

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36548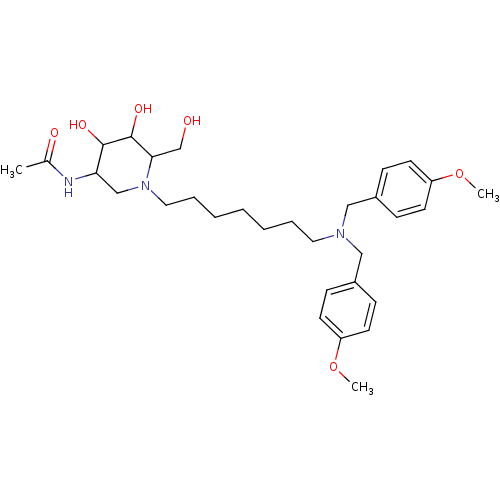 (N-[1-(7-{bis[(4-methoxyphenyl)methyl]amino}heptyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.690 | -53.2 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327904 (2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327907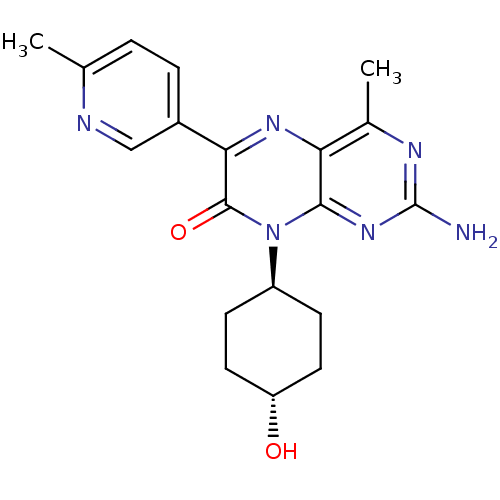 (CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36547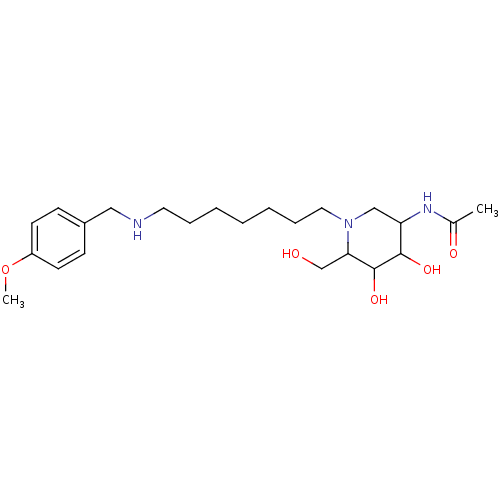 (N-[4,5-dihydroxy-6-(hydroxymethyl)-1-(7-{[(4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20 | -51.8 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101546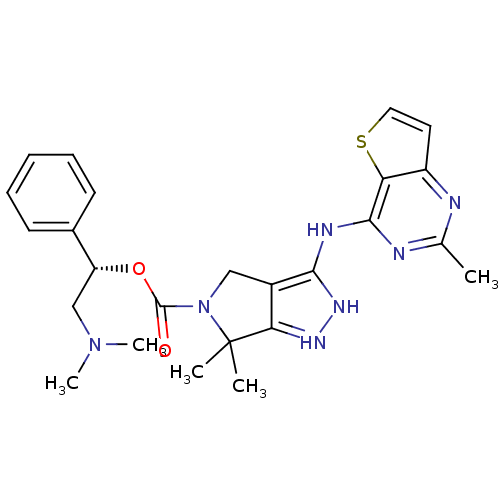 (US8530494, 211 | US8530652, 125 | US8530652, 73) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101542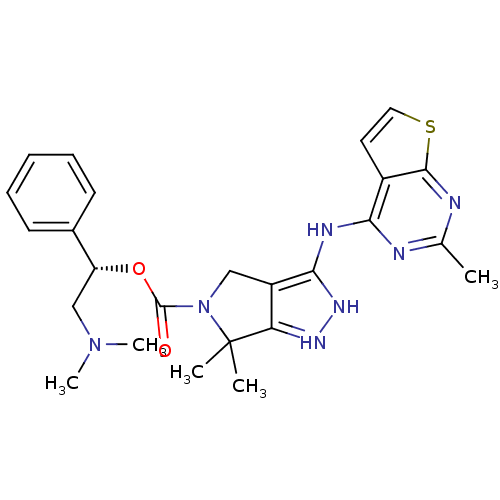 (US8530494, 207 | US8530652, 121 | US8530652, 69) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36546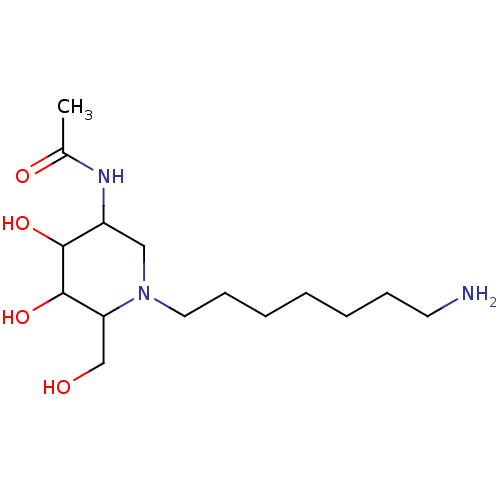 (N-[1-(7-aminoheptyl)-4,5-dihydroxy-6-(hydroxymethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.10 | -50.4 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389112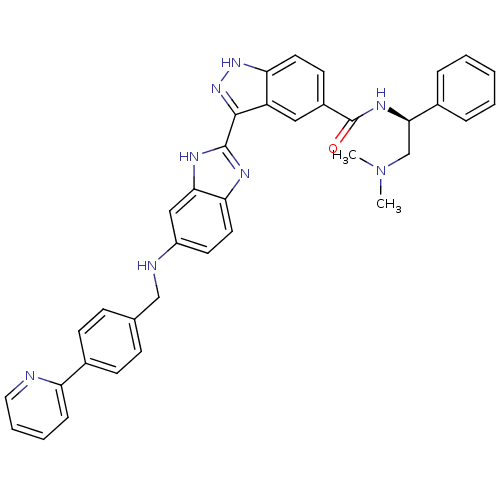 (CHEMBL2064556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101479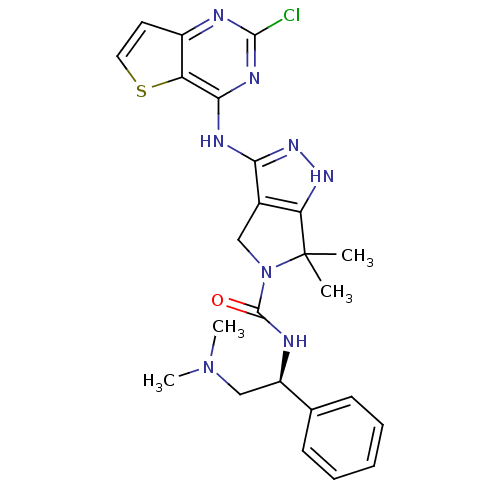 (US8524710, 56 | US8530652, 50 | US8530652, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.80 | n/a | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101537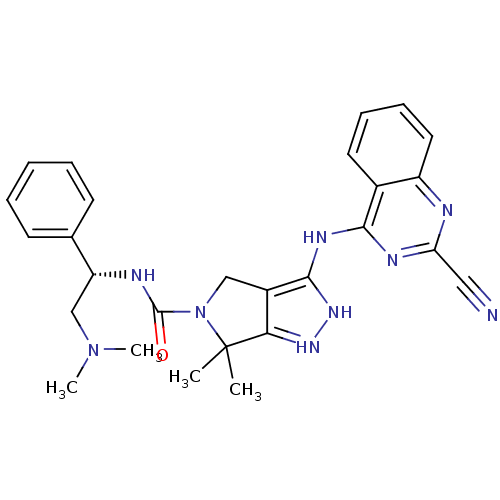 (US8530494, 204 | US8530652, 115 | US8530652, 64) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50389111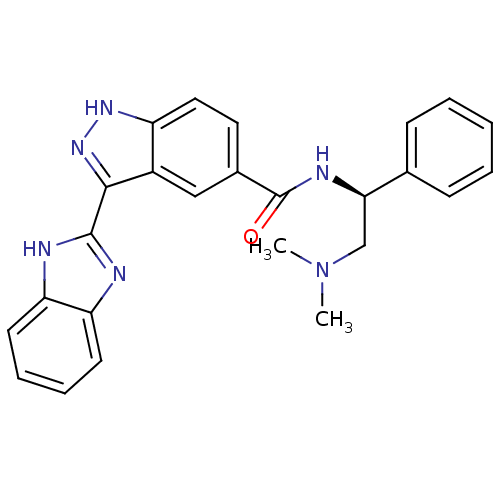 (CHEMBL2064555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of CDK2 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM4838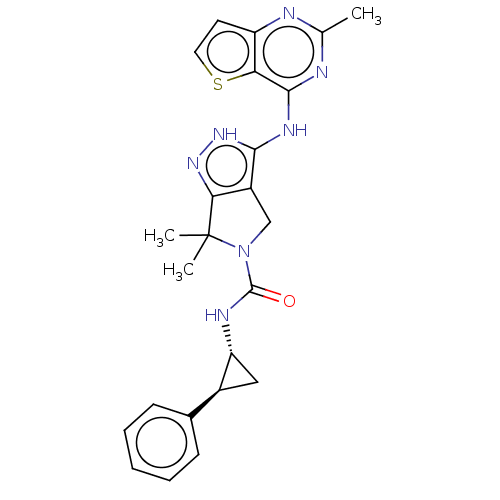 (US8524710, 83 | US8530494, 13 | US8530652, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM4838 (US8524710, 83 | US8530494, 13 | US8530652, 52) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101547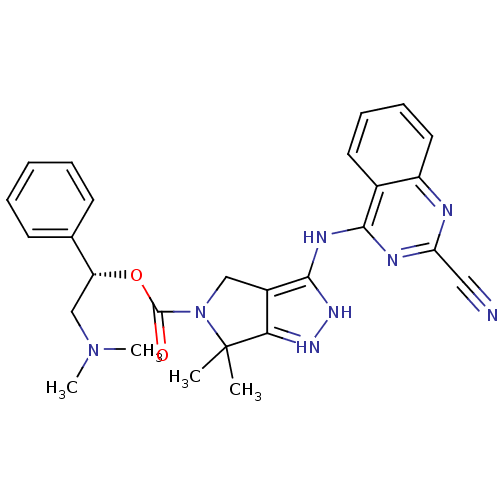 (US8530494, 212 | US8530652, 126 | US8530652, 74) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.60 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327894 (2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327906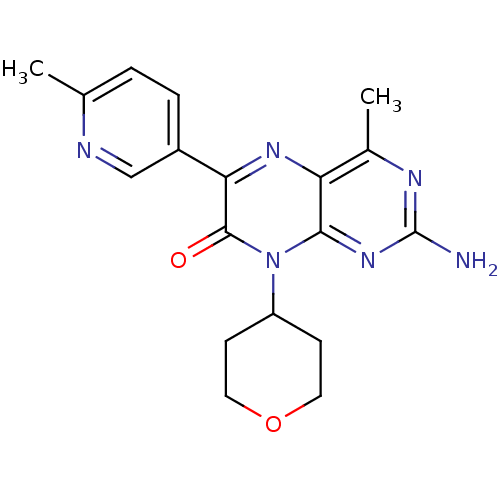 (2-amino-4-methyl-6-(6-methylpyridin-3-yl)-8-(tetra...