

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28206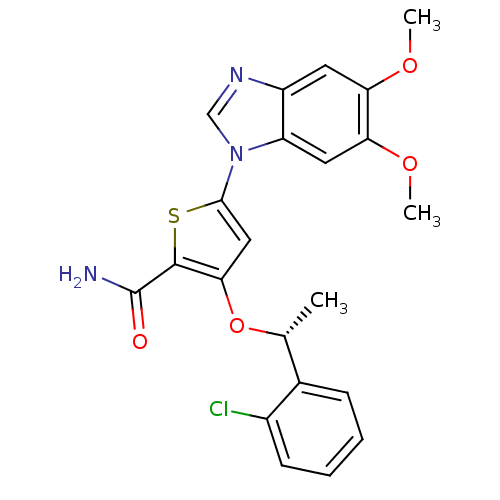 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28208 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-methoxy-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM25004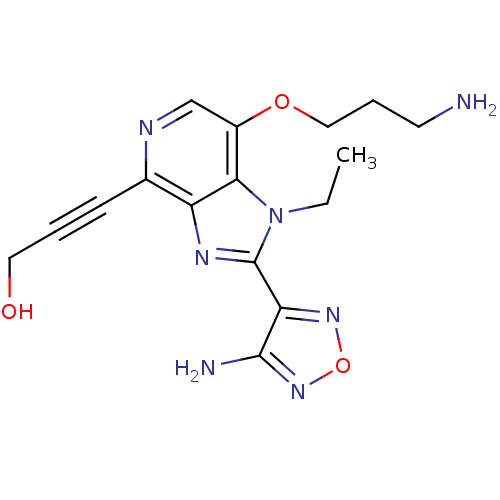 (3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM24994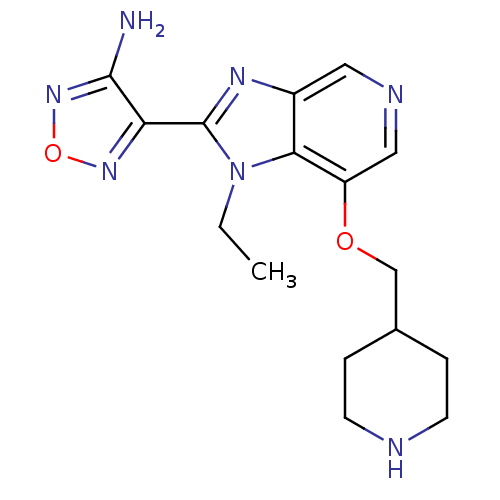 (4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28210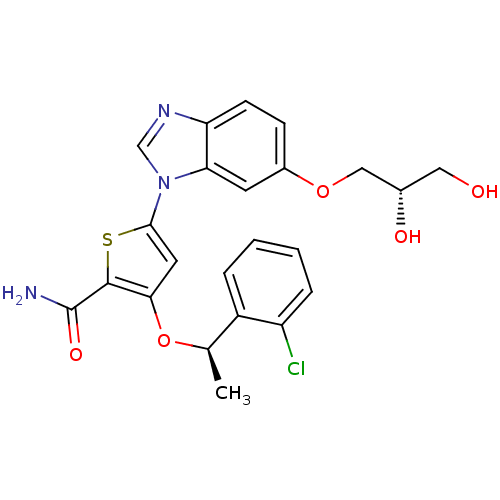 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2,3-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28175 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM25120 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28177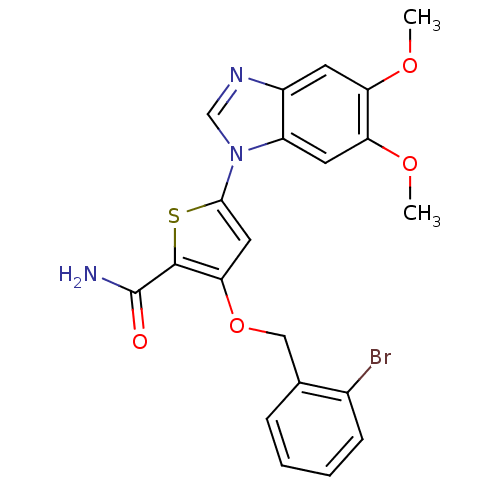 (3-[(2-bromophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178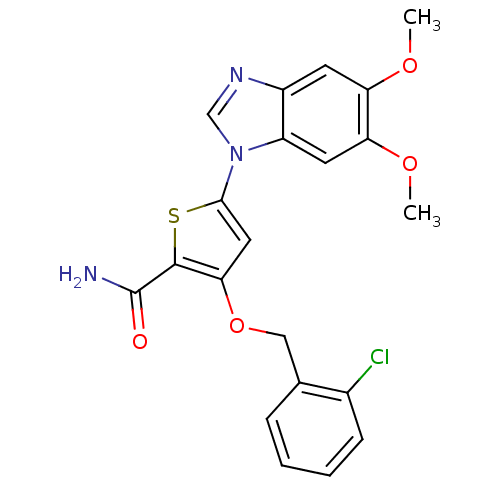 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28194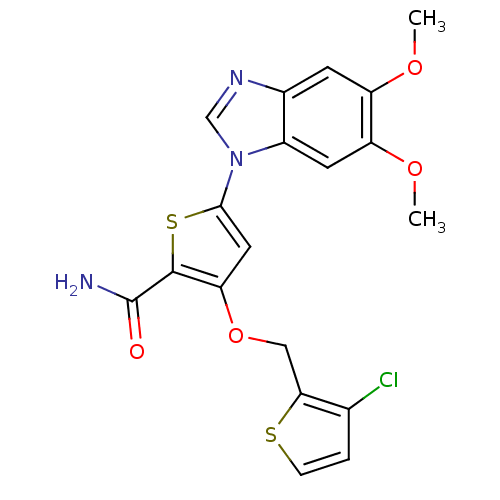 (3-[(3-chlorothiophen-2-yl)methoxy]-5-(5,6-dimethox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25013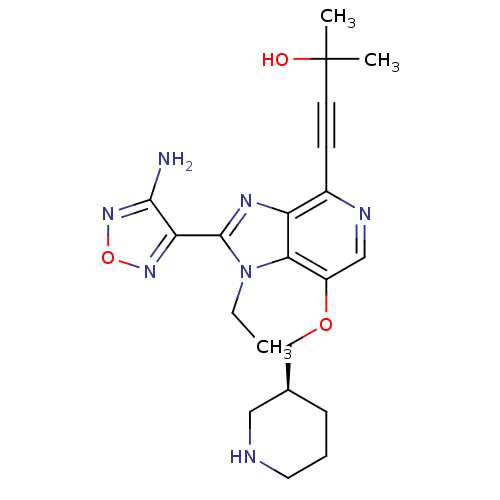 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28178 (3-[(2-chlorophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28216 (5-{6-[(1-methylpiperidin-4-yl)oxy]-1H-1,3-benzodia...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28217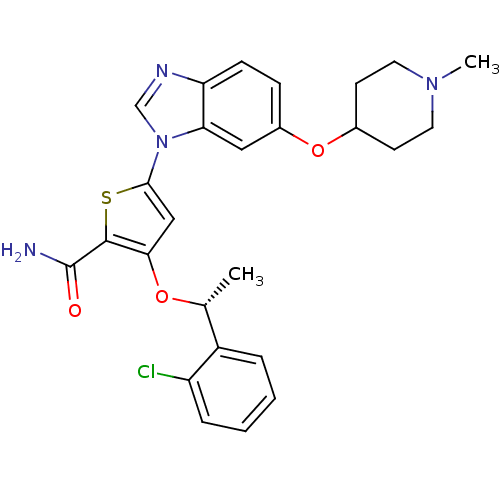 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25004 (3-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25009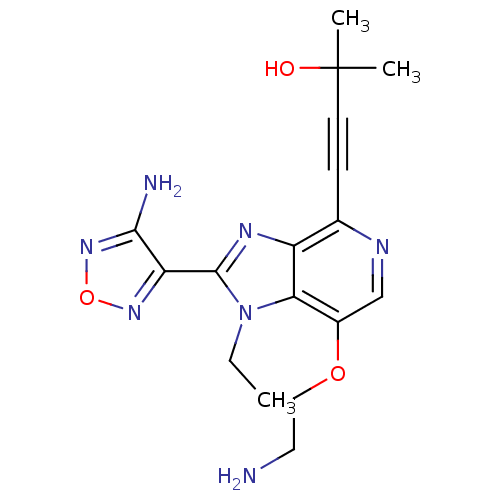 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(2-aminoetho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28200 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28209 (5-(6-methoxy-1H-1,3-benzodiazol-1-yl)-3-[(1R)-1-[2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28212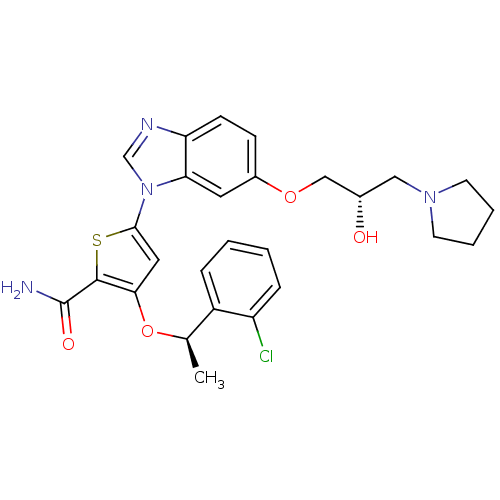 