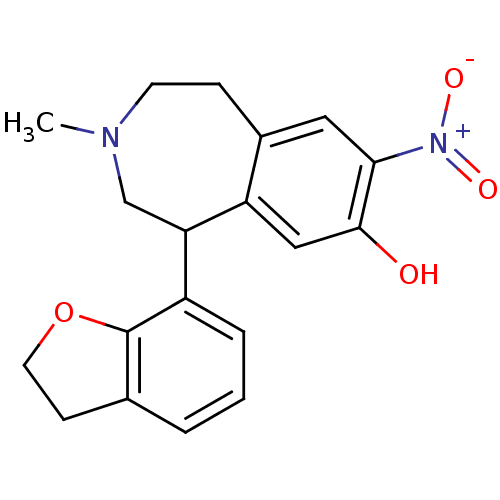
(CAS_164252 | NNC-687 | NSC_164252)Show SMILES CN1CCc2cc(c(O)cc2C(C1)c1cccc2CCOc12)[N+]([O-])=O Show InChI InChI=1S/C19H20N2O4/c1-20-7-5-13-9-17(21(23)24)18(22)10-15(13)16(11-20)14-4-2-3-12-6-8-25-19(12)14/h2-4,9-10,16,22H,5-8,11H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 113: 149-56 (1993)
Article DOI: 10.1007/BF02245691
BindingDB Entry DOI: 10.7270/Q29P3047 |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50073438

(CHEMBL278165 | [5-Bromo-2-(2-dimethylaminomethyl-p...)Show InChI InChI=1S/C16H18BrNOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-nisoxatine binding to norepinephrine transporter (NET) of rat cortical homogenates |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199005
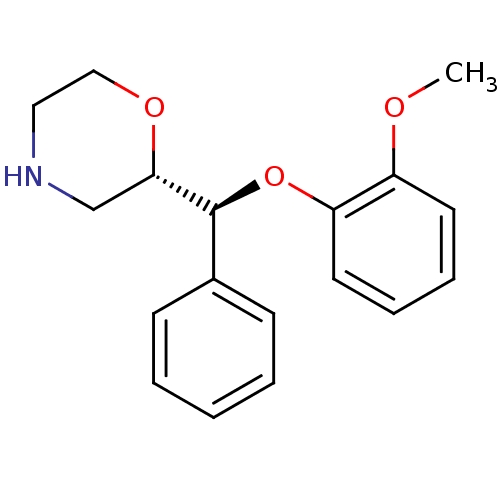
((S)-2-((S)-(2-methoxyphenoxy)(phenyl)methyl)morpho...)Show SMILES COc1ccccc1O[C@H]([C@@H]1CNCCO1)c1ccccc1 |r| Show InChI InChI=1S/C18H21NO3/c1-20-15-9-5-6-10-16(15)22-18(14-7-3-2-4-8-14)17-13-19-11-12-21-17/h2-10,17-19H,11-13H2,1H3/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50110783

(CHEMBL22743 | [2-(2-Dimethylaminomethyl-phenylsulf...)Show InChI InChI=1S/C16H18FNOS/c1-18(2)10-12-5-3-4-6-15(12)20-16-8-7-14(17)9-13(16)11-19/h3-9,19H,10-11H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 7.96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-paroxetine binding to serotonin transporter (SERT) of rat cortical membrane |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426189
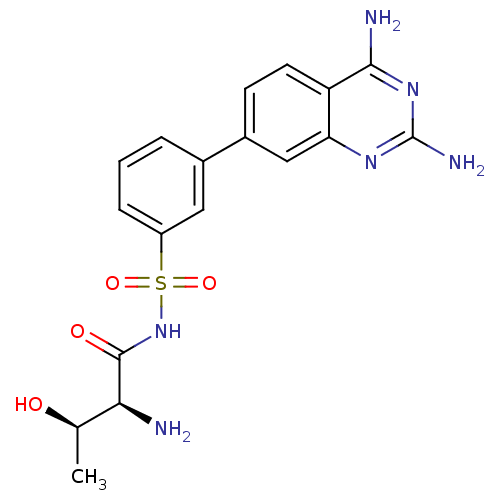
(CHEMBL2311919)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)nc(N)nc2c1 |r| Show InChI InChI=1S/C18H20N6O4S/c1-9(25)15(19)17(26)24-29(27,28)12-4-2-3-10(7-12)11-5-6-13-14(8-11)22-18(21)23-16(13)20/h2-9,15,25H,19H2,1H3,(H,24,26)(H4,20,21,22,23)/t9-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199003
