

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50241727 ((S)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic ac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase (unknown origin) in aerobic condition | Bioorg Med Chem 16: 8661-9 (2008) Article DOI: 10.1016/j.bmc.2008.07.087 BindingDB Entry DOI: 10.7270/Q2W66KKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247140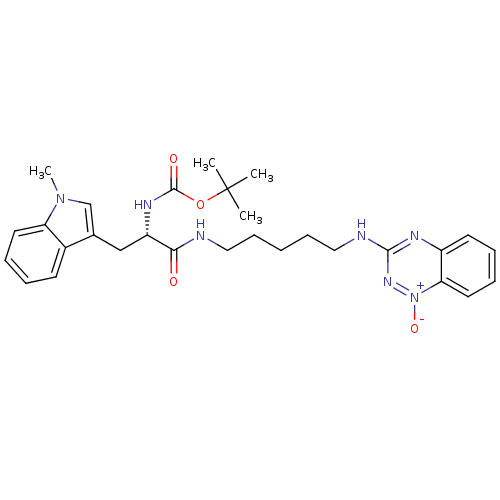 (CHEMBL470328 | TX-2236 | [5-(1-Oxido-1,2,4-benzotr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase (unknown origin) in aerobic condition | Bioorg Med Chem 16: 8661-9 (2008) Article DOI: 10.1016/j.bmc.2008.07.087 BindingDB Entry DOI: 10.7270/Q2W66KKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247142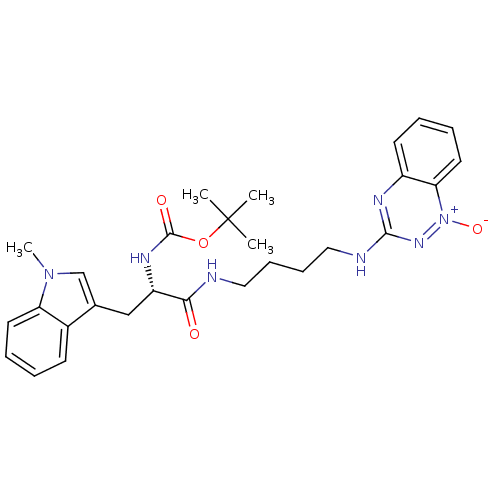 (CHEMBL470329 | TX-2228 | [4-(1-Oxido-1,2,4-benzotr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase (unknown origin) in aerobic condition | Bioorg Med Chem 16: 8661-9 (2008) Article DOI: 10.1016/j.bmc.2008.07.087 BindingDB Entry DOI: 10.7270/Q2W66KKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247141 (CHEMBL443424 | TX-2235 | [5-(1,4-Dioxido-1,2,4-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase (unknown origin) in aerobic condition | Bioorg Med Chem 16: 8661-9 (2008) Article DOI: 10.1016/j.bmc.2008.07.087 