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(2S)-2-hyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28215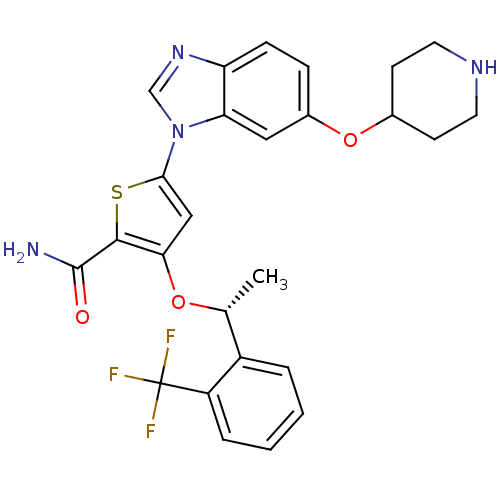 (5-[6-(piperidin-4-yloxy)-1H-1,3-benzodiazol-1-yl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28218 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4S)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24994 (4-[1-ethyl-7-(piperidin-4-ylmethoxy)-1H-imidazo[4,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25014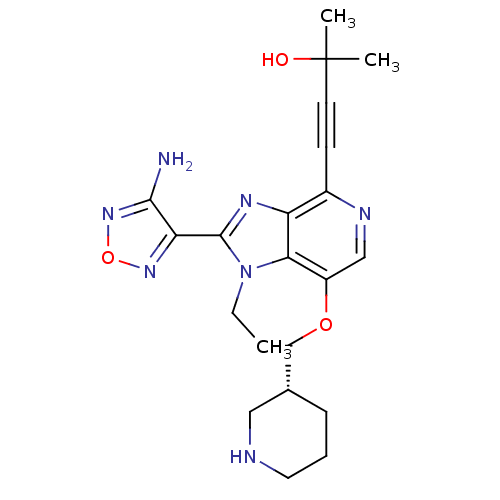 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25016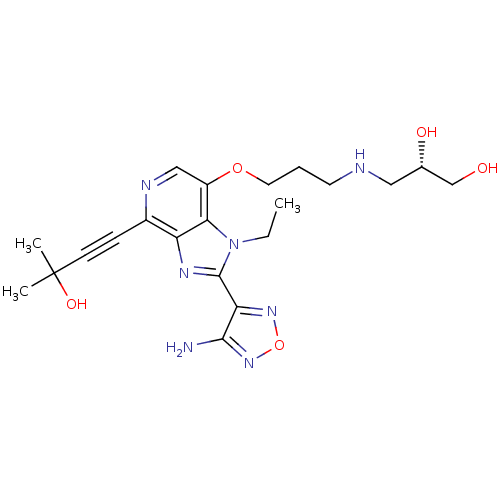 ((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-5 (Homo sapiens (Human)) | BDBM24991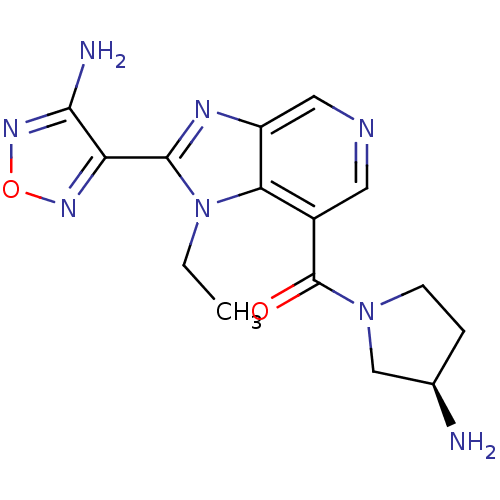 (4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25010 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28169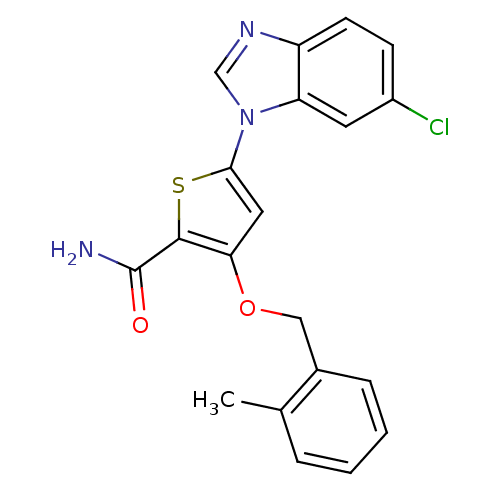 (5-(6-chloro-1H-1,3-benzodiazol-1-yl)-3-[(2-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25010 