 Found 68 hits with Last Name = 'zaheer-ul-haq' and Initial = 'na'
Found 68 hits with Last Name = 'zaheer-ul-haq' and Initial = 'na' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50484065
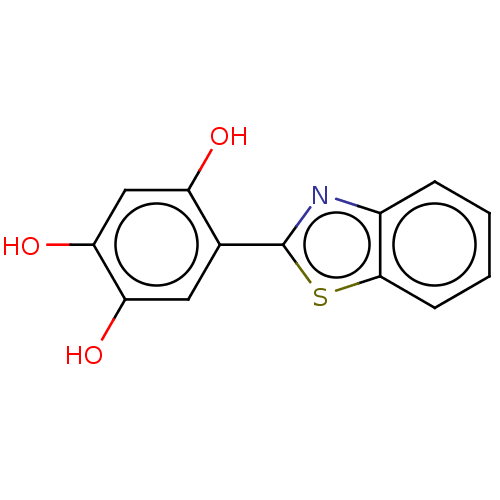
(CHEMBL1808311)Show InChI InChI=1S/C13H9NO3S/c15-9-6-11(17)10(16)5-7(9)13-14-8-3-1-2-4-12(8)18-13/h1-6,15-17H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50484063

(CHEMBL1650632)Show InChI InChI=1S/C13H9NOS/c15-11-7-3-1-5-9(11)13-14-10-6-2-4-8-12(10)16-13/h1-8,15H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50242345
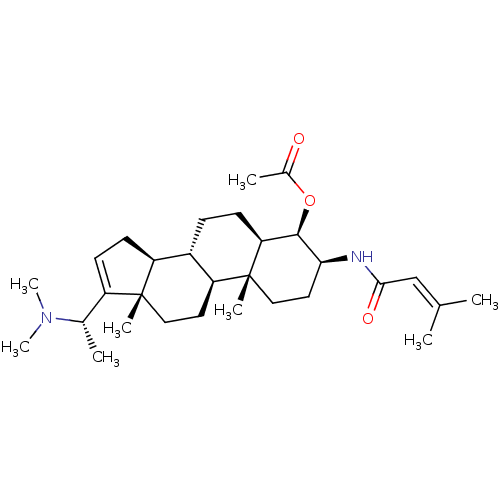
(CHEMBL460447 | [(20S)-20-(N,N-dimethylamino)-3 bet...)Show SMILES [#6]-[#6@H](-[#7](-[#6])-[#6])-[#6]1=[#6]-[#6]-[#6@H]2-[#6@@H]-3-[#6]-[#6]-[#6@H]4-[#6@@H](-[#8]-[#6](-[#6])=O)-[#6@H](-[#6]-[#6][C@]4([#6])[#6@H]-3-[#6]-[#6][C@]12[#6])-[#7]-[#6](=O)\[#6]=[#6](/[#6])-[#6] |r,t:5| Show InChI InChI=1S/C30H48N2O3/c1-18(2)17-27(34)31-26-14-16-30(6)24-13-15-29(5)22(19(3)32(7)8)11-12-23(29)21(24)9-10-25(30)28(26)35-20(4)33/h11,17,19,21,23-26,28H,9-10,12-16H2,1-8H3,(H,31,34)/t19-,21-,23-,24-,25-,26-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135147
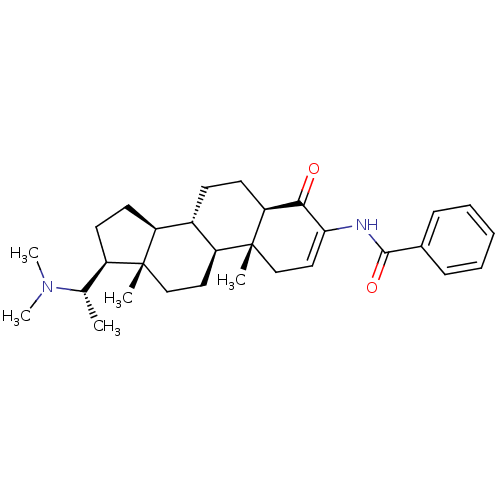
((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C(=O)C(NC(=O)c5ccccc5)=CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C |r,c:22| Show InChI InChI=1S/C30H42N2O2/c1-19(32(4)5)22-13-14-23-21-11-12-25-27(33)26(31-28(34)20-9-7-6-8-10-20)16-18-30(25,3)24(21)15-17-29(22,23)2/h6-10,16,19,21-25H,11-15,17-18H2,1-5H3,(H,31,34)/t19-,21-,22+,23-,24-,25-,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135146
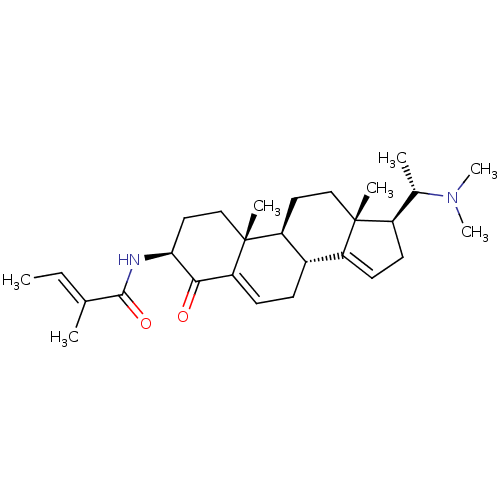
((E)-2-Methyl-but-2-enoic acid [(3S,8R,9S,10R,13R,1...)Show SMILES C\C=C(/C)C(=O)N[C@H]1CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC=C4[C@@H]3CC=C2C1=O)[C@H](C)N(C)C |c:19,25| Show InChI InChI=1S/C28H42N2O2/c1-8-17(2)26(32)29-24-14-16-28(5)22-13-15-27(4)20(18(3)30(6)7)11-12-21(27)19(22)9-10-23(28)25(24)31/h8,10,12,18-20,22,24H,9,11,13-16H2,1-7H3,(H,29,32)/b17-8+/t18-,19-,20+,22-,24-,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135148
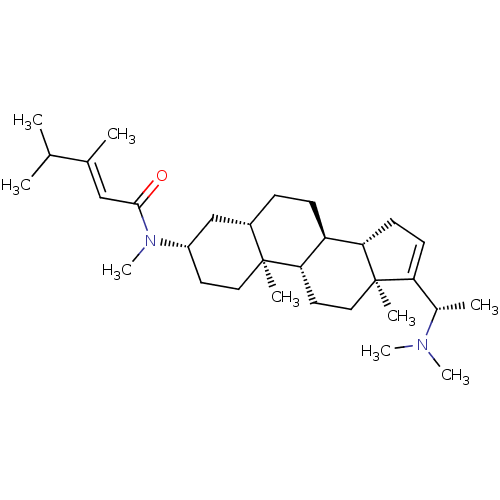
(1N-[14-(1-dimethylaminoethyl)-2,15-dimethyl-(1S,7S...)Show SMILES CC(C)C(\C)=C\C(=O)N(C)[C@H]1CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CC=C([C@H](C)N(C)C)[C@@]4(C)CC[C@H]23)C1 |t:21| Show InChI InChI=1S/C31H52N2O/c1-20(2)21(3)18-29(34)33(9)24-14-16-30(5)23(19-24)10-11-25-27-13-12-26(22(4)32(7)8)31(27,6)17-15-28(25)30/h12,18,20,22-25,27-28H,10-11,13-17,19H2,1-9H3/b21-18+/t22-,23-,24-,25-,27-,28-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421626
