 Found 319 hits with Last Name = 'hickory' and Initial = 'bs'
Found 319 hits with Last Name = 'hickory' and Initial = 'bs' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128131
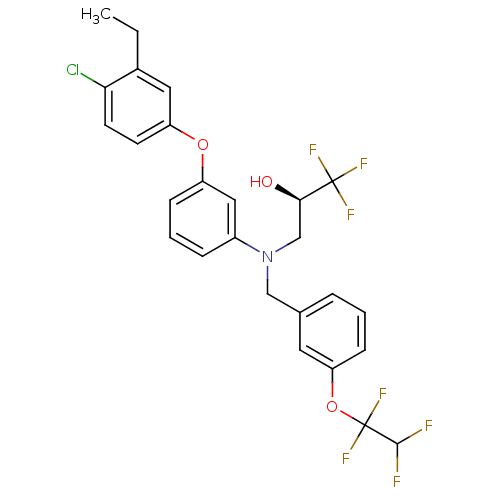
((R)-3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-...)Show SMILES CCc1cc(Oc2cccc(c2)N(C[C@@H](O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C26H23ClF7NO3/c1-2-17-12-20(9-10-22(17)27)37-19-7-4-6-18(13-19)35(15-23(36)25(30,31)32)14-16-5-3-8-21(11-16)38-26(33,34)24(28)29/h3-13,23-24,36H,2,14-15H2,1H3/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer with <1 nM [CETP] for 18 ... |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347099
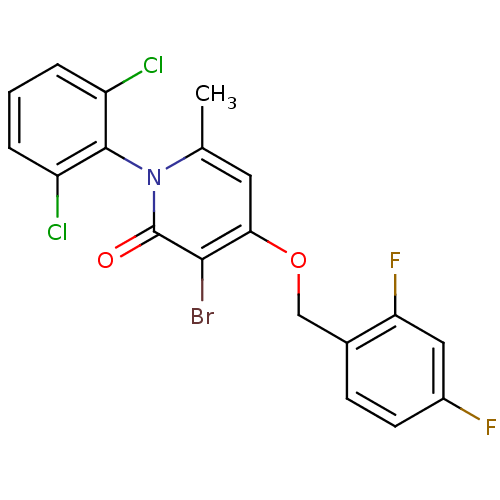
(CHEMBL1797202)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(Cl)cccc1Cl |(3.97,-20.05,;2.63,-20.81,;1.31,-20.04,;-.02,-20.81,;-1.36,-20.05,;-2.69,-20.82,;-4.03,-20.06,;-5.35,-20.83,;-6.68,-20.07,;-6.69,-18.52,;-8.03,-17.76,;-5.36,-17.75,;-4.02,-18.52,;-2.69,-17.75,;-.02,-22.35,;-1.36,-23.13,;1.31,-23.12,;1.31,-24.66,;2.63,-22.35,;3.97,-23.13,;5.29,-22.36,;5.29,-20.82,;6.62,-23.12,;6.63,-24.67,;5.29,-25.44,;3.96,-24.67,;2.62,-25.43,)| Show InChI InChI=1S/C19H12BrCl2F2NO2/c1-10-7-16(27-9-11-5-6-12(23)8-15(11)24)17(20)19(26)25(10)18-13(21)3-2-4-14(18)22/h2-8H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347100
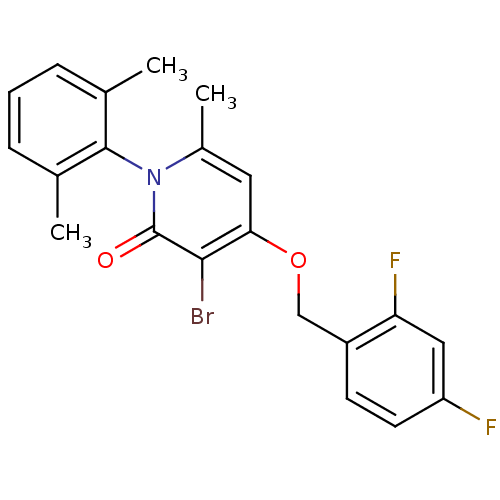
(CHEMBL1797203)Show SMILES Cc1cccc(C)c1-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.24,-20.12,;29.24,-21.66,;30.57,-22.42,;30.57,-23.97,;29.24,-24.74,;27.91,-23.97,;26.57,-24.73,;27.92,-22.43,;26.58,-21.65,;26.58,-20.11,;27.92,-19.35,;25.25,-19.34,;23.92,-20.11,;22.59,-19.35,;21.26,-20.12,;19.92,-19.36,;18.6,-20.13,;17.27,-19.37,;17.26,-17.82,;15.92,-17.06,;18.59,-17.05,;19.92,-17.82,;21.26,-17.05,;23.92,-21.65,;22.59,-22.43,;25.25,-22.42,;25.25,-23.96,)| Show InChI InChI=1S/C21H18BrF2NO2/c1-12-5-4-6-13(2)20(12)25-14(3)9-18(19(22)21(25)26)27-11-15-7-8-16(23)10-17(15)24/h4-10H,11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347929