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-[(3S)-3-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28219 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(6-{[(4R)-1-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28211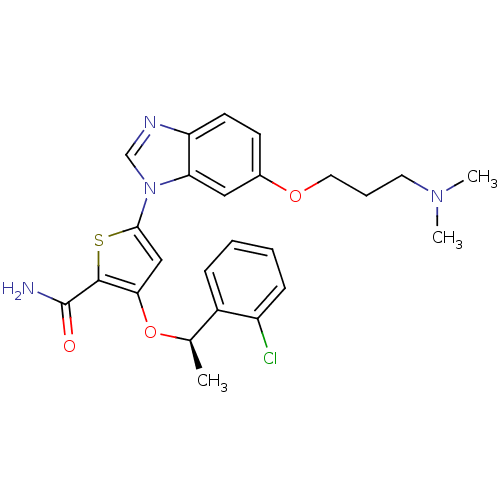 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[3-(dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28182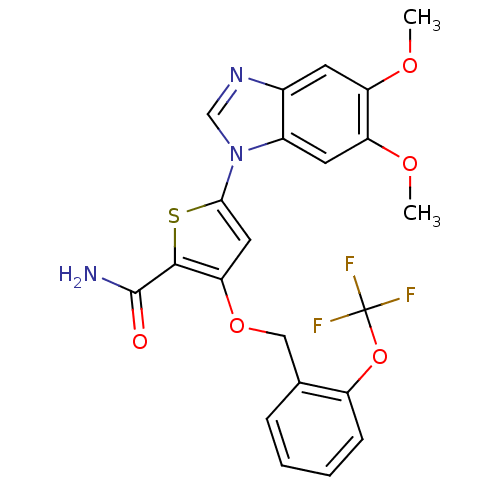 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25015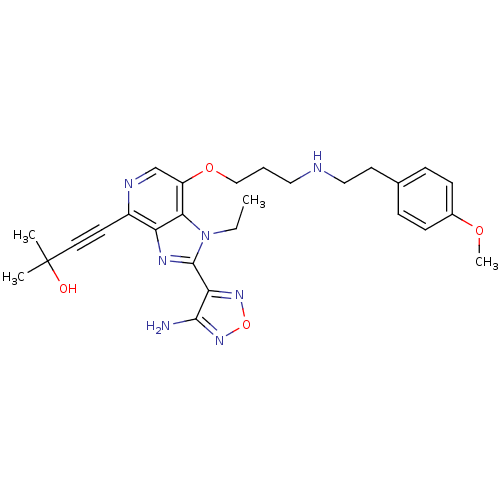 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(3-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24991 (4-(7-{[(3R)-3-aminopyrrolidin-1-yl]carbonyl}-1-eth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24990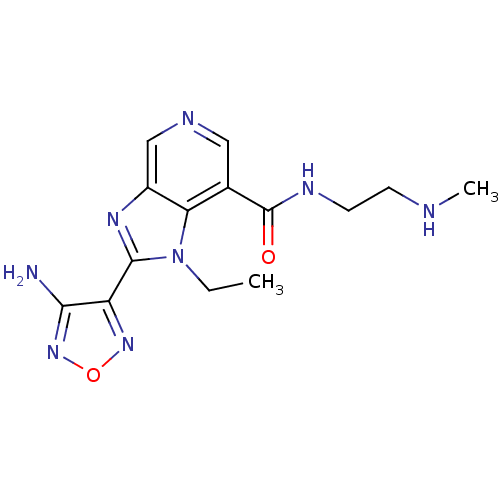 (2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-[2-(met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25016 ((2S)-3-[(3-{[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28179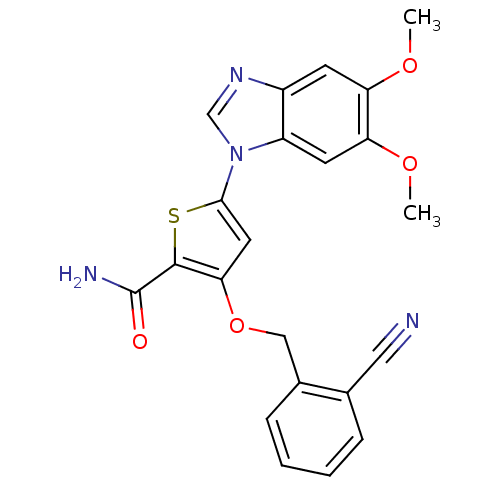 (3-[(2-cyanophenyl)methoxy]-5-(5,6-dimethoxy-1H-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28202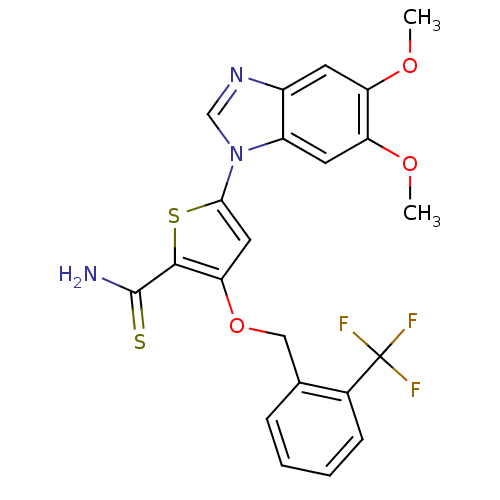 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28214 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-{6-[(1-methylp...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribosomal protein S6 kinase alpha-1 (Homo sapiens (Human)) | BDBM25005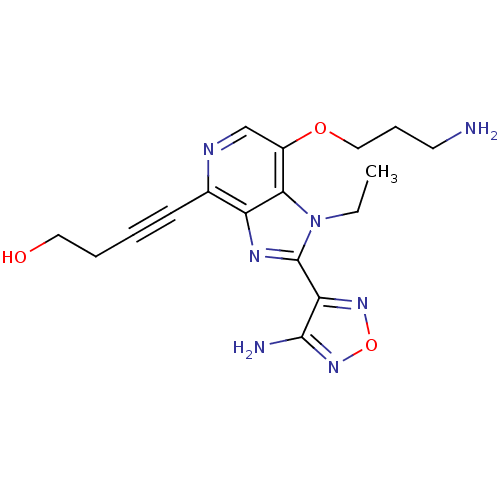 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25003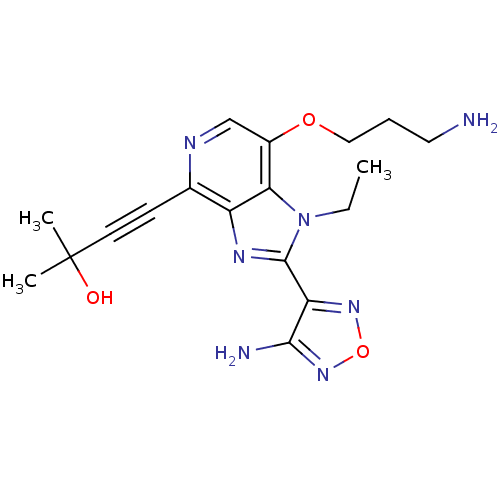 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-7-(3-aminoprop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25015 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(3-{...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Rattus norvegicus (Rat)) | BDBM24995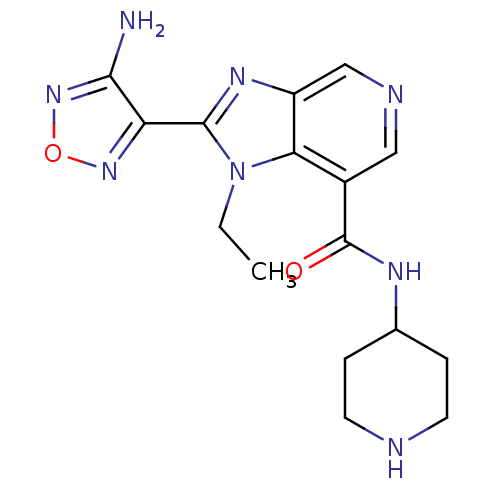 (2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-N-(piperi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK3 (Homo sapiens (Human)) | BDBM28206 (3-[(1R)-1-(2-chlorophenyl)ethoxy]-5-(5,6-dimethoxy...) | PDB NCI pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28213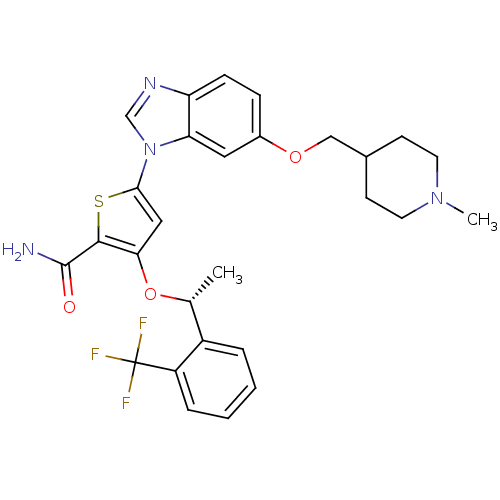 (5-{6-[(1-methylpiperidin-4-yl)methoxy]-1H-1,3-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1694-7 (2009) Article DOI: 10.1016/j.bmcl.2009.01.094 BindingDB Entry DOI: 10.7270/Q2319T6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28174 (5-(5-methoxy-1H-1,3-benzodiazol-1-yl)-3-{[2-(trifl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.2 | 22 |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PLK1 (Homo sapiens (Human)) | BDBM28183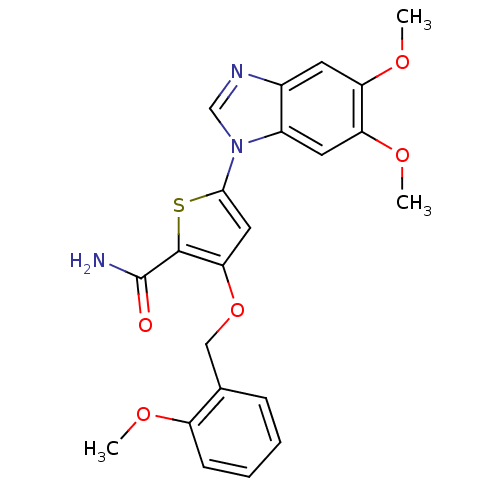 (5-(5,6-dimethoxy-1H-1,3-benzodiazol-1-yl)-3-[(2-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The compound inhibitory activity was determined by incubation with purified PLK enzyme and biotinylated peptide substrate in the presence ATP/[gamma-... | Bioorg Med Chem Lett 19: 