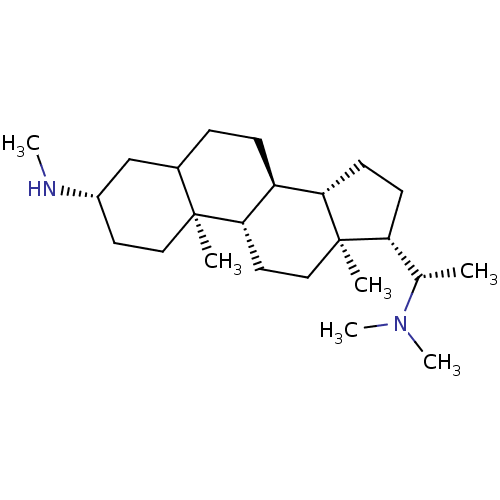
(CHEMBL139685)Show SMILES CN[C@H]1CC[C@@]2(C)C(CC[C@H]3[C@@H]4CC[C@H]([C@H](C)N(C)C)[C@@]4(C)CC[C@H]23)C1 Show InChI InChI=1S/C24H44N2/c1-16(26(5)6)20-9-10-21-19-8-7-17-15-18(25-4)11-13-23(17,2)22(19)12-14-24(20,21)3/h16-22,25H,7-15H2,1-6H3/t16-,17?,18-,19-,20+,21-,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421627
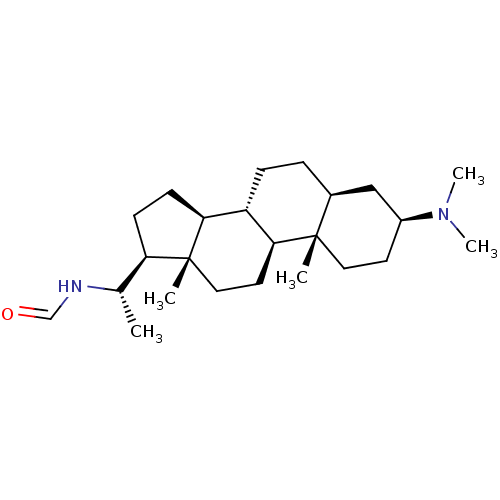
(CHEMBL136317)Show SMILES C[C@H](NC=O)[C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C Show InChI InChI=1S/C24H42N2O/c1-16(25-15-27)20-8-9-21-19-7-6-17-14-18(26(4)5)10-12-23(17,2)22(19)11-13-24(20,21)3/h15-22H,6-14H2,1-5H3,(H,25,27)/t16-,17-,18-,19-,20+,21-,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135153
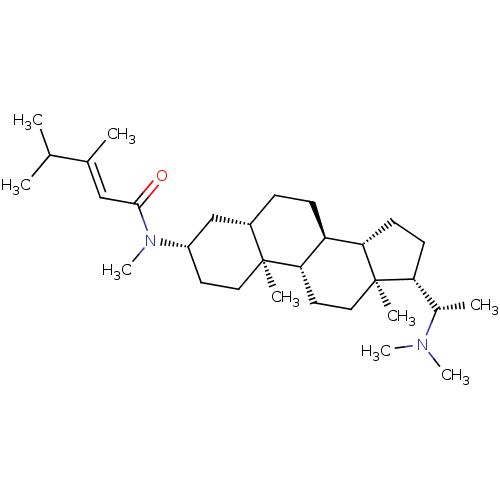
((E)-3,4-Dimethyl-pent-2-enoic acid [(3S,5S,8R,9S,1...)Show SMILES CC(C)C(\C)=C\C(=O)N(C)[C@H]1CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CC[C@H]([C@H](C)N(C)C)[C@@]4(C)CC[C@H]23)C1 Show InChI InChI=1S/C31H54N2O/c1-20(2)21(3)18-29(34)33(9)24-14-16-30(5)23(19-24)10-11-25-27-13-12-26(22(4)32(7)8)31(27,6)17-15-28(25)30/h18,20,22-28H,10-17,19H2,1-9H3/b21-18+/t22-,23-,24-,25-,26+,27-,28-,30-,31+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135158
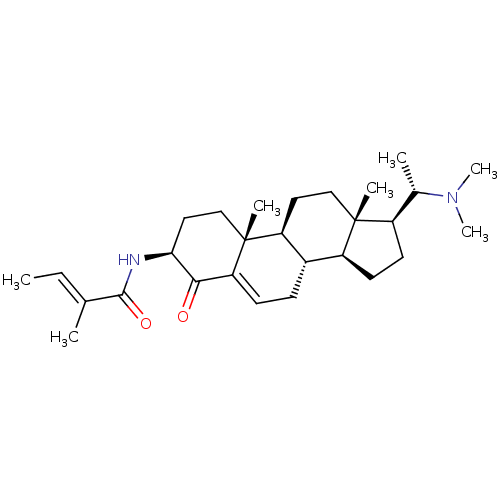
((E)-2-Methyl-but-2-enoic acid [(3S,8S,9S,10R,13S,1...)Show SMILES C\C=C(/C)C(=O)N[C@H]1CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CC=C2C1=O)[C@H](C)N(C)C |c:25| Show InChI InChI=1S/C28H44N2O2/c1-8-17(2)26(32)29-24-14-16-28(5)22-13-15-27(4)20(18(3)30(6)7)11-12-21(27)19(22)9-10-23(28)25(24)31/h8,10,18-22,24H,9,11-16H2,1-7H3,(H,29,32)/b17-8+/t18-,19-,20+,21-,22-,24-,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421630
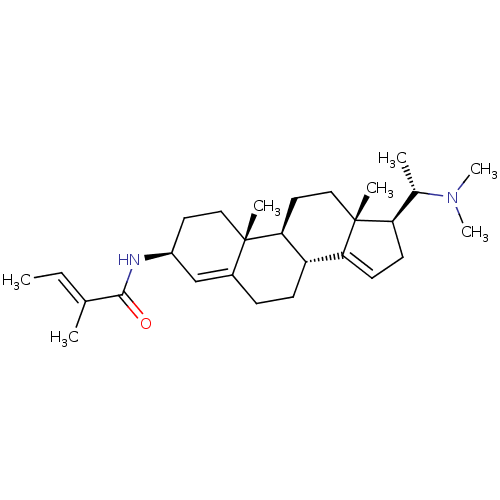
(CHEMBL136251)Show SMILES C\C=C(/C)C(=O)N[C@H]1CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC=C4[C@@H]3CCC2=C1)[C@H](C)N(C)C |c:19,27| Show InChI InChI=1S/C28H44N2O/c1-8-18(2)26(31)29-21-13-15-27(4)20(17-21)9-10-22-24-12-11-23(19(3)30(6)7)28(24,5)16-14-25(22)27/h8,12,17,19,21-23,25H,9-11,13-16H2,1-7H3,(H,29,31)/b18-8+/t19-,21-,22-,23+,25-,27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM33932

(4-(3H-1,3-benzothiazol-2-ylidene)-1-cyclohexa-2,5-...)Show InChI InChI=1S/C13H9NOS/c15-10-7-5-9(6-8-10)13-14-11-3-1-2-4-12(11)16-13/h1-8,15H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50484066

(CHEMBL1808308)Show InChI InChI=1S/C13H9NO2S/c15-8-5-6-11(16)9(7-8)13-14-10-3-1-2-4-12(10)17-13/h1-7,15-16H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421625
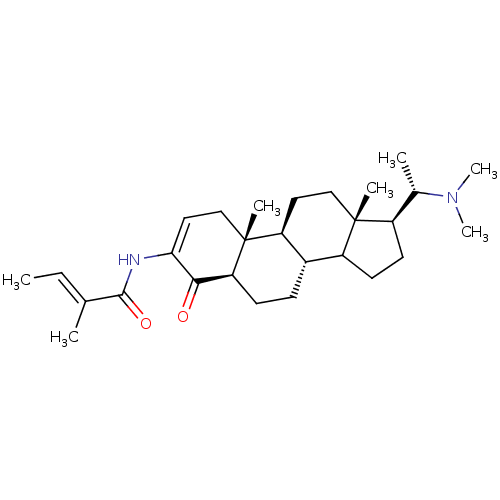
(CHEMBL136135)Show SMILES C\C=C(/C)C(=O)NC1=CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CCC4[C@@H]3CC[C@H]2C1=O)[C@H](C)N(C)C |t:7| Show InChI InChI=1S/C28H44N2O2/c1-8-17(2)26(32)29-24-14-16-28(5)22-13-15-27(4)20(18(3)30(6)7)11-12-21(27)19(22)9-10-23(28)25(24)31/h8,14,18-23H,9-13,15-16H2,1-7H3,(H,29,32)/b17-8+/t18-,19-,20+,21?,22-,23-,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM35525
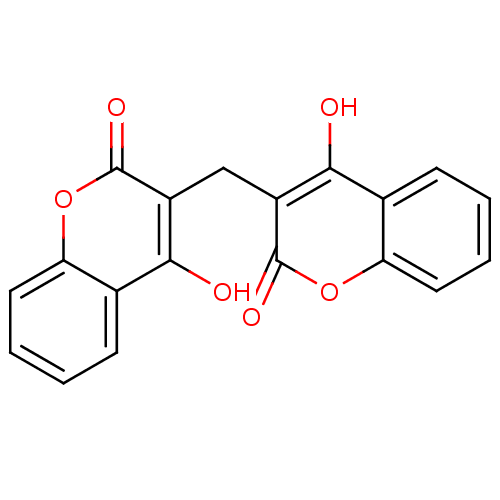
(3,3''''-methylenebis(4-hydroxy-coumarin | 3,3''''-...)Show InChI InChI=1S/C19H12O6/c20-16-10-5-1-3-7-14(10)24-18(22)12(16)9-13-17(21)11-6-2-4-8-15(11)25-19(13)23/h1-8,20-21H,9H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421636
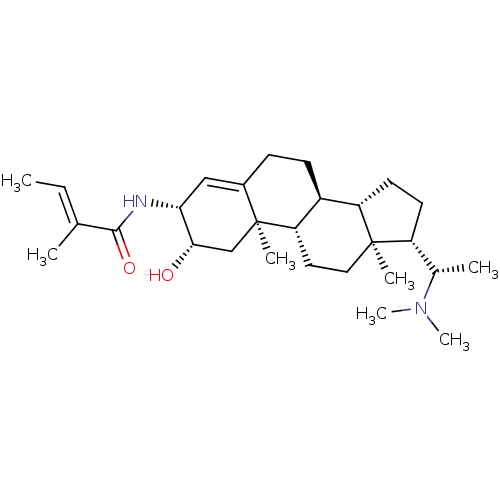
(CHEMBL139424)Show SMILES C\C=C(/C)C(=O)N[C@@H]1C=C2CC[C@H]3[C@@H]4CC[C@H]([C@H](C)N(C)C)[C@@]4(C)CC[C@@H]3[C@@]2(C)C[C@@H]1O |t:8| Show InChI InChI=1S/C28H46N2O2/c1-8-17(2)26(32)29-24-15-19-9-10-20-22-12-11-21(18(3)30(6)7)27(22,4)14-13-23(20)28(19,5)16-25(24)31/h8,15,18,20-25,31H,9-14,16H2,1-7H3,(H,29,32)/b17-8+/t18-,20-,21+,22-,23-,24+,25-,27+,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50339940
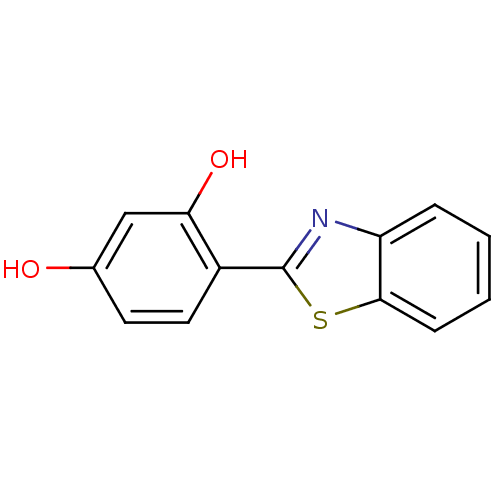
(4-(Benzo[d]thiazol-2-yl)benzene-1,3-diol | CHEMBL1...)Show InChI InChI=1S/C13H9NO2S/c15-8-5-6-9(11(16)7-8)13-14-10-3-1-2-4-12(10)17-13/h1-7,15-16H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135159
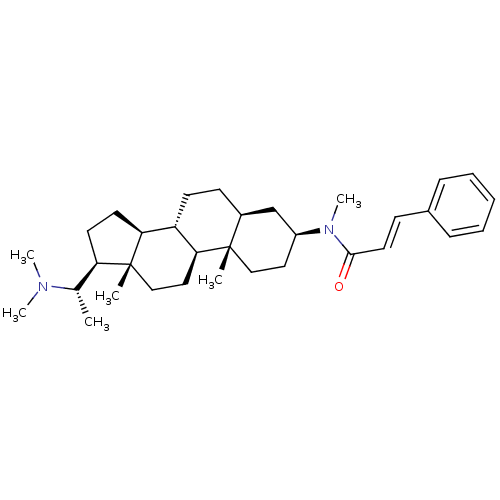
((20S,2'E)-20-(N,N-dimethylamino)-3beta-(3'-phenyl-...)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C(=O)\C=C\c1ccccc1)N(C)C |r| Show InChI InChI=1S/C33H50N2O/c1-23(34(4)5)28-15-16-29-27-14-13-25-22-26(18-20-32(25,2)30(27)19-21-33(28,29)3)35(6)31(36)17-12-24-10-8-7-9-11-24/h7-12,17,23,25-30H,13-16,18-22H2,1-6H3/b17-12+/t23-,25-,26-,27-,28+,29-,30-,32-,33+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421631
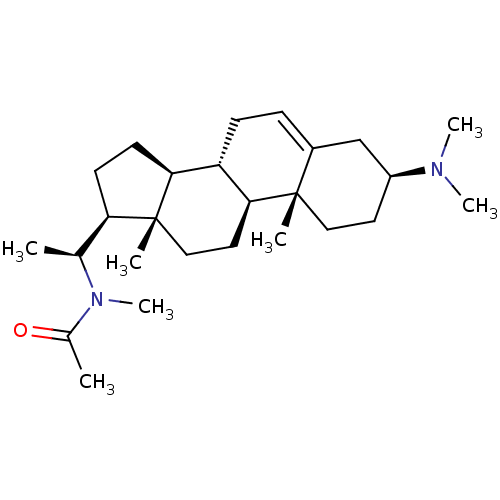