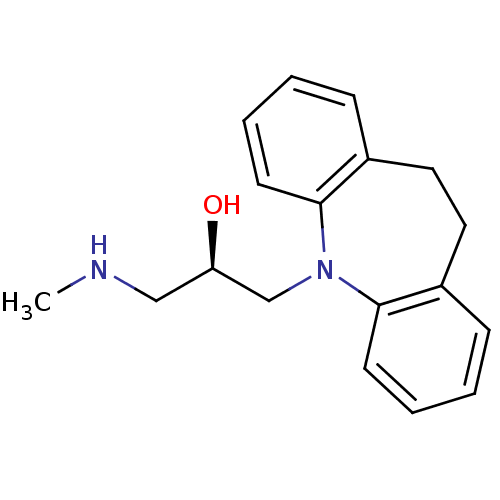
((R)-1-(10,11-dihydro-dibenzo[b,f]azepin-5-yl)-3-me...)Show InChI InChI=1S/C18H22N2O/c1-19-12-16(21)13-20-17-8-4-2-6-14(17)10-11-15-7-3-5-9-18(15)20/h2-9,16,19,21H,10-13H2,1H3/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426187
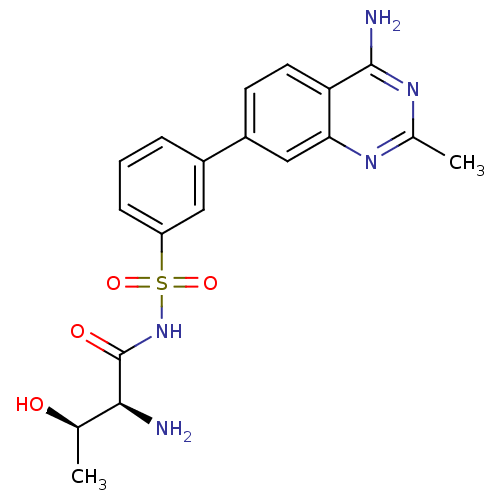
(CHEMBL2311921)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)nc(C)nc2c1 |r| Show InChI InChI=1S/C19H21N5O4S/c1-10(25)17(20)19(26)24-29(27,28)14-5-3-4-12(8-14)13-6-7-15-16(9-13)22-11(2)23-18(15)21/h3-10,17,25H,20H2,1-2H3,(H,24,26)(H2,21,22,23)/t10-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Threonine--tRNA ligase
(Yersinia pestis) | BDBM50426200
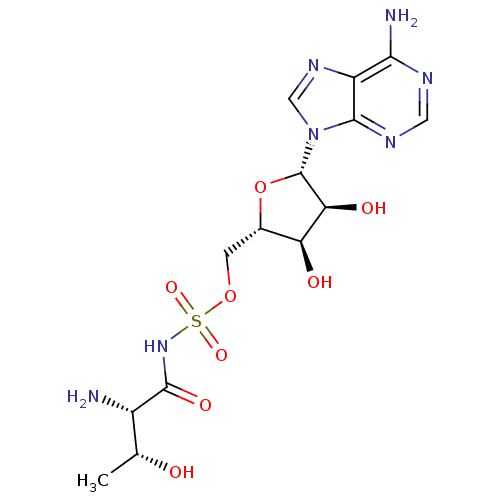
(CHEMBL2311917)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)OC[C@@H]1O[C@@H]([C@@H](O)[C@H]1O)n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C14H21N7O8S/c1-5(22)7(15)13(25)20-30(26,27)28-2-6-9(23)10(24)14(29-6)21-4-19-8-11(16)17-3-18-12(8)21/h3-7,9-10,14,22-24H,2,15H2,1H3,(H,20,25)(H2,16,17,18)/t5-,6+,7+,9+,10+,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to Yersinia pestis ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Threonine--tRNA ligase 1, cytoplasmic
(Homo sapiens (Human)) | BDBM50426189

(CHEMBL2311919)Show SMILES C[C@@H](O)[C@H](N)C(=O)NS(=O)(=O)c1cccc(c1)-c1ccc2c(N)nc(N)nc2c1 |r| Show InChI InChI=1S/C18H20N6O4S/c1-9(25)15(19)17(26)24-29(27,28)12-4-2-3-10(7-12)11-5-6-13-14(8-11)22-18(21)23-16(13)20/h2-9,15,25H,19H2,1H3,(H,24,26)(H4,20,21,22,23)/t9-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Trius Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human cytoplasmic ThrRS by coupled spectrophotometry |