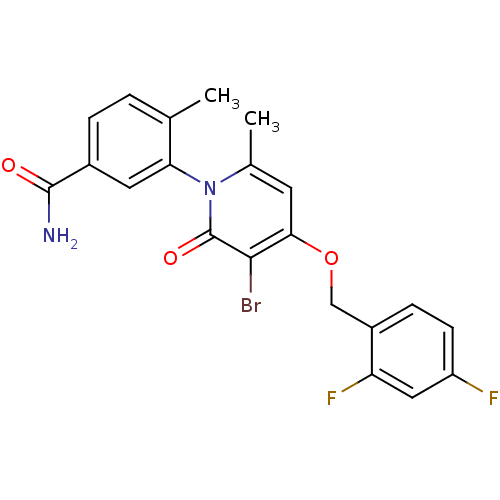
(CHEMBL1802632)Show SMILES Cc1ccc(cc1-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O)C(N)=O |(3.55,-6.94,;3.56,-8.48,;4.89,-9.25,;4.9,-10.79,;3.56,-11.57,;2.23,-10.8,;2.24,-9.26,;.9,-8.49,;.9,-6.94,;2.23,-6.16,;-.44,-6.17,;-1.77,-6.95,;-3.1,-6.18,;-4.44,-6.95,;-5.77,-6.18,;-7.09,-6.95,;-8.43,-6.18,;-8.43,-4.64,;-9.77,-3.87,;-7.1,-3.87,;-5.76,-4.64,;-4.43,-3.87,;-1.77,-8.49,;-3.1,-9.26,;-.44,-9.26,;-.44,-10.8,;3.56,-13.11,;4.9,-13.87,;2.23,-13.88,)| Show InChI InChI=1S/C21H17BrF2N2O3/c1-11-3-4-13(20(25)27)8-17(11)26-12(2)7-18(19(22)21(26)28)29-10-14-5-6-15(23)9-16(14)24/h3-9H,10H2,1-2H3,(H2,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314073
(3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.32,-4.45,;29.32,-2.91,;27.99,-2.14,;26.65,-2.91,;27.99,-.6,;29.32,.17,;29.31,1.72,;27.98,2.48,;27.97,4.02,;26.66,1.71,;26.65,.17,;25.32,2.48,;25.32,4.03,;26.65,4.8,;23.98,4.79,;22.65,4.02,;21.32,4.79,;19.99,4.02,;18.65,4.79,;18.65,6.33,;17.32,7.1,;16,6.33,;14.66,7.1,;15.99,4.78,;17.32,4.02,;17.33,2.48,;22.65,2.48,;21.32,1.71,;23.99,1.71,;23.99,.17,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314073

(3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.32,-4.45,;29.32,-2.91,;27.99,-2.14,;26.65,-2.91,;27.99,-.6,;29.32,.17,;29.31,1.72,;27.98,2.48,;27.97,4.02,;26.66,1.71,;26.65,.17,;25.32,2.48,;25.32,4.03,;26.65,4.8,;23.98,4.79,;22.65,4.02,;21.32,4.79,;19.99,4.02,;18.65,4.79,;18.65,6.33,;17.32,7.1,;16,6.33,;14.66,7.1,;15.99,4.78,;17.32,4.02,;17.33,2.48,;22.65,2.48,;21.32,1.71,;23.99,1.71,;23.99,.17,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50314073

(3-(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-ox...)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(29.32,-4.45,;29.32,-2.91,;27.99,-2.14,;26.65,-2.91,;27.99,-.6,;29.32,.17,;29.31,1.72,;27.98,2.48,;27.97,4.02,;26.66,1.71,;26.65,.17,;25.32,2.48,;25.32,4.03,;26.65,4.8,;23.98,4.79,;22.65,4.02,;21.32,4.79,;19.99,4.02,;18.65,4.79,;18.65,6.33,;17.32,7.1,;16,6.33,;14.66,7.1,;15.99,4.78,;17.32,4.02,;17.33,2.48,;22.65,2.48,;21.32,1.71,;23.99,1.71,;23.99,.17,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-4-5-14(21(28)26-3)9-18(12)27-13(2)8-19(20(23)22(27)29)30-11-15-6-7-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347096