(CHEMBL136765)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C)N(C)C(C)=O |t:8| Show InChI InChI=1S/C26H44N2O/c1-17(28(7)18(2)29)22-10-11-23-21-9-8-19-16-20(27(5)6)12-14-25(19,3)24(21)13-15-26(22,23)4/h8,17,20-24H,9-16H2,1-7H3/t17-,20-,21-,22+,23-,24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421634
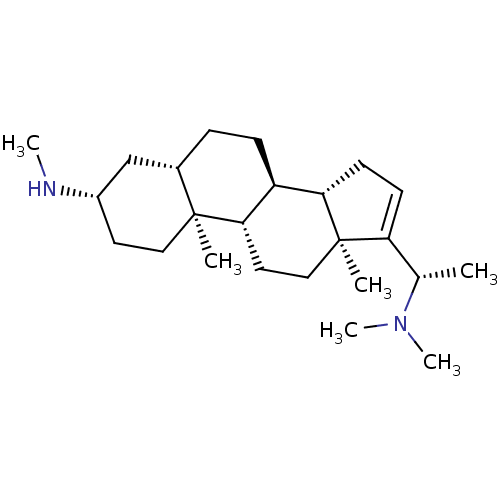
(CHEMBL137721)Show SMILES CN[C@H]1CC[C@@]2(C)[C@@H](CC[C@H]3[C@@H]4CC=C([C@H](C)N(C)C)[C@@]4(C)CC[C@H]23)C1 |t:13| Show InChI InChI=1S/C24H42N2/c1-16(26(5)6)20-9-10-21-19-8-7-17-15-18(25-4)11-13-23(17,2)22(19)12-14-24(20,21)3/h9,16-19,21-22,25H,7-8,10-15H2,1-6H3/t16-,17-,18-,19-,21-,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50412078
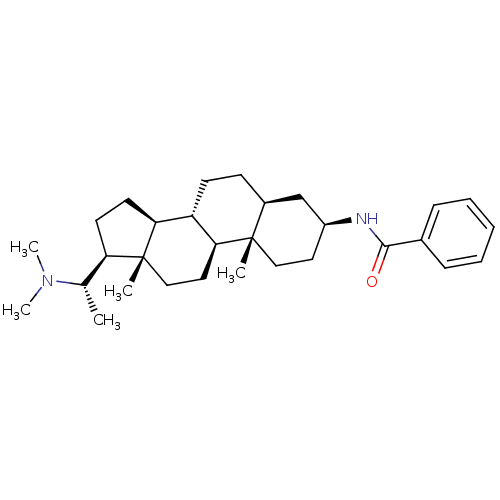
(EPIPACHYSAMINE D)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)NC(=O)c1ccccc1)N(C)C |r| Show InChI InChI=1S/C30H46N2O/c1-20(32(4)5)25-13-14-26-24-12-11-22-19-23(31-28(33)21-9-7-6-8-10-21)15-17-29(22,2)27(24)16-18-30(25,26)3/h6-10,20,22-27H,11-19H2,1-5H3,(H,31,33)/t20-,22-,23-,24-,25+,26-,27-,29-,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421635
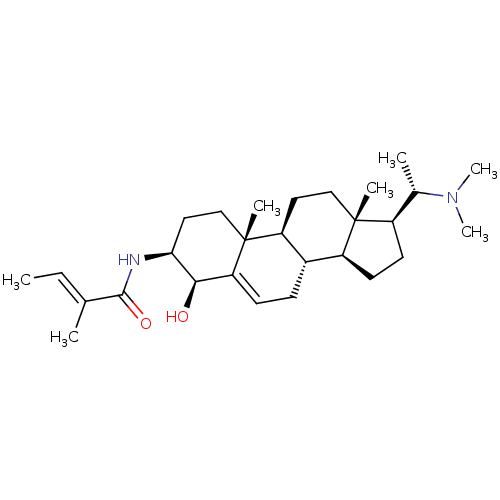
(CHEMBL2368067)Show SMILES [H][C@@](C)(N(C)C)[C@@]1([H])CC[C@@]2([H])[C@]3([H])CC=C4[C@@H](O)[C@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)NC(=O)C(\C)=C\C |t:15| Show InChI InChI=1S/C28H46N2O2/c1-8-17(2)26(32)29-24-14-16-28(5)22-13-15-27(4)20(18(3)30(6)7)11-12-21(27)19(22)9-10-23(28)25(24)31/h8,10,18-22,24-25,31H,9,11-16H2,1-7H3,(H,29,32)/b17-8+/t18-,19-,20+,21-,22-,24-,25+,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421629
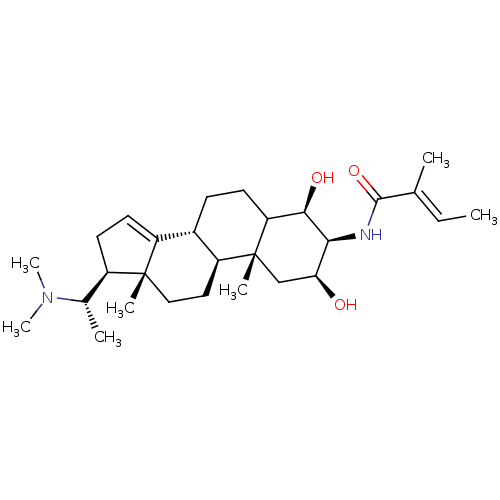
(CHEMBL337494)Show SMILES C\C=C(/C)C(=O)N[C@H]1[C@@H](O)C[C@@]2(C)C(CC[C@@H]3[C@@H]2CC[C@]2(C)[C@H](CC=C32)[C@H](C)N(C)C)[C@H]1O |t:25| Show InChI InChI=1S/C28H46N2O3/c1-8-16(2)26(33)29-24-23(31)15-28(5)21-13-14-27(4)19(17(3)30(6)7)11-12-20(27)18(21)9-10-22(28)25(24)32/h8,12,17-19,21-25,31-32H,9-11,13-15H2,1-7H3,(H,29,33)/b16-8+/t17-,18-,19+,21-,22?,23-,24-,25+,27+,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423628
(CHEMBL259477)Show SMILES Oc1c(C(c2cccnc2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C24H15NO6/c26-21-14-7-1-3-9-16(14)30-23(28)19(21)18(13-6-5-11-25-12-13)20-22(27)15-8-2-4-10-17(15)31-24(20)29/h1-12,18,26-27H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM33577
(3-(1,3-benzothiazol-2-yl)phenol | MLS000063319 | S...)Show InChI InChI=1S/C13H9NOS/c15-10-5-3-4-9(8-10)13-14-11-6-1-2-7-12(11)16-13/h1-8,15H | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421628

(CHEMBL344384)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC=C4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C)N(C)C |t:8| Show InChI InChI=1S/C25H44N2/c1-17(26(4)5)21-10-11-22-20-9-8-18-16-19(27(6)7)12-14-24(18,2)23(20)13-15-25(21,22)3/h8,17,19-23H,9-16H2,1-7H3/t17-,19-,20-,21+,22-,23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50412080
(CHEMBL342394)Show SMILES CO[C@H]1CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CC=C2C1)[C@H](C)N(C)C |r,c:20| Show InChI InChI=1S/C24H41NO/c1-16(25(4)5)20-9-10-21-19-8-7-17-15-18(26-6)11-13-23(17,2)22(19)12-14-24(20,21)3/h7,16,18-22H,8-15H2,1-6H3/t16-,18-,19-,20+,21-,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423637
(CHEMBL409740)Show SMILES C\C=C\C(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C22H16O6/c1-2-7-14(17-19(23)12-8-3-5-10-15(12)27-21(17)25)18-20(24)13-9-4-6-11-16(13)28-22(18)26/h2-11,14,23-24H,1H3/b7-2+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423625
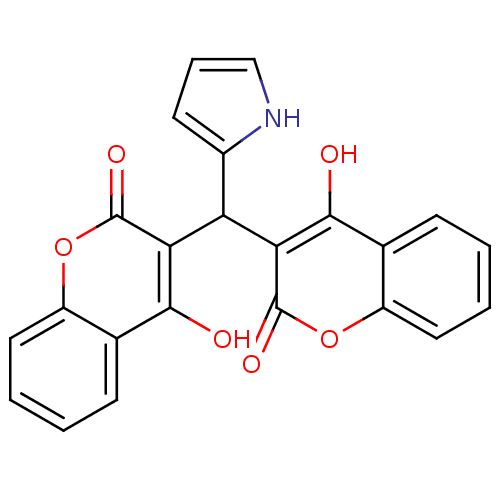
(CHEMBL260998)Show SMILES Oc1c(C(c2ccc[nH]2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C23H15NO6/c25-20-12-6-1-3-9-15(12)29-22(27)18(20)17(14-8-5-11-24-14)19-21(26)13-7-2-4-10-16(13)30-23(19)28/h1-11,17,24-26H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Beta-glucuronidase
(Bos taurus (Bovine)) | BDBM50008907
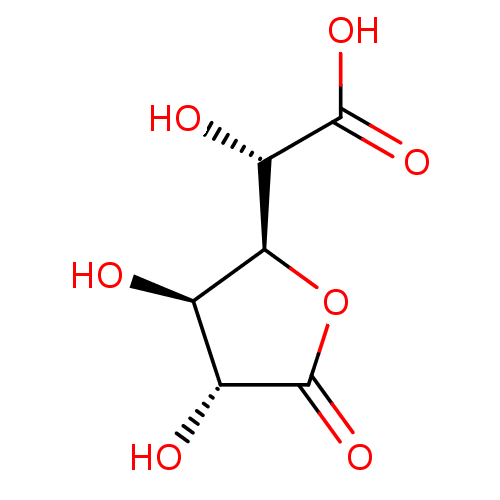
(D-Saccharic Acid 1,4-Lactone)Show SMILES [H][C@@]1(OC(=O)[C@H](O)[C@H]1O)[C@H](O)C(O)=O |r| Show InChI InChI=1S/C6H8O7/c7-1-2(8)6(12)13-4(1)3(9)5(10)11/h1-4,7-9H,(H,10,11)/t1-,2-,3+,4+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of bovine liver beta glucuronidase assessed as p-nitrophenol formation after 30 mins by spectrophotometric method |
Bioorg Med Chem 19: 4286-94 (2011)
Article DOI: 10.1016/j.bmc.2011.05.052
BindingDB Entry DOI: 10.7270/Q2FB55SC |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421623
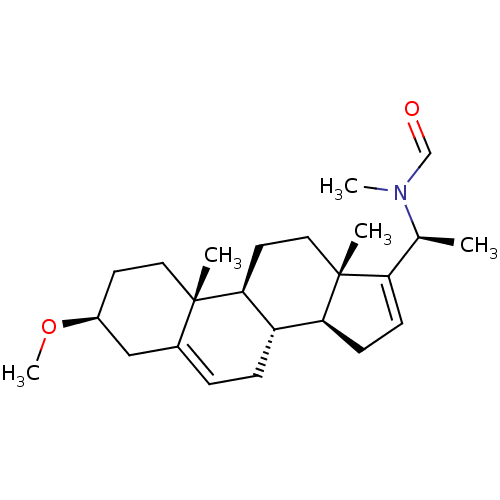
(CHEMBL140241)Show SMILES CO[C@H]1CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](CC=C4[C@H](C)N(C)C=O)[C@@H]3CC=C2C1 |c:14,26| Show InChI InChI=1S/C24H37NO2/c1-16(25(4)15-26)20-8-9-21-19-7-6-17-14-18(27-5)10-12-23(17,2)22(19)11-13-24(20,21)3/h6,8,15-16,18-19,21-22H,7,9-14H2,1-5H3/t16-,18-,19-,21-,22-,23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421632
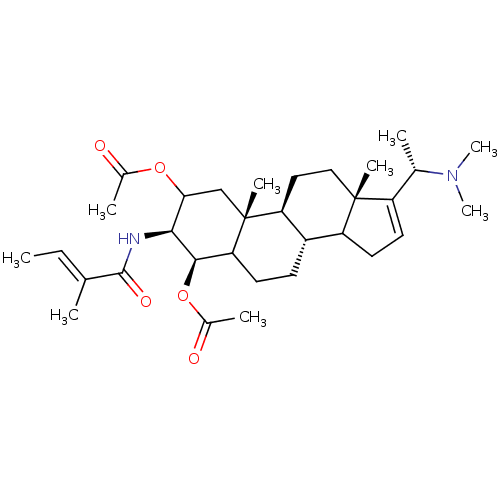