BindingDB Entry DOI: 10.7270/Q2W66KKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM50247143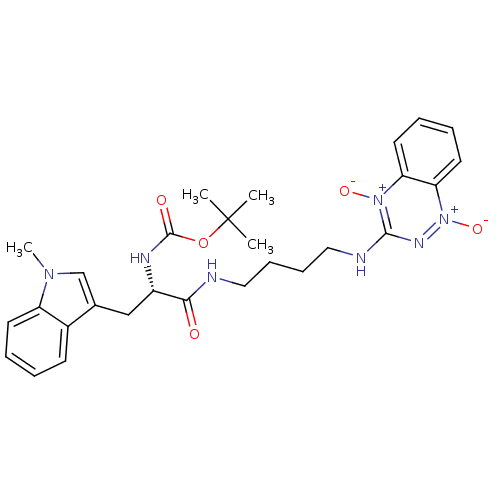 (CHEMBL513210 | TX-2234 | [4-(1,4-Dioxido-1,2,4-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokushima Curated by ChEMBL | Assay Description Inhibition of indoleamine 2,3-dioxygenase (unknown origin) in aerobic condition | Bioorg Med Chem 16: 8661-9 (2008) Article DOI: 10.1016/j.bmc.2008.07.087 BindingDB Entry DOI: 10.7270/Q2W66KKP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29994 (2-Cyclohexylcarbonylbenzimidazole, 7e) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 2.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29992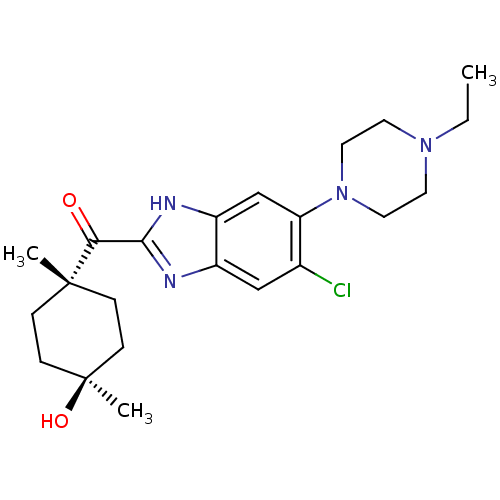 (2-Cyclohexylcarbonylbenzimidazole, 7c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293856 (1-(2,2-dimethyl-1,3-dioxan-5-yl)-3-{[1S,3R,6S)-2,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29990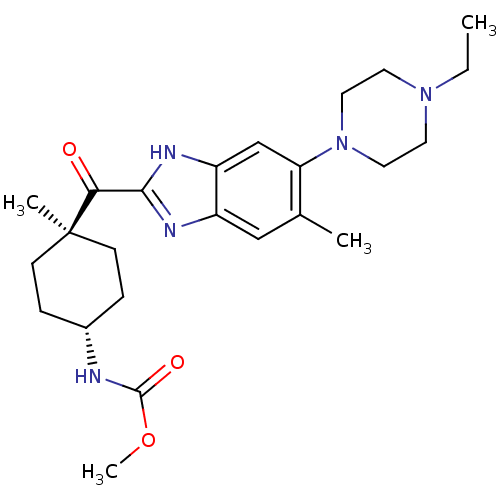 (2-Cyclohexylcarbonylbenzimidazole, 7b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | 5.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293857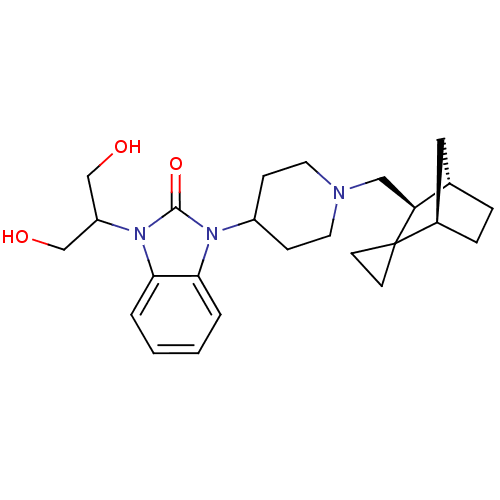 (1-(1,3-dihydroxypropan-2-yl)-3-(1-((1R,3S,4S)-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293855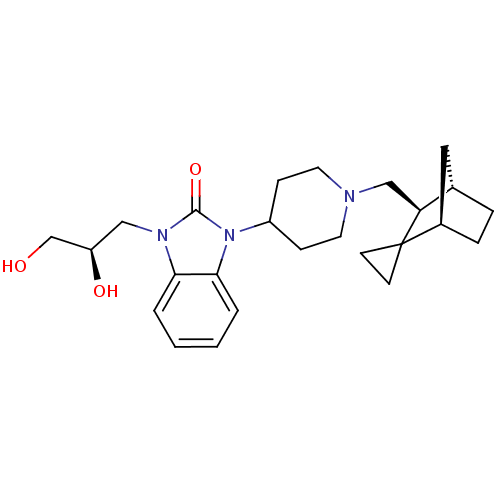 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230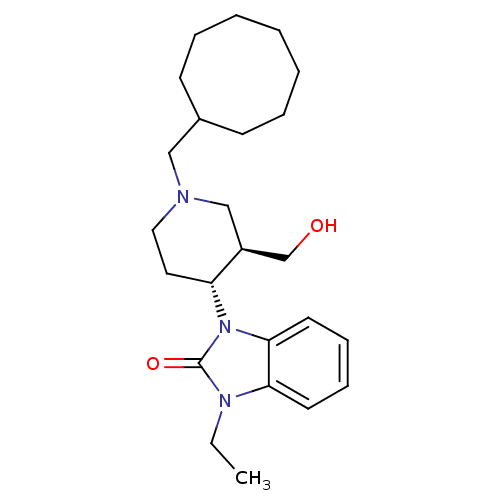 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from human cloned ORL1 receptor expressed in CHO cells | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29987 (benzimidazole analogue, 7e | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 0.720 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202356 (CHEMBL218806 | cyclo(-D-Tyr-D-MeArg-L-Arg-L-Nal-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50159214 (CHEMBL438934) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 expressed in CHO cells | J Med Chem 48: 380-91 (2005) Article DOI: 10.1021/jm049429h BindingDB Entry DOI: 10.7270/Q27S7N98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202350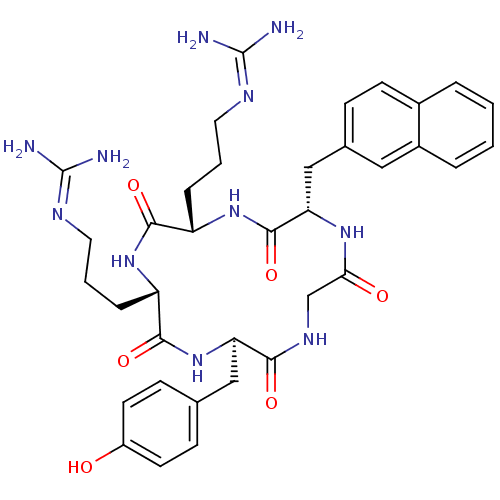 (CHEMBL219474 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-Gly-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166087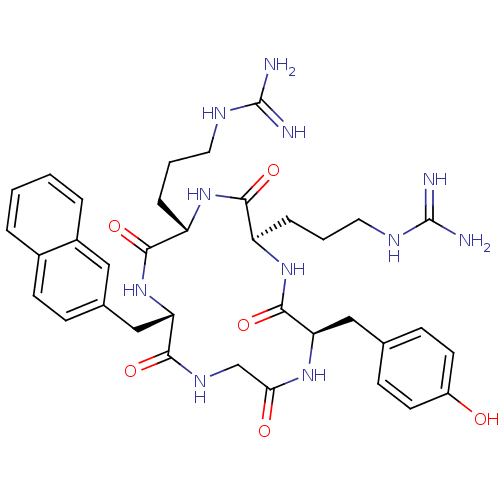 (CHEMBL436097 | N-{3-[(2R,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50159213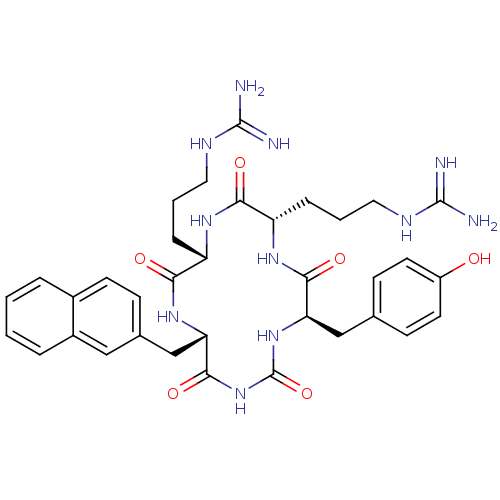 (CHEMBL370001 | N-{3-[10-(3-Guanidino-propyl)-4-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 expressed in CHO cells | J Med Chem 48: 380-91 (2005) Article DOI: 10.1021/jm049429h BindingDB Entry DOI: 10.7270/Q27S7N98 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083230 (1-((3R,4R)-1-Cyclooctylmethyl-3-hydroxymethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29989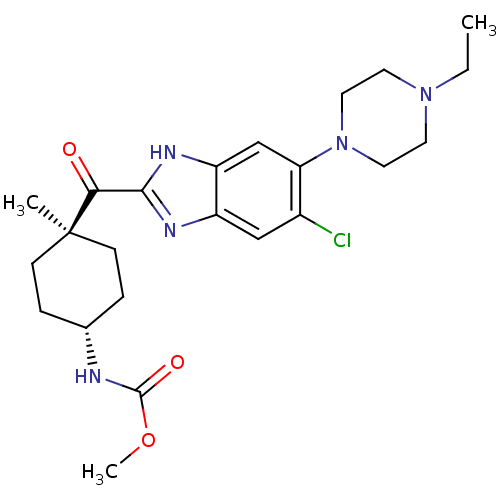 (2-Cyclohexylcarbonylbenzimidazole, 7a) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | 7.5 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083227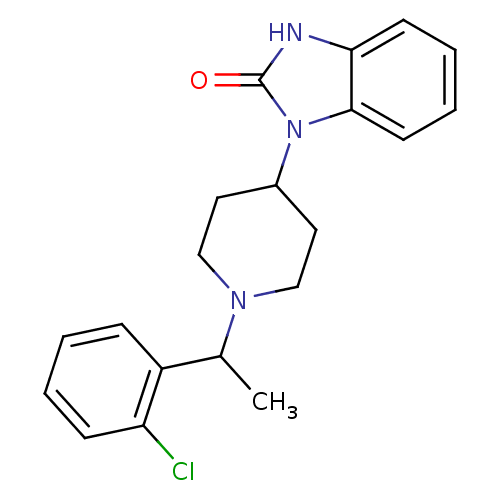 (1-{1-[1-(2-Chloro-phenyl)-ethyl]-piperidin-4-yl}-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound in CHO cells stably expressing cloned human Opioid receptor like 1 by displacing radioligand [125I]-Tyr14-nociceptin | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232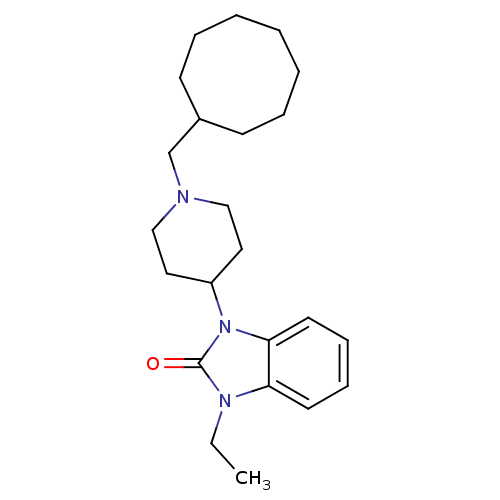 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [125I]-Tyr14-nociceptin binding to human Opioid receptor like 1 (opioid receptor like 1) in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102044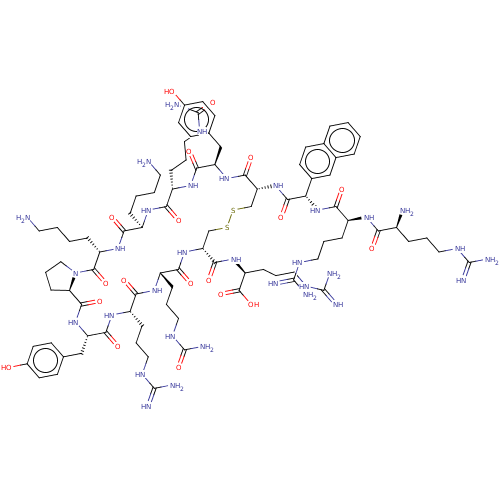 (CHEMBL2373002 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202346 (CHEMBL219339 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-Gly-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166106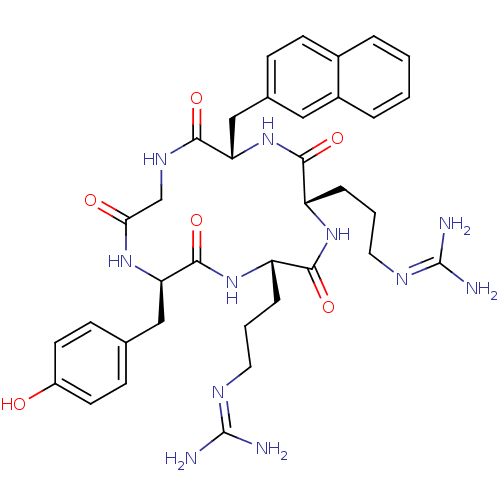 (CHEMBL436283 | N-{3-[(2S,5S,8S,14R)-5-(3-Guanidino...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29991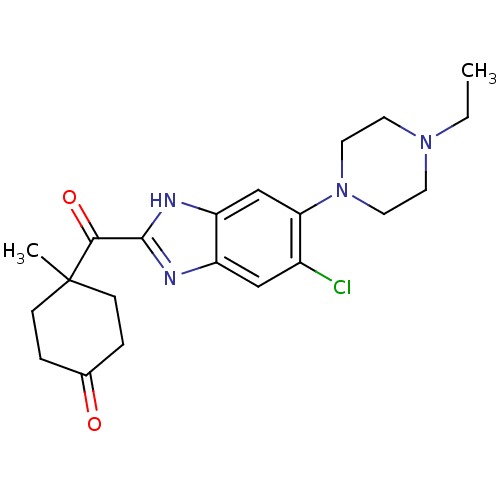 (2-Cyclohexylcarbonylbenzimidazole, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | 38 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166096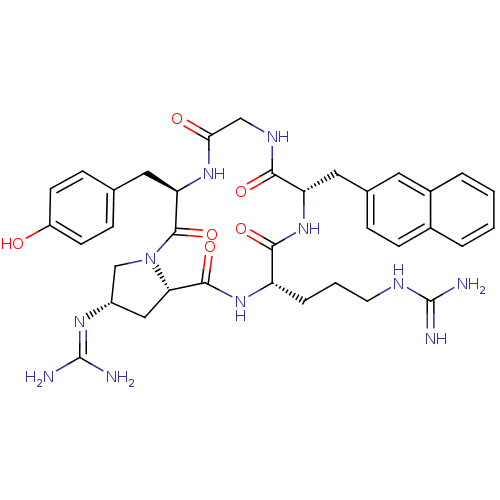 (CHEMBL372874 | N-[(2S,5R,11S,14S,16aS)-14-(3-Guani...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166079 (CHEMBL192183 | N-[(2R,5R,11S,14S,16aS)-14-(3-Guani...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50096735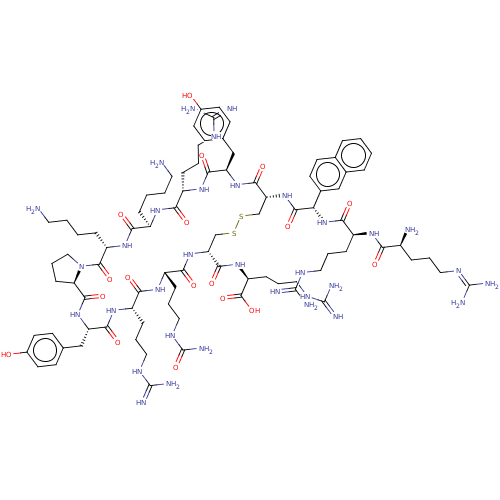 (CHEMBL2372983 | Compound T140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202345 (CHEMBL373636 | cyclo(-D-Tyr-D-Arg-L-Arg-L-Nal-D-Al...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202359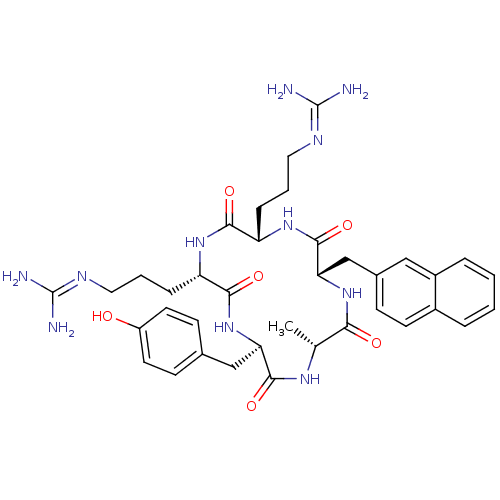 (CHEMBL375990 | cyclo(-D-Tyr-L-Arg-L-Arg-L-Nal-D-Al...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102037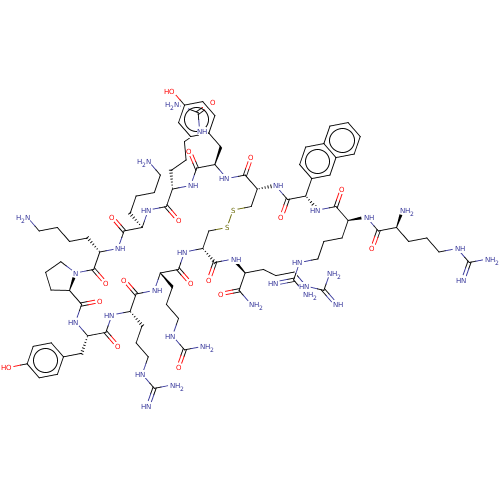 (CHEMBL2372993 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity in CHO cells stably expressing cloned human Opioid receptor kappa 1 by displacing radioligand [3H]U-69593 | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293857 (1-(1,3-dihydroxypropan-2-yl)-3-(1-((1R,3S,4S)-spir...