J Med Chem 56: 1748-60 (2013)
Article DOI: 10.1021/jm301756m
BindingDB Entry DOI: 10.7270/Q2GQ7036 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199004
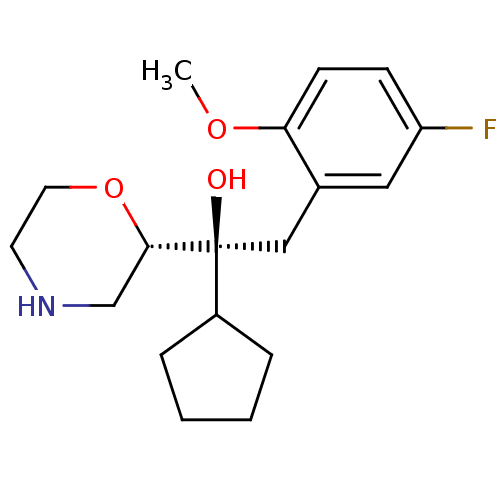
((S,S)-1-cyclopentyl-2-(5-fluoro-2-methoxy-phenyl)-...)Show SMILES COc1ccc(F)cc1C[C@](O)(C1CCCC1)[C@@H]1CNCCO1 Show InChI InChI=1S/C18H26FNO3/c1-22-16-7-6-15(19)10-13(16)11-18(21,14-4-2-3-5-14)17-12-20-8-9-23-17/h6-7,10,14,17,20-21H,2-5,8-9,11-12H2,1H3/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50296783
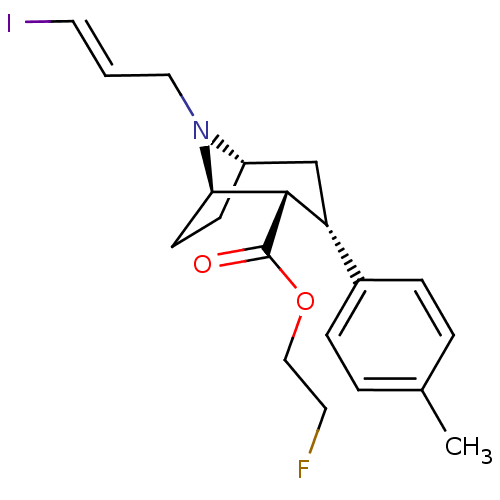
((E)-N-(3-iodoprop-2-enyl)-2beta-carbofluoroethoxy-...)Show SMILES Cc1ccc(cc1)[C@H]1C[C@@H]2CC[C@H]([C@H]1C(=O)OCCF)N2C\C=C\I |r,THB:21:20:13.7.8:11.10| Show InChI InChI=1S/C20H25FINO2/c1-14-3-5-15(6-4-14)17-13-16-7-8-18(23(16)11-2-10-22)19(17)20(24)25-12-9-21/h2-6,10,16-19H,7-9,11-13H2,1H3/b10-2+/t16-,17+,18+,19-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Displacement of [125I]PE2I from rat DAT |
Bioorg Med Chem Lett 19: 4843-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.032
BindingDB Entry DOI: 10.7270/Q2VH5NWZ |
More data for this
Ligand-Target Pair | |
Transporter
(Rattus norvegicus) | BDBM50110782

(CHEMBL429065 | [5-Iodo-2-(2-methylaminomethyl-phen...)Show InChI InChI=1S/C15H16INOS/c1-17-9-11-4-2-3-5-14(11)19-15-7-6-13(16)8-12(15)10-18/h2-8,17-18H,9-10H2,1H3 | Reactome pathway
KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 12.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-nisoxatine binding to norepinephrine transporter (NET) of rat cortical homogenates |
J Med Chem 45: 1253-8 (2002)
BindingDB Entry DOI: 10.7270/Q2TH8M15 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data