(CHEMBL342245)Show SMILES C\C=C(/C)C(=O)N[C@H]1C(C[C@]2(C)[C@H]3CC[C@@]4(C)C(CC=C4[C@H](C)N(C)C)[C@@H]3CCC2[C@H]1OC(C)=O)OC(C)=O |c:19| Show InChI InChI=1S/C32H50N2O5/c1-10-18(2)30(37)33-28-27(38-20(4)35)17-32(7)25-15-16-31(6)23(19(3)34(8)9)13-14-24(31)22(25)11-12-26(32)29(28)39-21(5)36/h10,13,19,22,24-29H,11-12,14-17H2,1-9H3,(H,33,37)/b18-10+/t19-,22-,24?,25-,26?,27?,28-,29+,31+,32+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421624
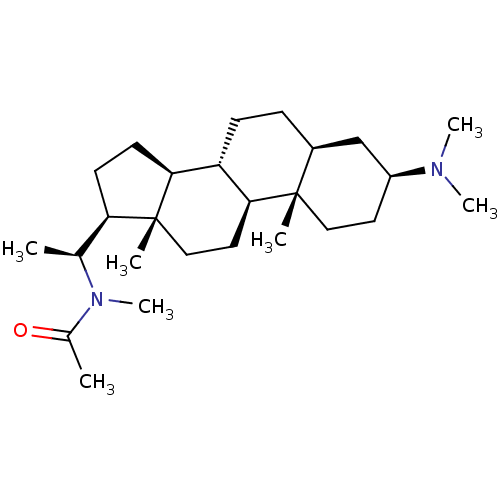
(CHEMBL136052)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C)N(C)C(C)=O Show InChI InChI=1S/C26H46N2O/c1-17(28(7)18(2)29)22-10-11-23-21-9-8-19-16-20(27(5)6)12-14-25(19,3)24(21)13-15-26(22,23)4/h17,19-24H,8-16H2,1-7H3/t17-,19-,20-,21-,22+,23-,24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423624
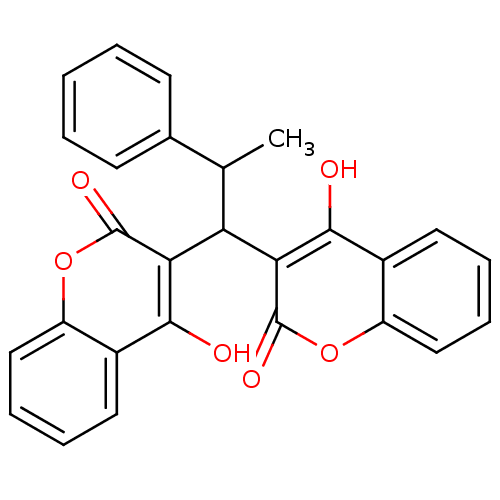
(CHEMBL260178)Show SMILES CC(C(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O)c1ccccc1 Show InChI InChI=1S/C27H20O6/c1-15(16-9-3-2-4-10-16)21(22-24(28)17-11-5-7-13-19(17)32-26(22)30)23-25(29)18-12-6-8-14-20(18)33-27(23)31/h2-15,21,28-29H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423639
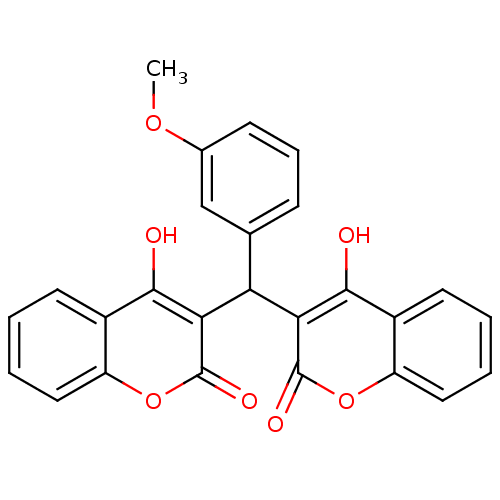
(CHEMBL264936)Show SMILES COc1cccc(c1)C(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C26H18O7/c1-31-15-8-6-7-14(13-15)20(21-23(27)16-9-2-4-11-18(16)32-25(21)29)22-24(28)17-10-3-5-12-19(17)33-26(22)30/h2-13,20,27-28H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.62E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM85754

(Dicoumarol derivative, 7)Show SMILES Oc1c(C(c2ccncc2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C24H15NO6/c26-21-14-5-1-3-7-16(14)30-23(28)19(21)18(13-9-11-25-12-10-13)20-22(27)15-6-2-4-8-17(15)31-24(20)29/h1-12,18,26-27H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50226816
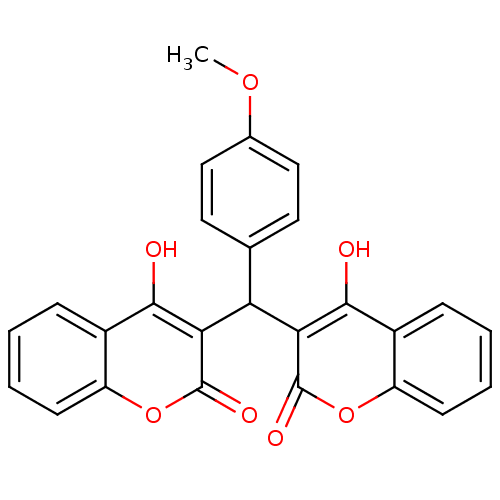
(4-Hydroxy-3-[(4-hydroxy-2-oxo-4a,8a-dihydro-2H-chr...)Show SMILES COc1ccc(cc1)C(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C26H18O7/c1-31-15-12-10-14(11-13-15)20(21-23(27)16-6-2-4-8-18(16)32-25(21)29)22-24(28)17-7-3-5-9-19(17)33-26(22)30/h2-13,20,27-28H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423629
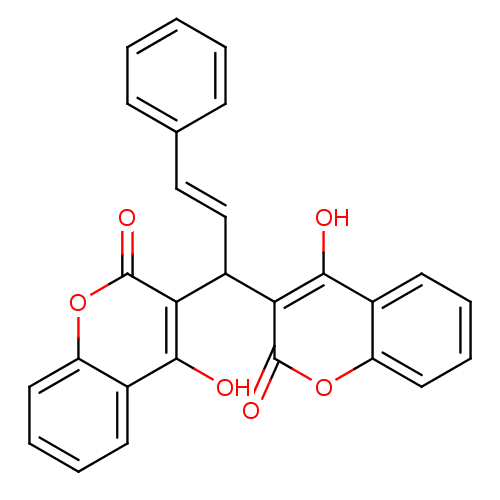
(CHEMBL259154)Show SMILES Oc1c(C(\C=C\c2ccccc2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C27H18O6/c28-24-17-10-4-6-12-20(17)32-26(30)22(24)19(15-14-16-8-2-1-3-9-16)23-25(29)18-11-5-7-13-21(18)33-27(23)31/h1-15,19,28-29H/b15-14+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135156
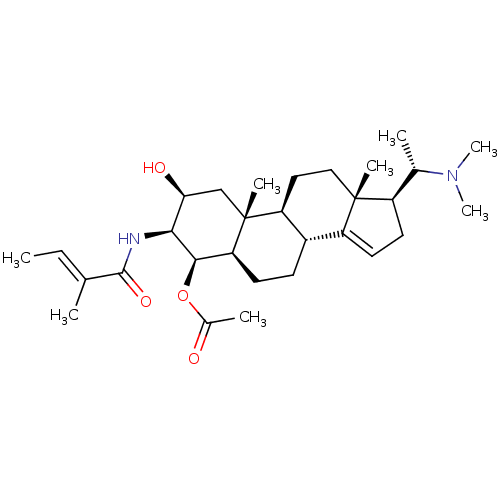
(Acetic acid (2S,3S,4R,5R,8R,9S,10R,13R,17S)-17-((S...)Show SMILES C\C=C(/C)C(=O)N[C@H]1[C@@H](O)C[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC=C4[C@@H]3CC[C@H]2[C@H]1OC(C)=O)[C@H](C)N(C)C |c:20| Show InChI InChI=1S/C30H48N2O4/c1-9-17(2)28(35)31-26-25(34)16-30(6)23-14-15-29(5)21(18(3)32(7)8)12-13-22(29)20(23)10-11-24(30)27(26)36-19(4)33/h9,13,18,20-21,23-27,34H,10-12,14-16H2,1-8H3,(H,31,35)/b17-9+/t18-,20-,21+,23-,24-,25-,26-,27+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423635
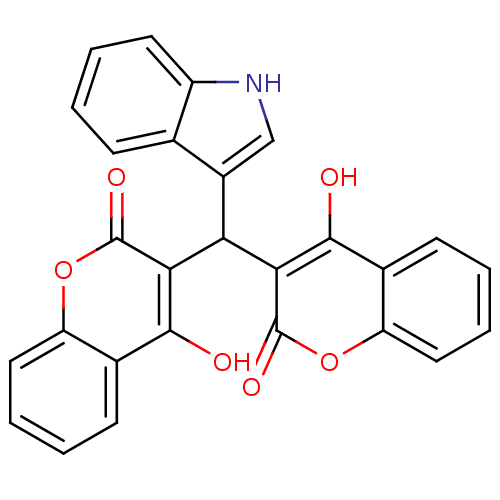
(CHEMBL258733)Show SMILES Oc1c(C(c2c[nH]c3ccccc23)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C27H17NO6/c29-24-15-8-2-5-11-19(15)33-26(31)22(24)21(17-13-28-18-10-4-1-7-14(17)18)23-25(30)16-9-3-6-12-20(16)34-27(23)32/h1-13,21,28-30H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423623
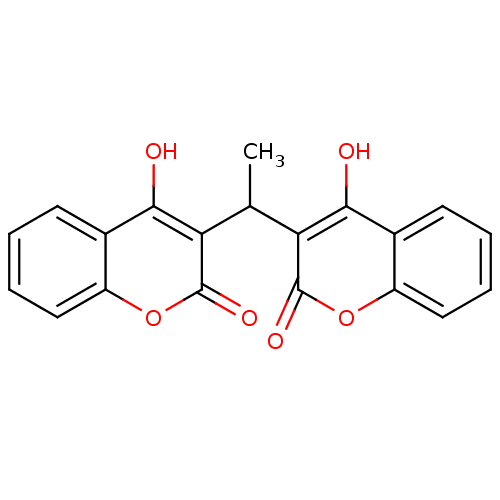
(CHEMBL402459)Show InChI InChI=1S/C20H14O6/c1-10(15-17(21)11-6-2-4-8-13(11)25-19(15)23)16-18(22)12-7-3-5-9-14(12)26-20(16)24/h2-10,21-22H,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423630
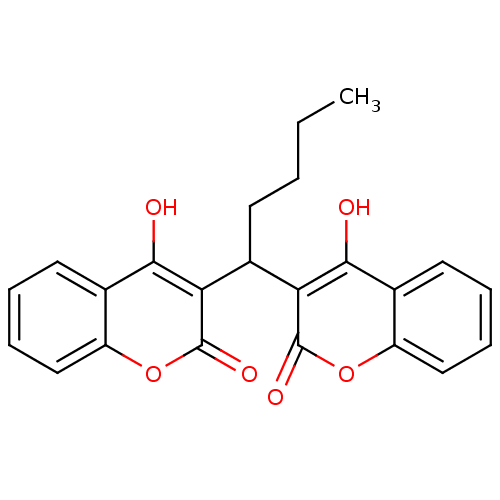
(CHEMBL261558)Show SMILES CCCCC(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C23H20O6/c1-2-3-8-15(18-20(24)13-9-4-6-11-16(13)28-22(18)26)19-21(25)14-10-5-7-12-17(14)29-23(19)27/h4-7,9-12,15,24-25H,2-3,8H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423631
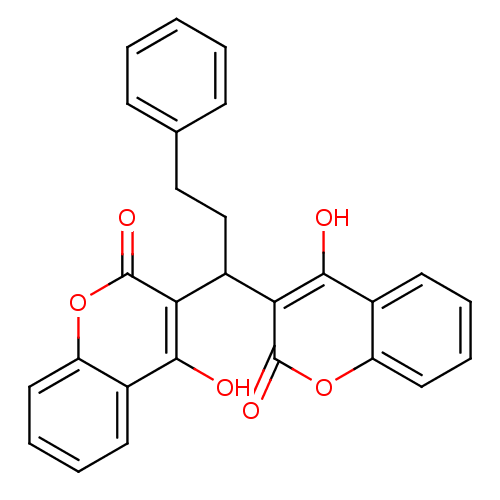
(CHEMBL261789)Show SMILES Oc1c(C(CCc2ccccc2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C27H20O6/c28-24-17-10-4-6-12-20(17)32-26(30)22(24)19(15-14-16-8-2-1-3-9-16)23-25(29)18-11-5-7-13-21(18)33-27(23)31/h1-13,19,28-29H,14-15H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM35539
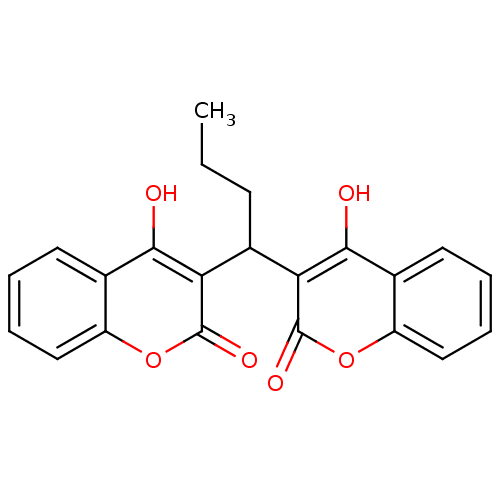
(symmetric dicoumarol analogue, 15)Show SMILES CCCC(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C22H18O6/c1-2-7-14(17-19(23)12-8-3-5-10-15(12)27-21(17)25)18-20(24)13-9-4-6-11-16(13)28-22(18)26/h3-6,8-11,14,23-24H,2,7H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50135152
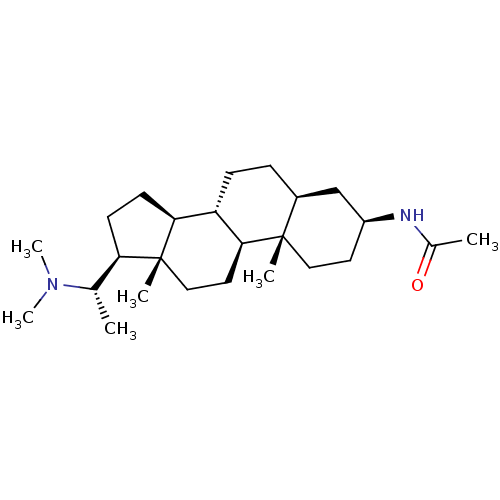
(CHEMBL422098 | N-[(3S,5S,8R,9S,10S,13S,14S,17S)-17...)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)NC(C)=O)N(C)C Show InChI InChI=1S/C25H44N2O/c1-16(27(5)6)21-9-10-22-20-8-7-18-15-19(26-17(2)28)11-13-24(18,3)23(20)12-14-25(21,22)4/h16,18-23H,7-15H2,1-6H3,(H,26,28)/t16-,18-,19-,20-,21+,22-,23-,24-,25+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423626