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327903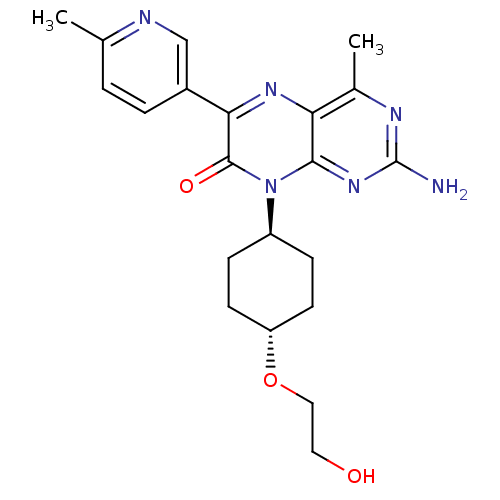 (CHEMBL1258910 | trans-2-amino-8-(4-(2-hydroxyethox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101548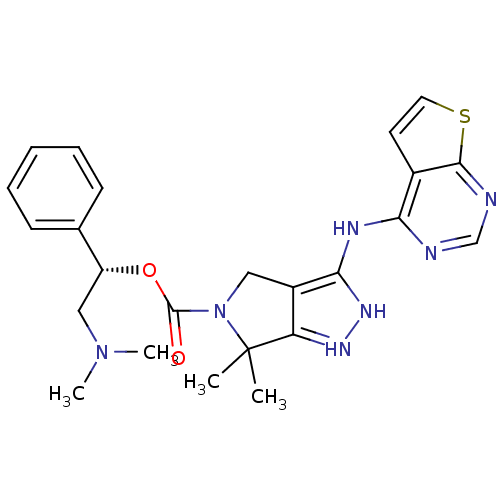 (US8530494, 108 | US8530652, 127 | US8530652, 75) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.20 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101544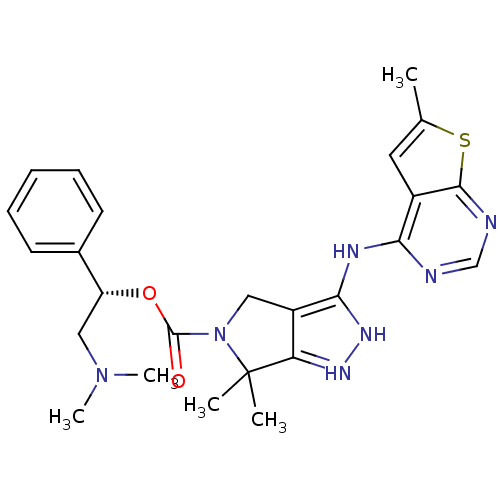 (US8530494, 105 | US8530652, 123 | US8530652, 71) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389111 (CHEMBL2064555) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327897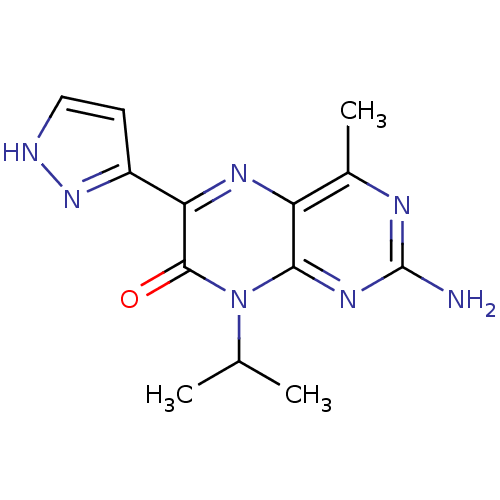 (2-amino-8-isopropyl-4-methyl-6-(1H-pyrazol-5-yl)pt...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101541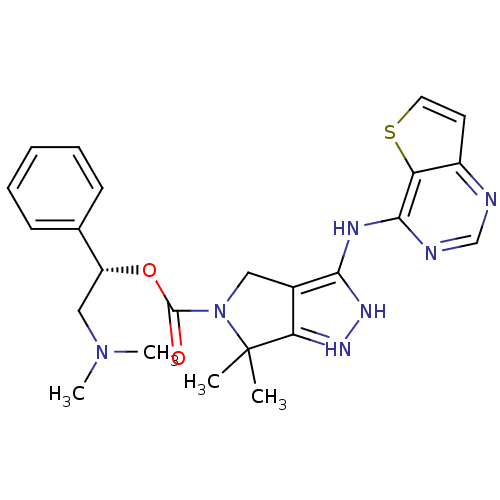 (US8530494, 104 | US8530652, 120 | US8530652, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.70 | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327905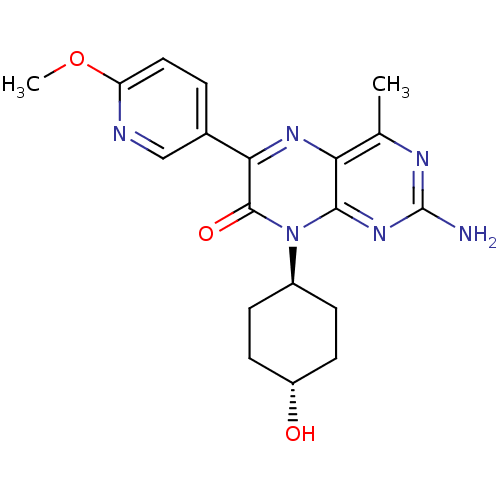 (CHEMBL1257177 | trans-2-amino-8-((1r,4r)-4-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101504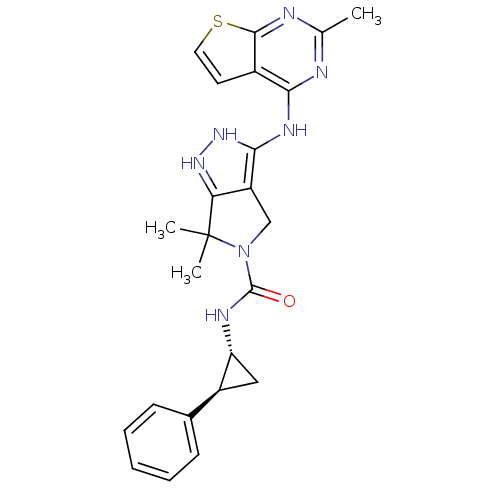 (US8524699, 91 | US8524710, 82 | US8530652, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7.20 | n/a | n/a | n/a | 21 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101549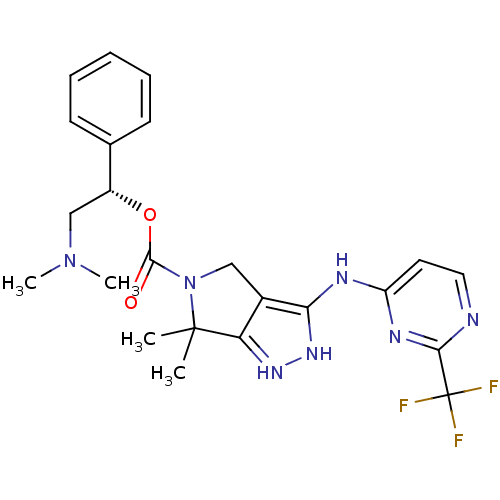 (US8530494, 109 | US8530652, 128 | US8530652, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.30 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101543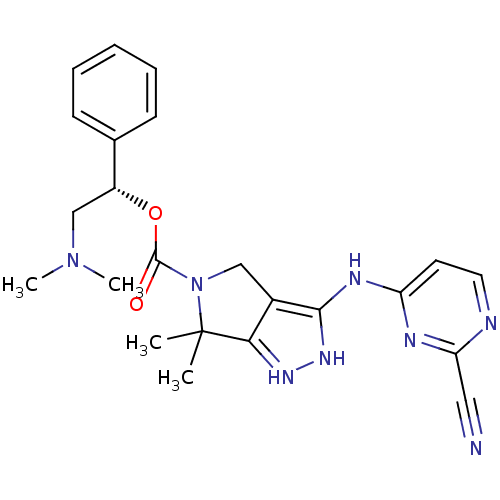 (US8530494, 206 | US8530652, 122 | US8530652, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8.80 | n/a | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327896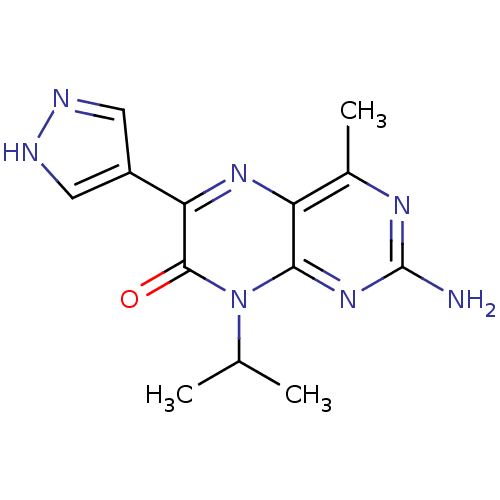 (2-amino-4-methyl-8-(1-methylethyl)-6-(1H-pyrazol-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101476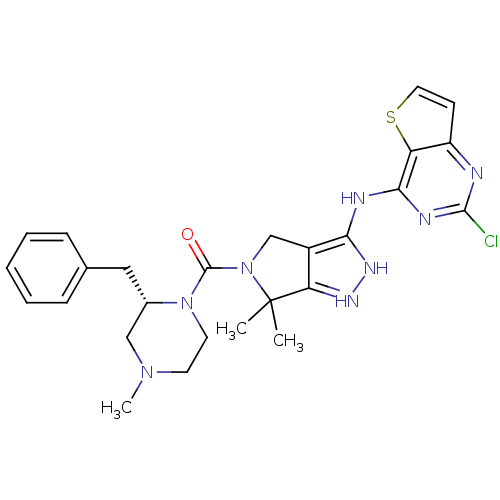 (US8524710, 53 | US8530652, 3 | US8530652, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11 | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389116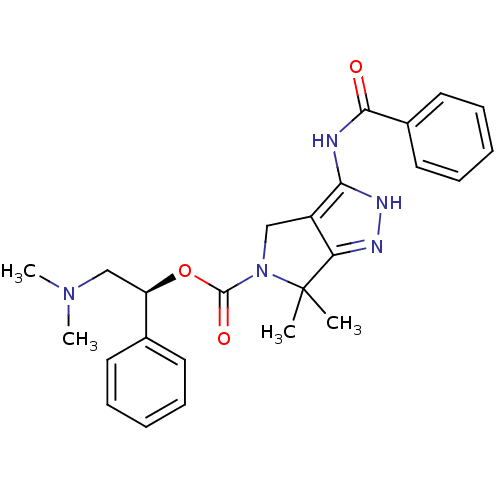 (CHEMBL2064561) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389117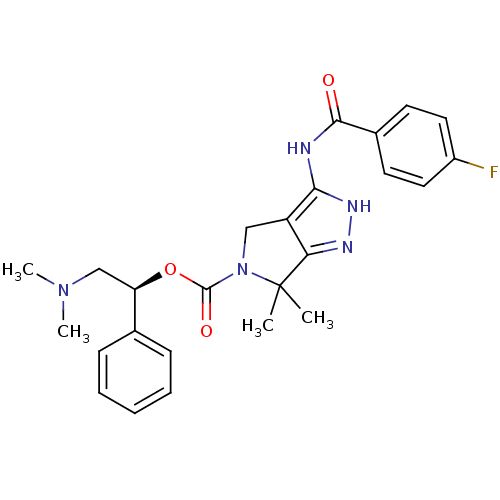 (CHEMBL2064562) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101530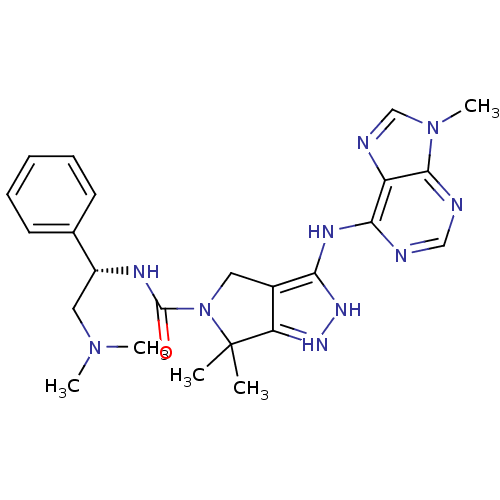 (US8530494, 29 | US8530494, 7 | US8530652, 57) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101475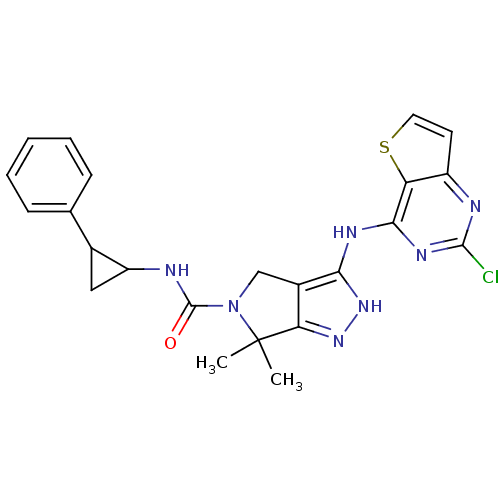 (US8524710, 52 | US8530652, 2 | US8530652, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | n/a | n/a | n/a | 36 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101535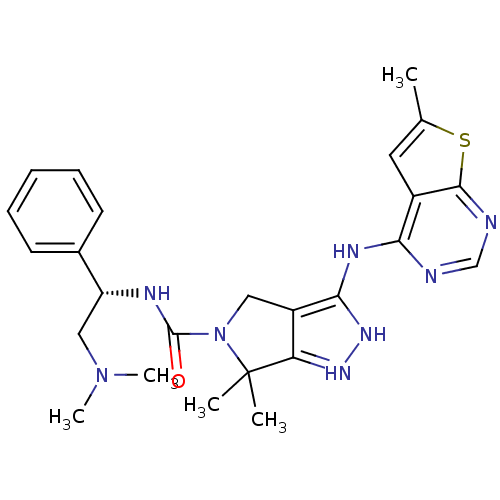 (US8530494, 104 | US8530652, 113 | US8530652, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15 | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101515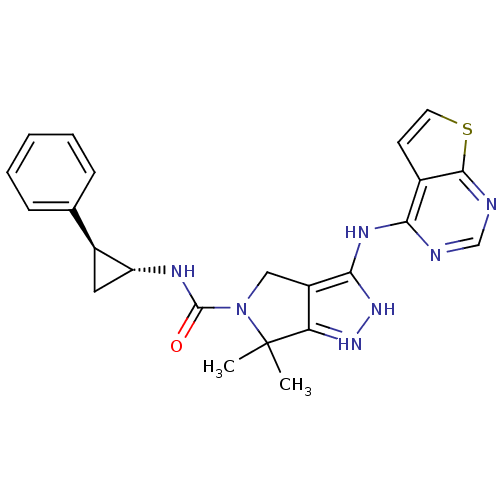 (US8524699, 26 | US8530652, 42 | US8530670, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | 340 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101545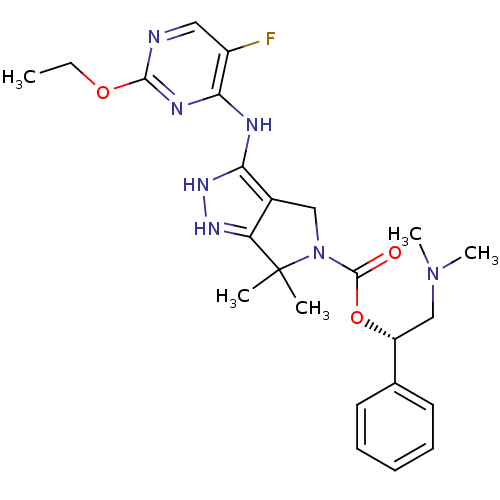 (US8530494, 107 | US8530652, 124 | US8530652, 72) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 16 | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101513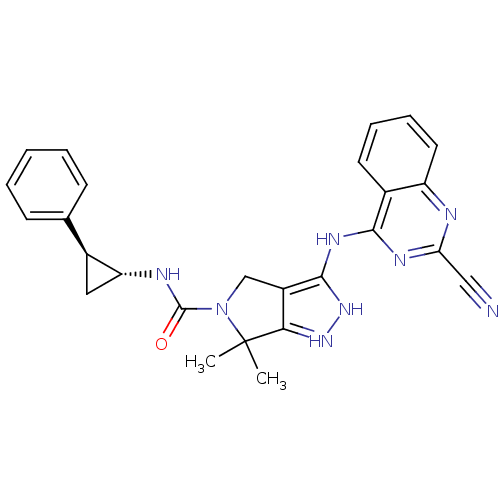 (US8524699, 18 | US8530652, 40 | US8530652, 85) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | 98 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101524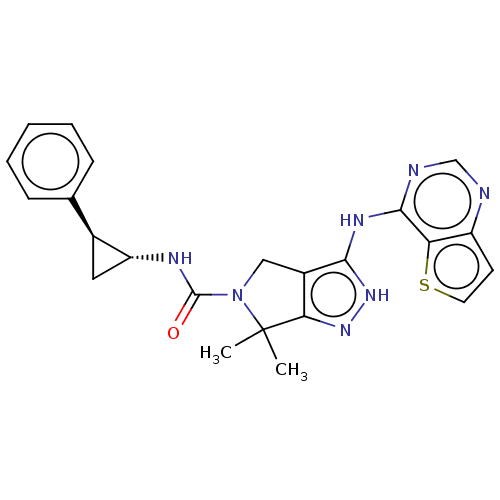 (US8530652, 51 | US8530652, 96) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 18 | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327901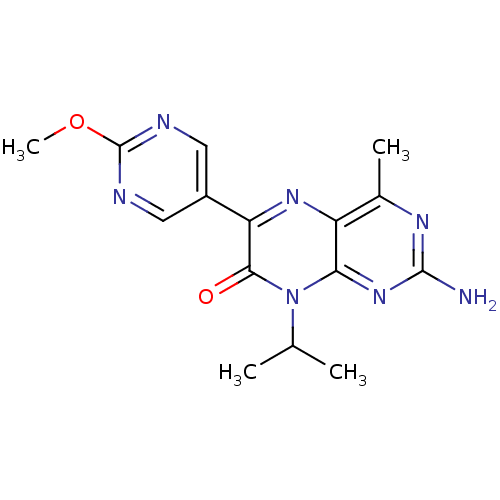 (2-amino-8-isopropyl-6-(2-methoxypyrimidin-5-yl)-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101504 (US8524699, 91 | US8524710, 82 | US8530652, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | 103 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101536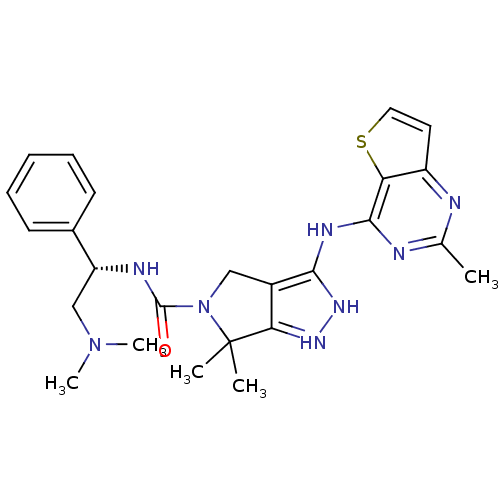 (CHEMBL3128043 | US8530494, 203 | US8530652, 114 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 20 | n/a | n/a | n/a | <3.90 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101507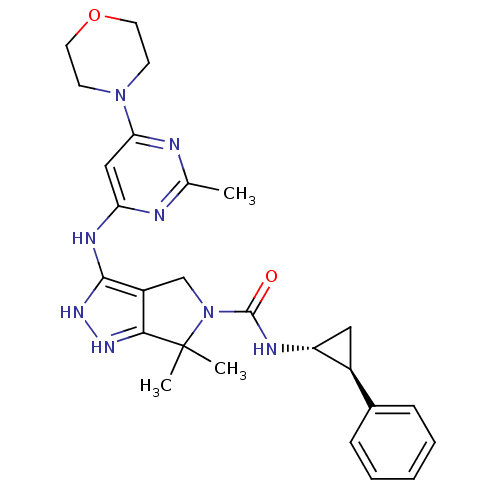 (US8524710, 86 | US8530652, 34 | US8530652, 79) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 21 | n/a | n/a | n/a | 778 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101534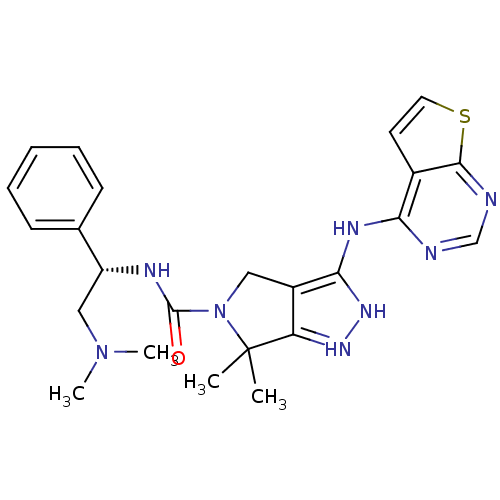 (US8530494, 202 | US8530652, 112 | US8530652, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 22 | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101514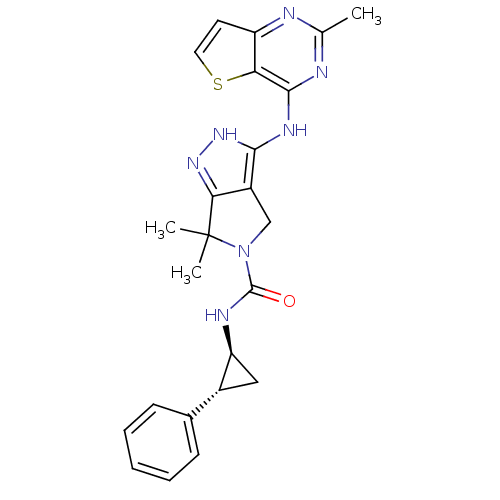 (US8524699, 22 | US8530652, 41 | US8530652, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 24 | n/a | n/a | n/a | 147 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101518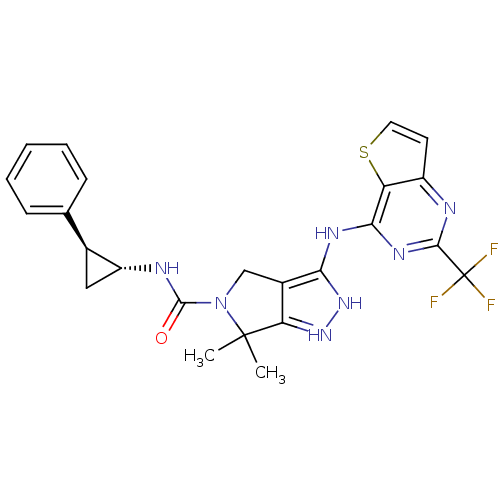 (US8524699, 46 | US8530652, 45 | US8530652, 90) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-hexosaminidase subunit beta (Homo sapiens (Human)) | BDBM36549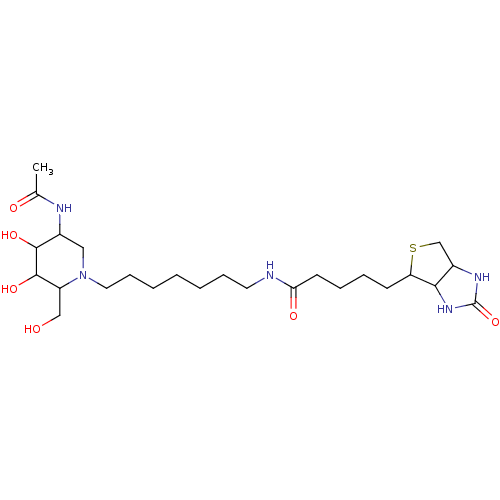 (N-{7-[5-acetamido-3,4-dihydroxy-2-(hydroxymethyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26.7 | -44.0 | n/a | n/a | n/a | n/a | n/a | 4.25 | 30 |
Academia Sinica | Assay Description Enzyme inhibition activity assay using 4-methylumbelliferyl N-acetylglucosamine (MUG) as substrate. | ACS Chem Biol 5: 489-97 (2010) Article DOI: 10.1021/cb100011u BindingDB Entry DOI: 10.7270/Q2JQ0ZCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389113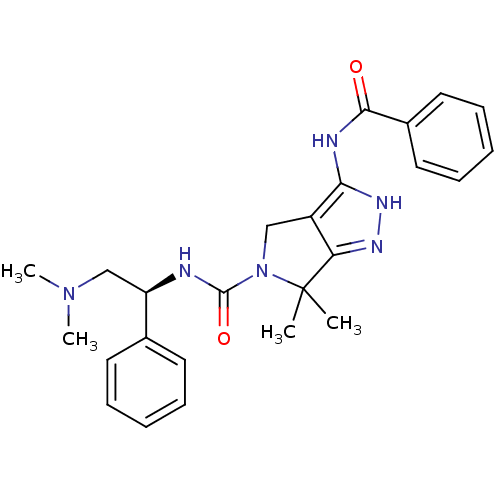 (CHEMBL2064558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase mTOR (Homo sapiens (Human)) | BDBM50327900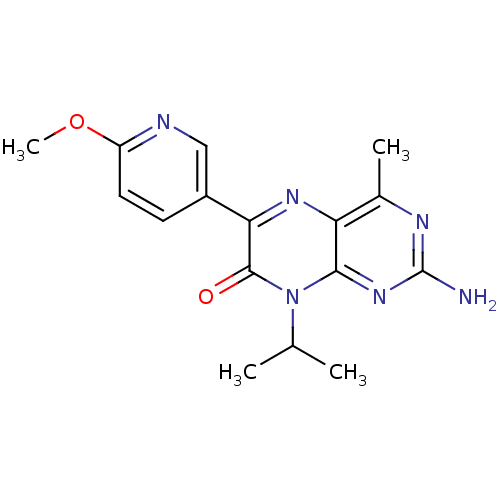 (2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of