(CHEMBL1797123)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cccc1F |(2.41,3.39,;1.07,2.62,;-.26,3.4,;-1.59,2.62,;-2.92,3.39,;-4.25,2.61,;-5.59,3.38,;-6.91,2.6,;-8.25,3.36,;-8.26,4.91,;-9.59,5.67,;-6.92,5.68,;-5.59,4.92,;-4.25,5.69,;-1.59,1.08,;-2.92,.31,;-.26,.32,;-.26,-1.22,;1.07,1.08,;2.4,.31,;3.73,1.08,;3.73,2.62,;5.06,.31,;5.06,-1.23,;3.73,-2,;2.4,-1.23,;1.06,-2,)| Show InChI InChI=1S/C19H12BrF4NO2/c1-10-7-16(27-9-11-5-6-12(21)8-15(11)24)17(20)19(26)25(10)18-13(22)3-2-4-14(18)23/h2-8H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347938
(CHEMBL1802637)Show SMILES CNC(=O)c1ccc(Cl)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(35.79,-26.5,;35.75,-24.96,;37.07,-24.16,;38.42,-24.9,;37.04,-22.63,;38.37,-21.85,;38.37,-20.31,;37.03,-19.54,;37.02,-18,;35.71,-20.32,;35.71,-21.86,;34.38,-19.55,;34.37,-18,;35.7,-17.22,;33.04,-17.23,;31.71,-18.01,;30.37,-17.24,;29.04,-18.01,;27.71,-17.24,;26.38,-18.01,;25.05,-17.24,;25.04,-15.7,;23.71,-14.93,;26.38,-14.93,;27.71,-15.7,;29.05,-14.93,;31.71,-19.55,;30.37,-20.32,;33.04,-20.32,;33.04,-21.86,)| Show InChI InChI=1S/C21H16BrClF2N2O3/c1-11-7-18(30-10-13-3-5-14(24)9-16(13)25)19(22)21(29)27(11)17-8-12(20(28)26-2)4-6-15(17)23/h3-9H,10H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347106
(CHEMBL1797209)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cc(OCC(N)=O)cc1F |(24.13,3.5,;22.79,2.74,;21.47,3.52,;20.14,2.74,;18.8,3.5,;17.47,2.73,;16.13,3.5,;14.81,2.72,;13.48,3.48,;13.47,5.03,;12.13,5.79,;14.8,5.8,;16.14,5.03,;17.47,5.8,;20.14,1.2,;18.8,.43,;21.47,.44,;21.47,-1.1,;22.79,1.2,;24.13,.43,;25.45,1.19,;25.45,2.73,;26.78,.43,;26.79,-1.12,;28.12,-1.89,;29.45,-1.12,;30.79,-1.89,;32.12,-1.12,;30.79,-3.43,;25.45,-1.89,;24.12,-1.11,;22.78,-1.88,)| Show InChI InChI=1S/C21H15BrF4N2O4/c1-10-4-17(32-8-11-2-3-12(23)5-14(11)24)19(22)21(30)28(10)20-15(25)6-13(7-16(20)26)31-9-18(27)29/h2-7H,8-9H2,1H3,(H2,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347126