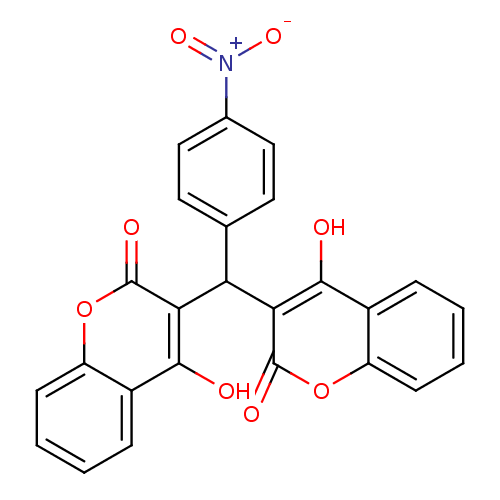
(CHEMBL410935)Show SMILES Oc1c(C(c2ccc(cc2)[N+]([O-])=O)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C25H15NO8/c27-22-15-5-1-3-7-17(15)33-24(29)20(22)19(13-9-11-14(12-10-13)26(31)32)21-23(28)16-6-2-4-8-18(16)34-25(21)30/h1-12,19,27-28H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50421622
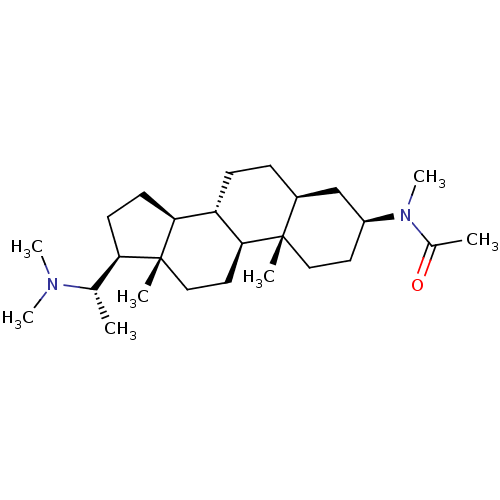
(CHEMBL434042)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@H](CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C(C)=O)N(C)C Show InChI InChI=1S/C26H46N2O/c1-17(27(5)6)22-10-11-23-21-9-8-19-16-20(28(7)18(2)29)12-14-25(19,3)24(21)13-15-26(22,23)4/h17,19-24H,8-16H2,1-7H3/t17-,19-,20-,21-,22+,23-,24-,25-,26+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Compound was tested for the in silico inhibition of acetylcholinesterase |
Bioorg Med Chem Lett 13: 4375-80 (2003)
BindingDB Entry DOI: 10.7270/Q29C6ZQG |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423636
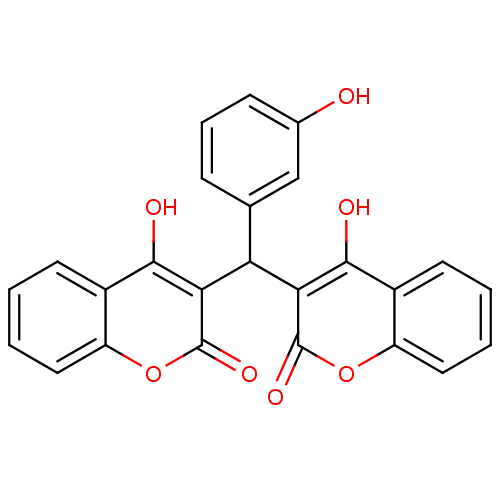
(CHEMBL409439)Show SMILES Oc1cccc(c1)C(c1c(O)c2ccccc2oc1=O)c1c(O)c2ccccc2oc1=O Show InChI InChI=1S/C25H16O7/c26-14-7-5-6-13(12-14)19(20-22(27)15-8-1-3-10-17(15)31-24(20)29)21-23(28)16-9-2-4-11-18(16)32-25(21)30/h1-12,19,26-28H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423638
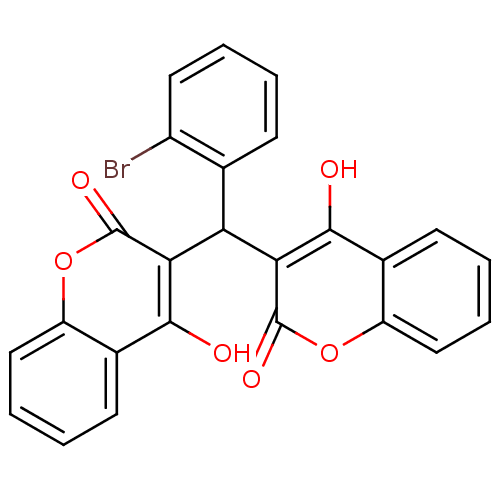
(CHEMBL410266)Show SMILES Oc1c(C(c2ccccc2Br)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C25H15BrO6/c26-16-10-4-1-7-13(16)19(20-22(27)14-8-2-5-11-17(14)31-24(20)29)21-23(28)15-9-3-6-12-18(15)32-25(21)30/h1-12,19,27-28H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |
Urease
(Canavalia ensiformis (Jack bean) (Horse bean)) | BDBM50423632
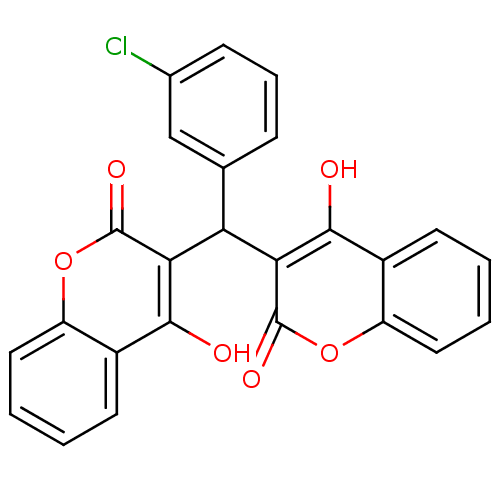
(CHEMBL412094)Show SMILES Oc1c(C(c2cccc(Cl)c2)c2c(O)c3ccccc3oc2=O)c(=O)oc2ccccc12 Show InChI InChI=1S/C25H15ClO6/c26-14-7-5-6-13(12-14)19(20-22(27)15-8-1-3-10-17(15)31-24(20)29)21-23(28)16-9-2-4-11-18(16)32-25(21)30/h1-12,19,27-28H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Karachi
Curated by ChEMBL
| Assay Description
Inhibition of jack bean urease |
Bioorg Med Chem 16: 3456-61 (2008)
Article DOI: 10.1016/j.bmc.2005.09.048
BindingDB Entry DOI: 10.7270/Q2474C5K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data