mTOR | Bioorg Med Chem Lett 20: 6096-9 (2010) Article DOI: 10.1016/j.bmcl.2010.08.045 BindingDB Entry DOI: 10.7270/Q2ZK5GWH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50389120 (CHEMBL2064565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PAK4 | J Med Chem 55: 4728-39 (2012) Article DOI: 10.1021/jm300204j BindingDB Entry DOI: 10.7270/Q2BZ673W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101639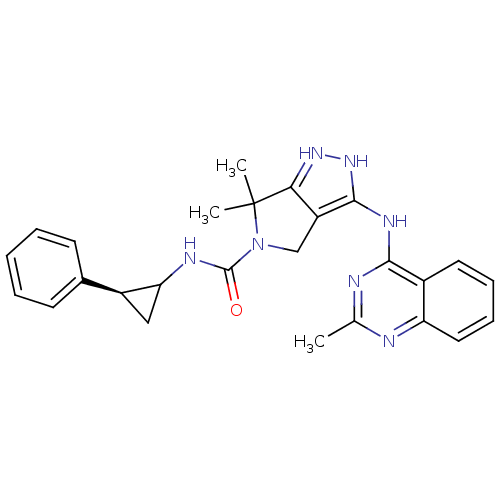 (US8530652, 84) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 34 | n/a | n/a | n/a | 455 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101485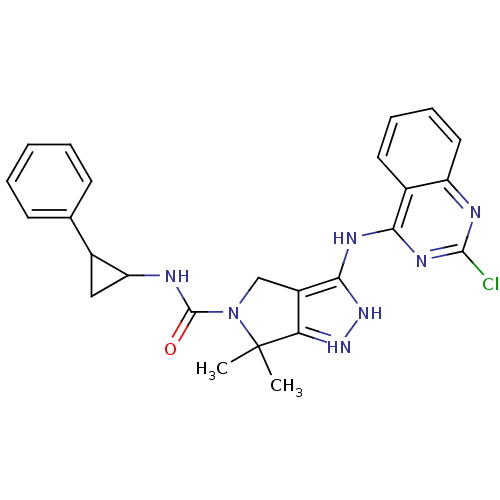 (US8524710, 62 | US8530652, 12 | US8530652, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 38 | n/a | n/a | n/a | 78 | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.; Pfizer Inc. US Patent | Assay Description The enzymatic activity of PAK4 KD was measured by its ability to catalyzed the transfer of a phosphate residue from a nucleoside triphosphate to an a... | US Patent US8530652 (2013) BindingDB Entry DOI: 10.7270/Q2F18XC4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 585 total ) | Next | Last >> |