1018-21 (2009) Article DOI: 10.1016/j.bmcl.2008.11.041 BindingDB Entry DOI: 10.7270/Q26T0JZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25011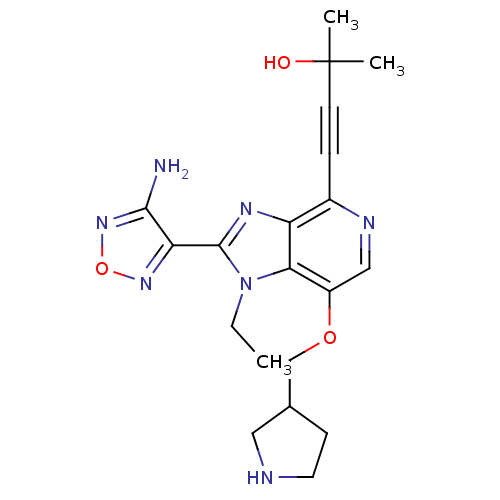 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | 22 |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM24989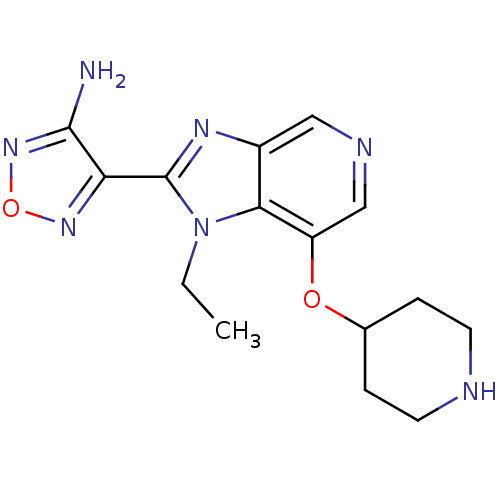 (4-[1-ethyl-7-(piperidin-4-yloxy)-1H-imidazo[4,5-c]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25008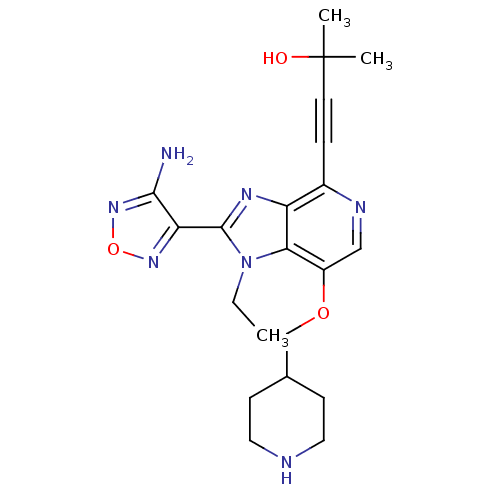 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM25012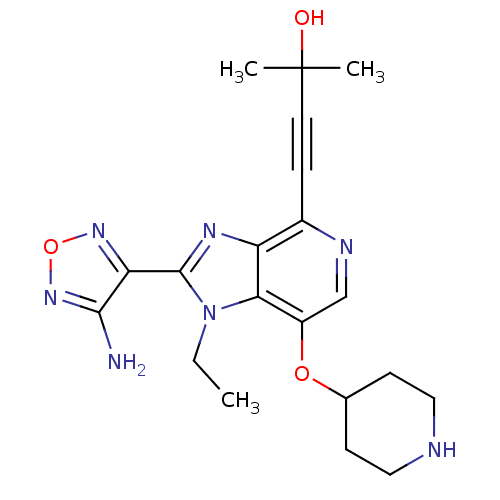 (4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-(pip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description IC50 is the inhibitor concentration, which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from radiolabeled AT... | J Med Chem 51: 5663-79 (2008) Article DOI: 10.1021/jm8004527 BindingDB Entry DOI: 10.7270/Q29G5K3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |