

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322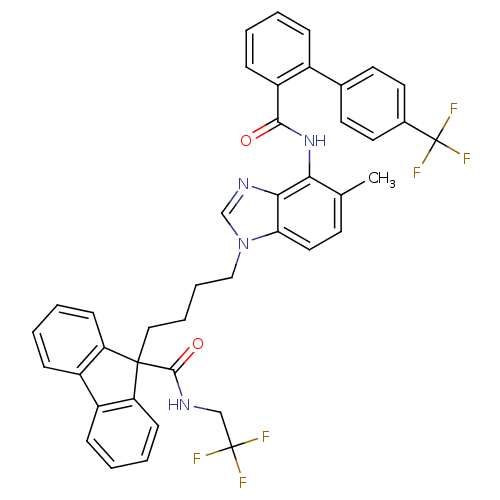 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326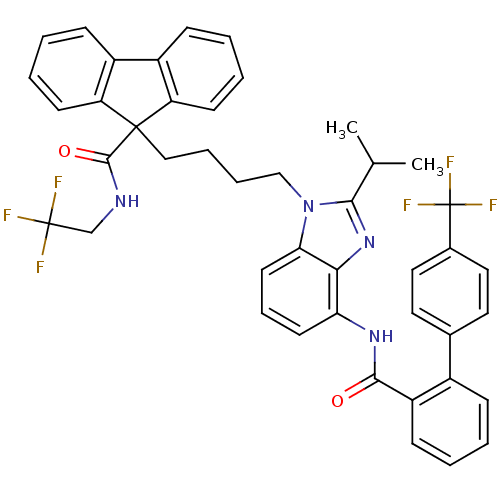 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324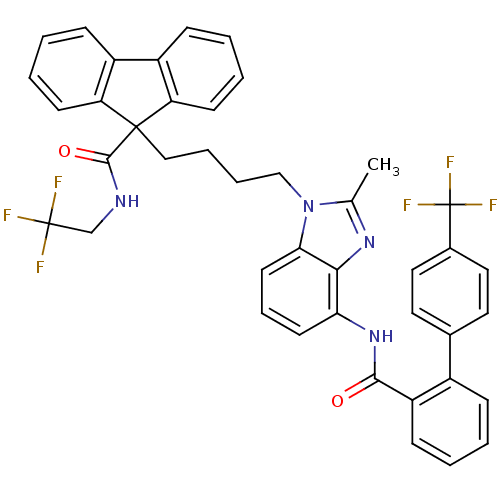 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325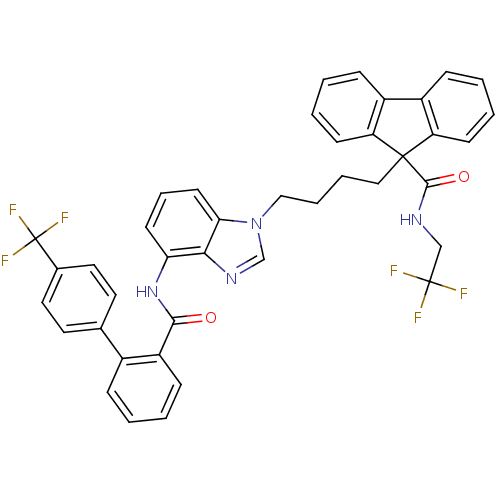 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217812 (CHEMBL322526) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217626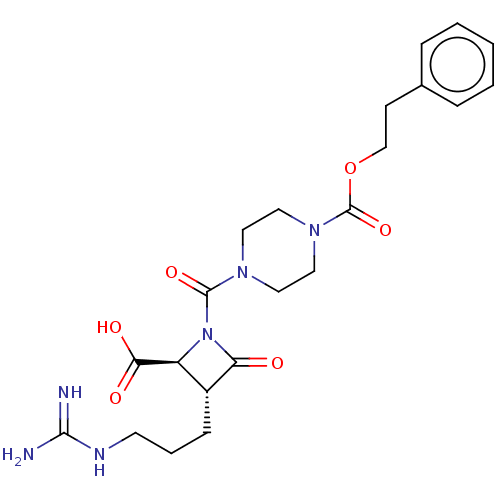 (CHEMBL322538) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217627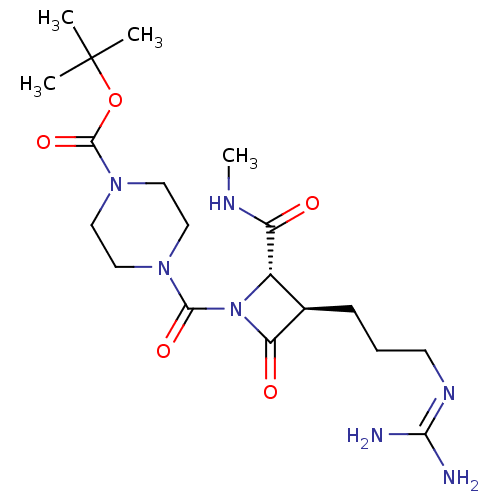 (CHEMBL110061) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320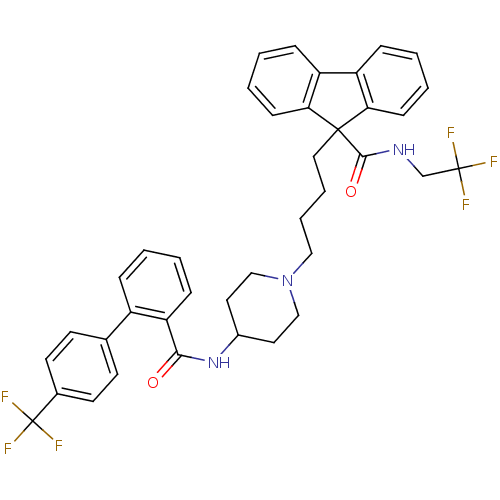 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217599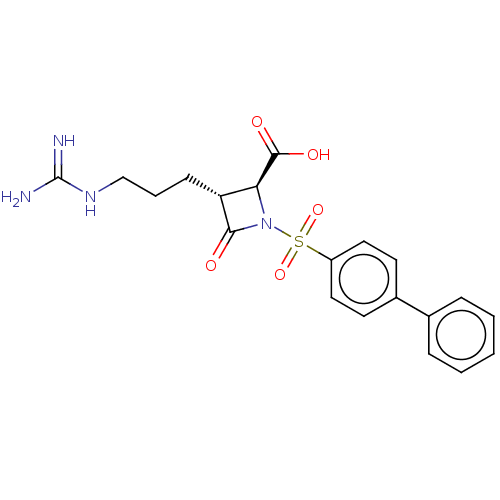 (CHEMBL109888) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144535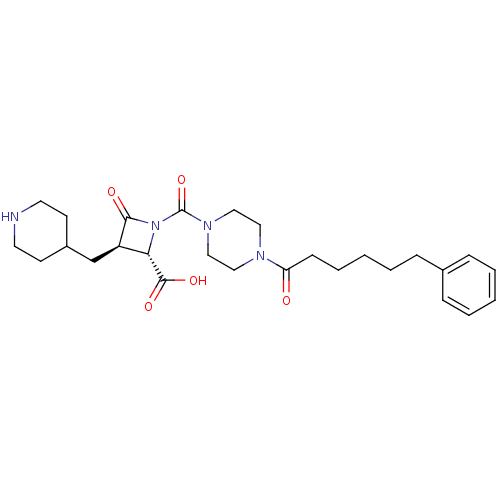 ((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144582 ((R)-1-[4-(6-Naphthalen-2-yl-hexanoyl)-piperazine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144555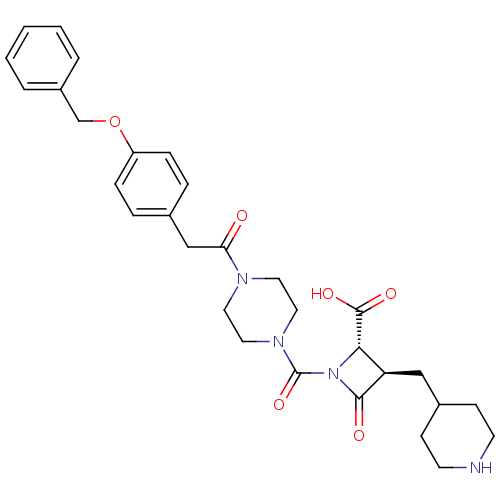 ((R)-1-{4-[2-(4-Benzyloxy-phenyl)-acetyl]-piperazin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144577 ((R)-1-[4-(6-Naphthalen-1-yl-hexanoyl)-piperazine-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144586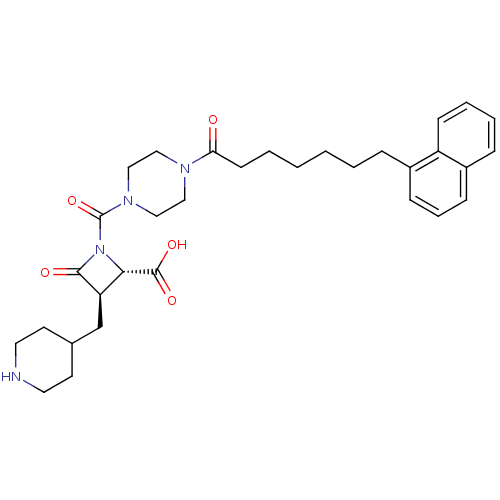 ((R)-1-[4-(7-Naphthalen-1-yl-heptanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144535 ((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217622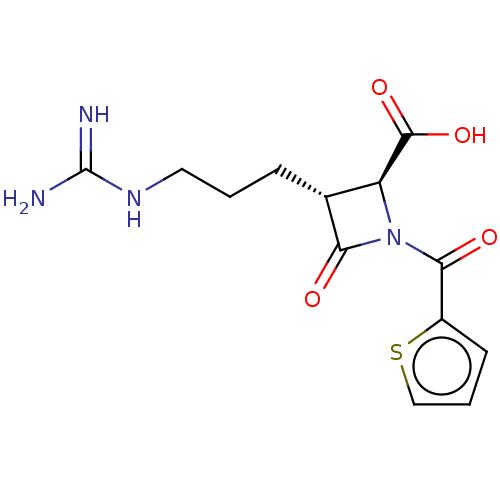 (CHEMBL443539) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217801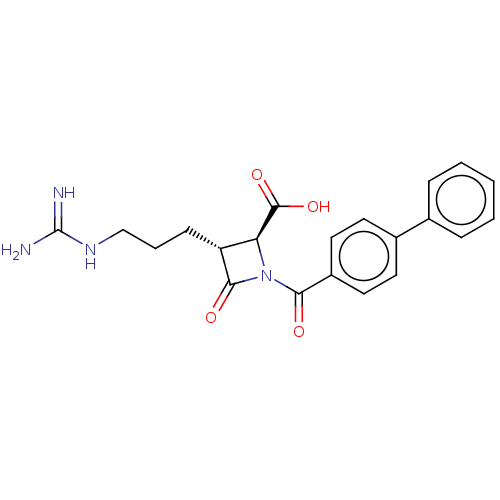 (CHEMBL111250) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092365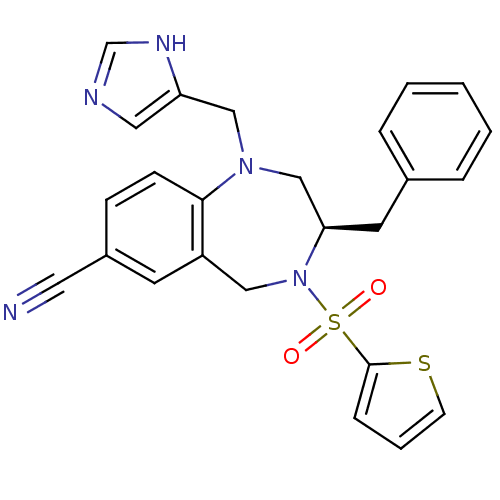 ((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092366 (3-Benzyl-4-(2-dimethylamino-ethanesulfonyl)-1-(3H-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.53 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50120387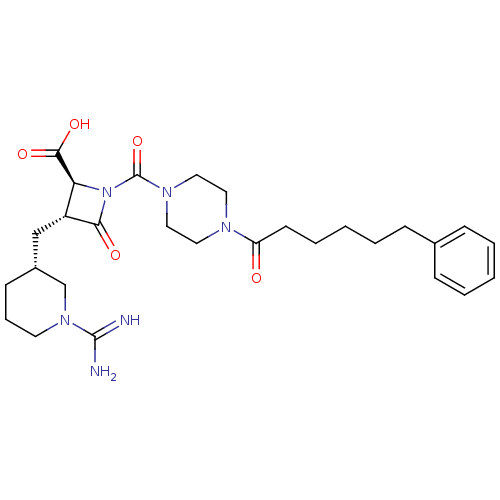 ((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50120387 ((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50221046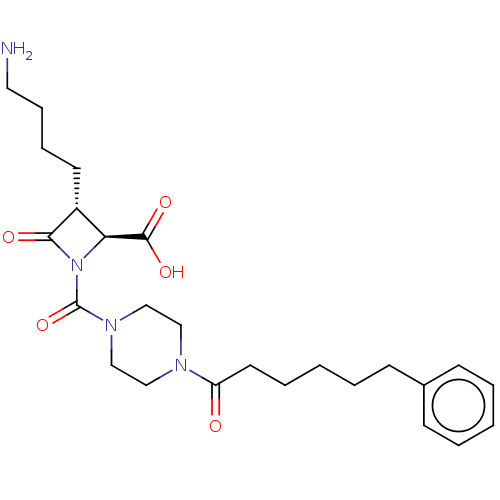 (CHEMBL72282) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50217824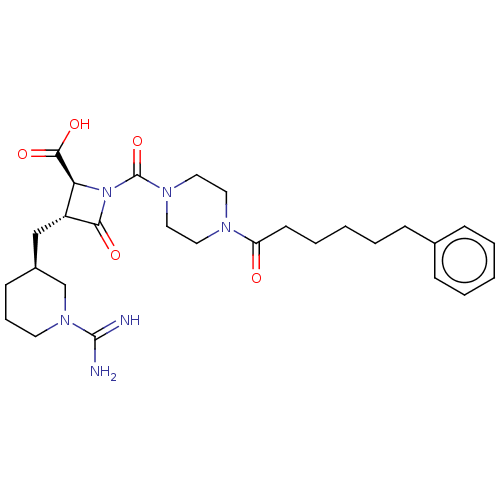 (BMS-363130 | CHEMBL70738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217824 (BMS-363130 | CHEMBL70738) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217823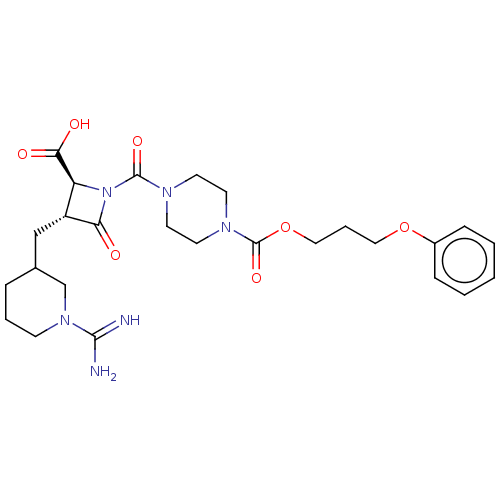 (CHEMBL111630) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217822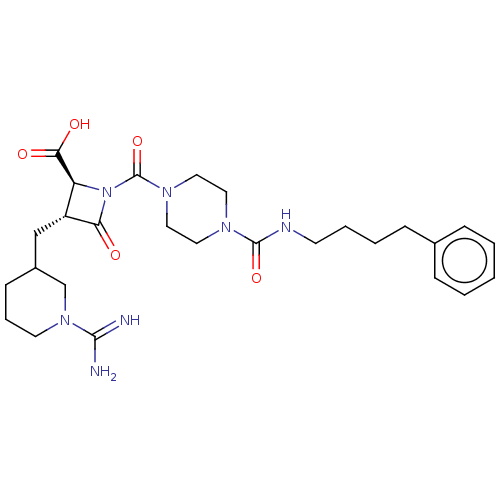 (CHEMBL111173) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217818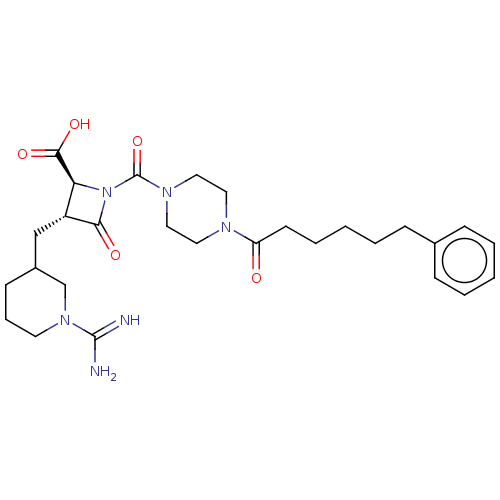 (CHEMBL440515) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217817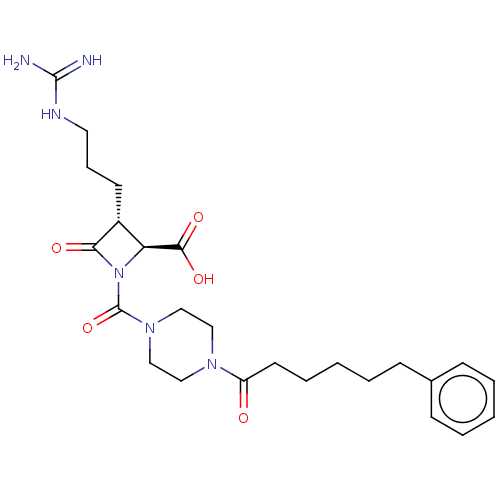 (CHEMBL326209) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120387 ((2S,3R)-3-((R)-1-Carbamimidoyl-piperidin-3-ylmethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092363 (3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-(propane-1-s...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092377 (4-Benzenesulfonyl-3-benzyl-1-(3H-imidazol-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.77 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144532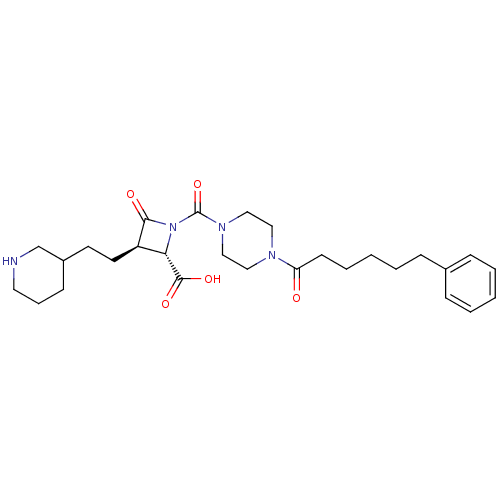 ((2S,3R)-4-Oxo-1-[4-(6-phenyl-hexanoyl)-piperazine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217819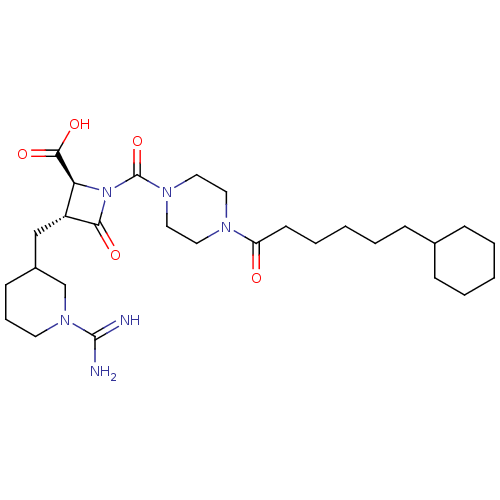 (CHEMBL109504) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098323 (9-(4-{2-Propyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092372 (3-Benzyl-7-cyano-1-(3H-imidazol-4-ylmethyl)-1,2,3,...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.85 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50092357 (3-Benzyl-1-(3H-imidazol-4-ylmethyl)-4-methanesulfo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of purified recombinant human farnesyltransferase (FT) | J Med Chem 43: 3587-95 (2000) BindingDB Entry DOI: 10.7270/Q2WD3ZSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217628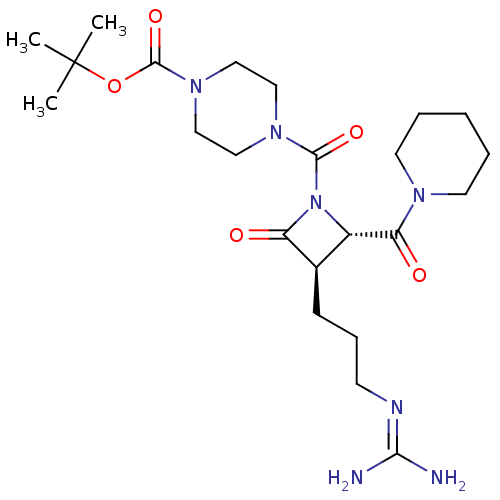 (CHEMBL107493) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217624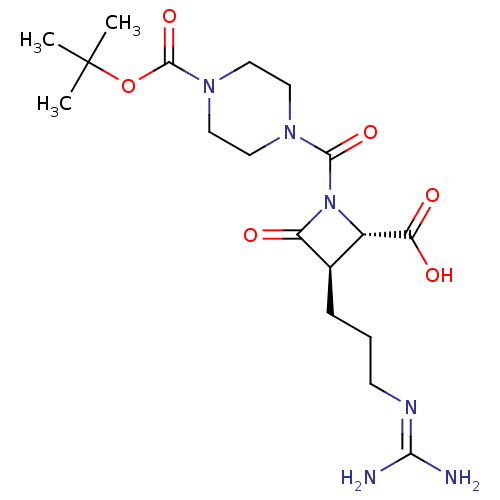 (CHEMBL321622) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50217813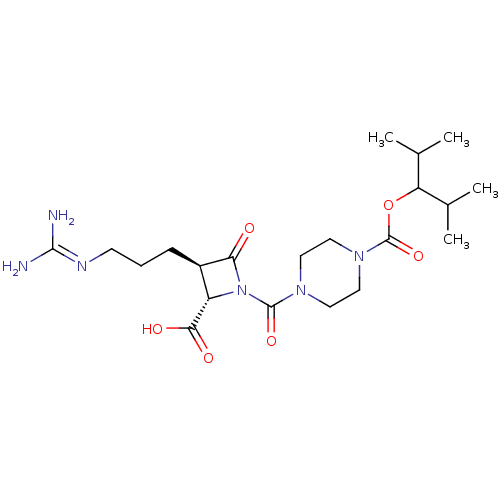 (CHEMBL302058) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120368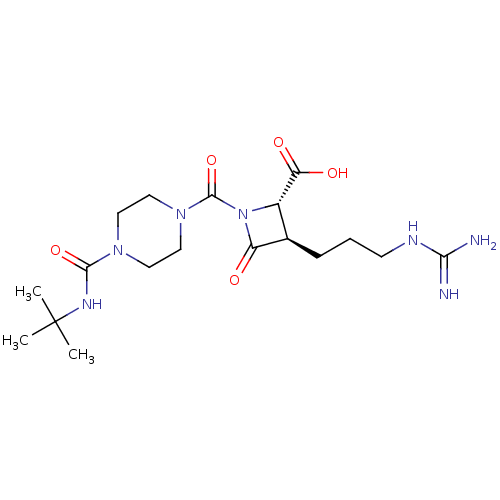 ((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human tryptase. | Bioorg Med Chem Lett 12: 3229-33 (2002) BindingDB Entry DOI: 10.7270/Q2QC05PZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50220841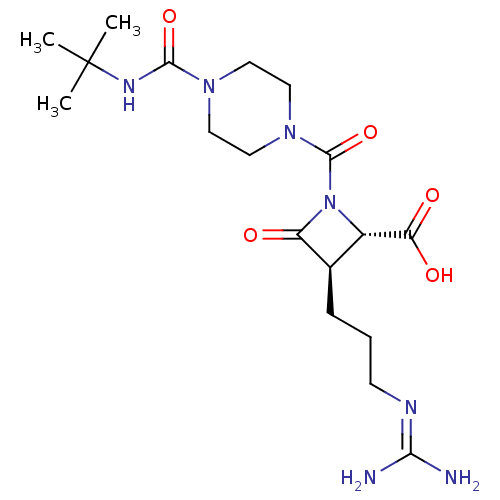 (BMS-262084 | CHEMBL71037) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50217813 (CHEMBL302058) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of compound against human tryptase was determined | Bioorg Med Chem Lett 14: 2227-31 (2004) Article DOI: 10.1016/j.bmcl.2004.02.011 BindingDB Entry DOI: 10.7270/Q2GM86Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50120368 ((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2/delta/gamma (Homo sapiens (Human)) | BDBM50144558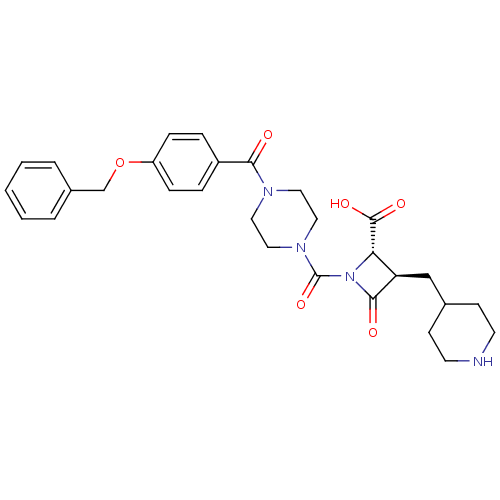 ((R)-1-[4-(4-Benzyloxy-benzoyl)-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human tryptase | Bioorg Med Chem Lett 14: 2233-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.012 BindingDB Entry DOI: 10.7270/Q2SB4697 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase delta (Homo sapiens (Human)) | BDBM50120368 ((2S,3R)-1-(4-tert-Butylcarbamoyl-piperazine-1-carb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory concentration against human tryptase | Bioorg Med Chem Lett 12: 3235-8 (2002) BindingDB Entry DOI: 10.7270/Q2KK9DZP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 354 total ) | Next | Last >> |