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293855 (4-{3-[(2R)-2,3-dihydroxypropyl]-2-oxo-2,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166101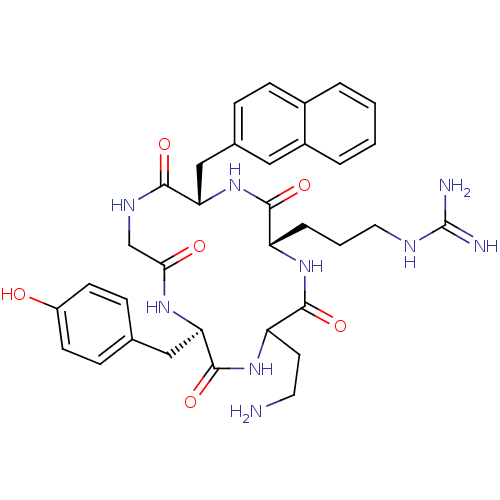 (CHEMBL372422 | N-{3-[(2S,5S,11R)-14-(2-Amino-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166086 (CHEMBL192594 | N-{3-[(2S,5S,11R)-14-(3-Amino-propy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102036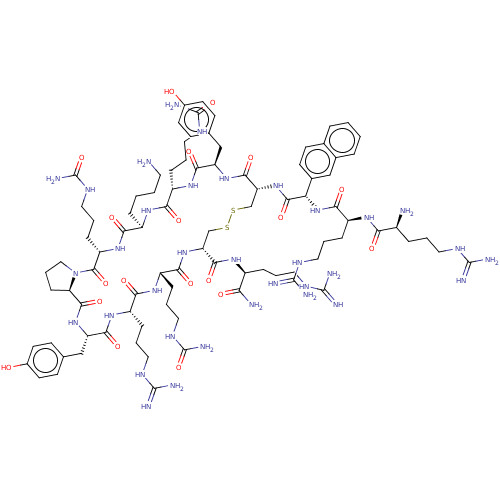 (CHEMBL2372985 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083232 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-3-ethyl-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50083231 (1-(1-Cyclooctylmethyl-piperidin-4-yl)-1,3-dihydro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Antagonistic activity of the compound measured by GTPgammaS binding against Opioid receptor like 1 in CHO cells | J Med Chem 42: 5061-3 (2000) BindingDB Entry DOI: 10.7270/Q2MC8Z69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202347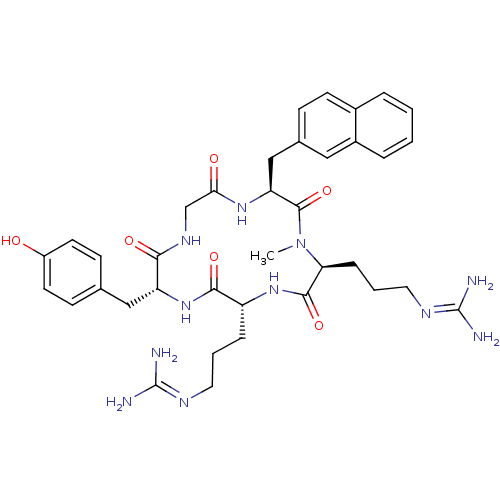 (CHEMBL376219 | cyclo(-D-Tyr-D-Arg-L-MeArg-L-Nal-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50202365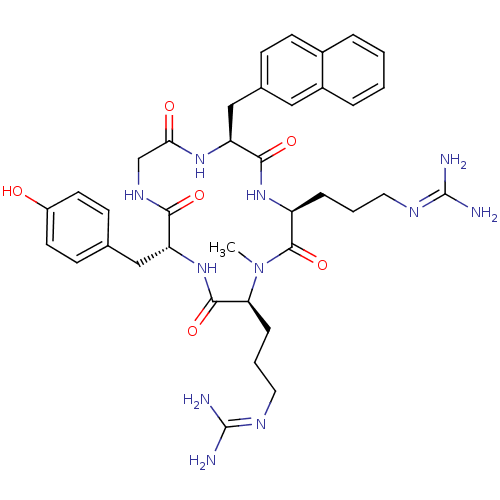 (CHEMBL374421 | cyclo(-D-Tyr-L-MeArg-L-Arg-L-Nal-Gl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]SDF1 binding to CXCR4 transfected in CHO cells | J Med Chem 50: 192-8 (2007) Article DOI: 10.1021/jm0607350 BindingDB Entry DOI: 10.7270/Q25M65D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102040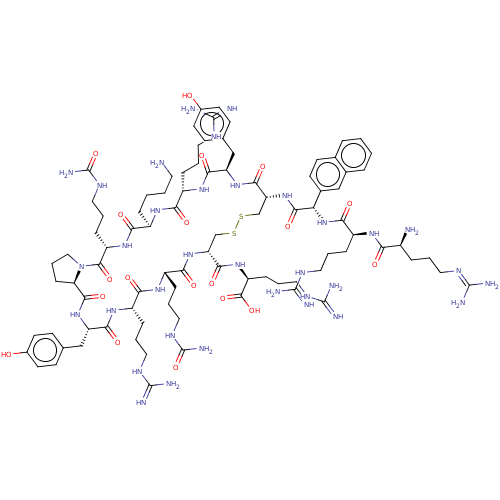 (CHEMBL2372994 | T140) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50166093 (CHEMBL193217 | N-{3-[(2S,5S,11R,14S)-14-(2-Guanidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [125I]-SDF-1 binding to C-X-C chemokine receptor type 4 (CXCR4) expressed in CHO cells | J Med Chem 48: 3280-9 (2005) Article DOI: 10.1021/jm050009h BindingDB Entry DOI: 10.7270/Q24X5790 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50102032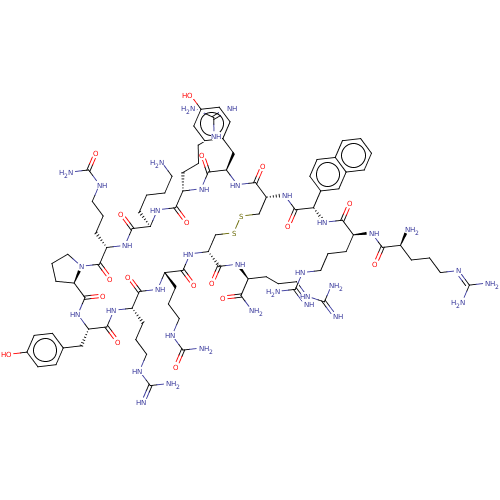 (CHEMBL2373001 | Derivative of T140 peptide) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibitory concentration determined on an HIV infection model mediated by CXCR4 | Bioorg Med Chem Lett 11: 1897-902 (2001) BindingDB Entry DOI: 10.7270/Q25X29FS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29993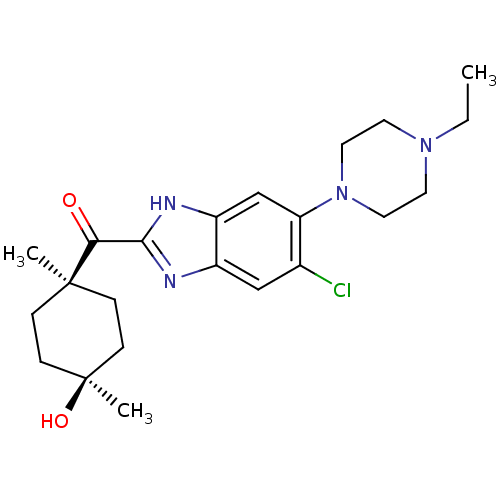 (2-Cyclohexylcarbonylbenzimidazole, 7d) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50293856 (1-(2,2-dimethyl-1,3-dioxan-5-yl)-3-{[1S,3R,6S)-2,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cells by [35S]GTPgammaS binding assay | J Med Chem 52: 4091-4 (2009) Article DOI: 10.1021/jm900581g BindingDB Entry DOI: 10.7270/Q2V98835 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 235 total ) | Next | Last >> |