(CHEMBL1797124)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Cl)c(=O)n1-c1c(F)cccc1F |(27.98,3.45,;26.64,2.68,;25.32,3.46,;23.99,2.68,;22.65,3.45,;21.32,2.68,;19.98,3.44,;18.66,2.66,;17.33,3.42,;17.32,4.97,;15.98,5.74,;18.65,5.75,;19.99,4.98,;21.32,5.75,;23.99,1.14,;22.65,.37,;25.32,.38,;25.32,-1.16,;26.64,1.14,;27.98,.37,;29.3,1.14,;29.3,2.68,;30.63,.38,;30.64,-1.17,;29.3,-1.94,;27.97,-1.17,;26.63,-1.93,)| Show InChI InChI=1S/C19H12ClF4NO2/c1-10-7-16(27-9-11-5-6-12(21)8-15(11)24)17(20)19(26)25(10)18-13(22)3-2-4-14(18)23/h2-8H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347937
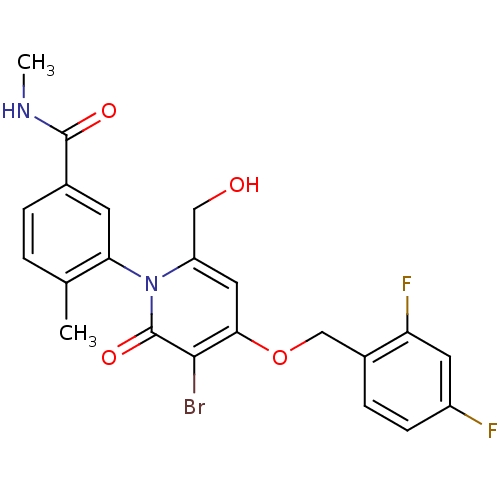
(CHEMBL1802641)Show SMILES CNC(=O)c1ccc(C)c(c1)-n1c(CO)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(2.58,-49.12,;2.54,-47.58,;3.86,-46.78,;5.21,-47.52,;3.83,-45.24,;5.16,-44.47,;5.15,-42.92,;3.82,-42.16,;3.81,-40.62,;2.5,-42.93,;2.49,-44.48,;1.17,-42.17,;1.16,-40.62,;2.49,-39.84,;2.49,-38.3,;-.17,-39.85,;-1.5,-40.62,;-2.84,-39.85,;-4.17,-40.62,;-5.5,-39.85,;-6.83,-40.62,;-8.16,-39.86,;-8.17,-38.31,;-9.5,-37.55,;-6.83,-37.54,;-5.5,-38.31,;-4.16,-37.55,;-1.51,-42.17,;-2.84,-42.94,;-.17,-42.94,;-.17,-44.48,)| Show InChI InChI=1S/C22H19BrF2N2O4/c1-12-3-4-13(21(29)26-2)7-18(12)27-16(10-28)9-19(20(23)22(27)30)31-11-14-5-6-15(24)8-17(14)25/h3-9,28H,10-11H2,1-2H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347107
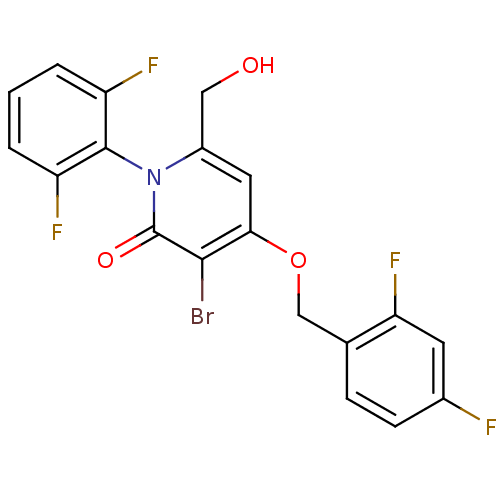
(CHEMBL1797210)Show SMILES OCc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cccc1F |(3.15,-8.82,;3.15,-10.36,;1.81,-11.13,;.48,-10.35,;-.85,-11.13,;-2.18,-10.36,;-3.52,-11.14,;-4.85,-10.37,;-6.17,-11.15,;-7.51,-10.39,;-7.52,-8.84,;-8.85,-8.08,;-6.18,-8.07,;-4.85,-8.83,;-3.51,-8.06,;-.85,-12.67,;-2.18,-13.44,;.48,-13.43,;.48,-14.97,;1.81,-12.67,;3.14,-13.44,;4.47,-12.67,;4.46,-11.13,;5.8,-13.44,;5.8,-14.99,;4.47,-15.75,;3.13,-14.98,;1.8,-15.75,)| Show InChI InChI=1S/C19H12BrF4NO3/c20-17-16(28-9-10-4-5-11(21)6-15(10)24)7-12(8-26)25(19(17)27)18-13(22)2-1-3-14(18)23/h1-7,26H,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347109
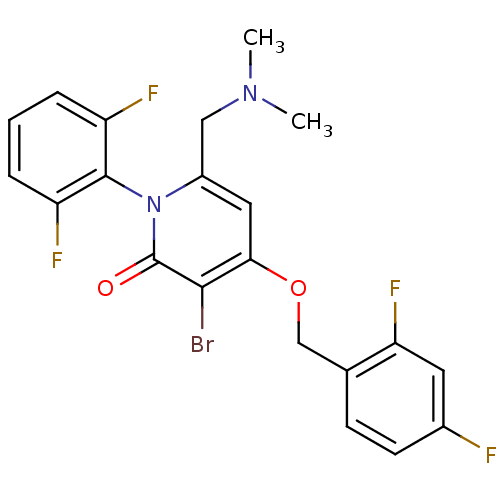
(CHEMBL1797212)Show SMILES CN(C)Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cccc1F |(3.68,-20.77,;2.34,-21.54,;1.01,-20.77,;2.34,-23.08,;1,-23.85,;-.33,-23.07,;-1.66,-23.85,;-2.99,-23.08,;-4.32,-23.85,;-5.66,-23.09,;-6.98,-23.87,;-8.32,-23.11,;-8.32,-21.56,;-9.66,-20.79,;-6.99,-20.78,;-5.66,-21.55,;-4.32,-20.78,;-1.66,-25.39,;-2.99,-26.16,;-.33,-26.15,;-.33,-27.69,;1,-25.39,;2.33,-26.16,;3.66,-25.39,;3.65,-23.85,;4.99,-26.15,;4.99,-27.7,;3.66,-28.47,;2.33,-27.7,;.99,-28.46,)| Show InChI InChI=1S/C21H17BrF4N2O2/c1-27(2)10-14-9-18(30-11-12-6-7-13(23)8-17(12)26)19(22)21(29)28(14)20-15(24)4-3-5-16(20)25/h3-9H,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347098
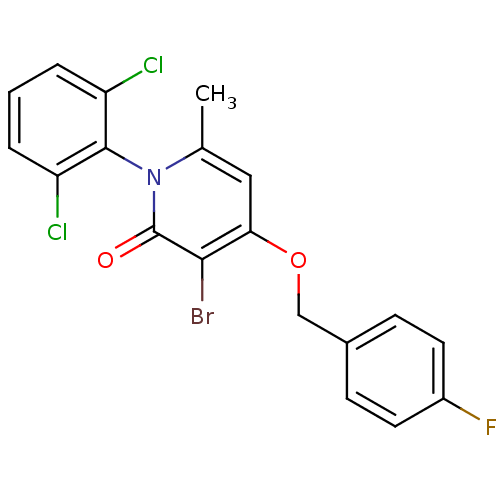
(CHEMBL1797201)Show SMILES Cc1cc(OCc2ccc(F)cc2)c(Br)c(=O)n1-c1c(Cl)cccc1Cl |(28.09,-8.26,;26.75,-9.03,;25.42,-8.25,;24.1,-9.03,;22.76,-8.26,;21.43,-9.04,;20.09,-8.27,;20.1,-6.73,;18.76,-5.97,;17.43,-6.74,;16.09,-5.98,;17.44,-8.29,;18.77,-9.05,;24.1,-10.57,;22.76,-11.34,;25.42,-11.33,;25.42,-12.87,;26.75,-10.57,;28.09,-11.34,;29.41,-10.57,;29.41,-9.03,;30.74,-11.34,;30.75,-12.89,;29.41,-13.65,;28.08,-12.88,;26.74,-13.65,)| Show InChI InChI=1S/C19H13BrCl2FNO2/c1-11-9-16(26-10-12-5-7-13(23)8-6-12)17(20)19(25)24(11)18-14(21)3-2-4-15(18)22/h2-9H,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347105
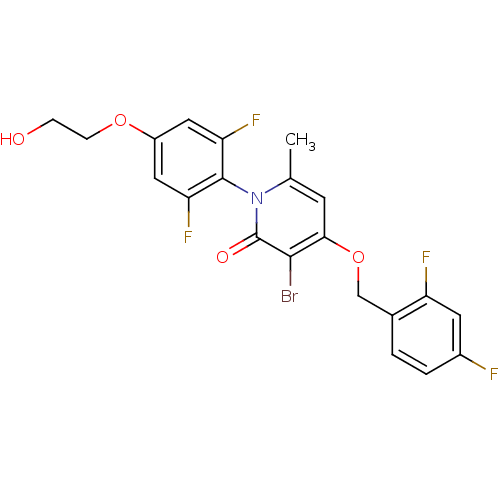
(CHEMBL1797208)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cc(OCCO)cc1F |(2.39,2.52,;1.06,1.75,;-.27,2.53,;-1.6,1.75,;-2.94,2.52,;-4.27,1.74,;-5.61,2.51,;-6.93,1.73,;-8.26,2.49,;-8.27,4.04,;-9.61,4.8,;-6.94,4.81,;-5.6,4.05,;-4.27,4.82,;-1.6,.21,;-2.93,-.56,;-.27,-.55,;-.27,-2.09,;1.06,.21,;2.39,-.56,;3.71,.21,;3.71,1.75,;5.05,-.56,;5.05,-2.11,;6.38,-2.88,;7.72,-2.11,;9.05,-2.88,;10.38,-2.11,;3.71,-2.87,;2.38,-2.1,;1.04,-2.87,)| Show InChI InChI=1S/C21H16BrF4NO4/c1-11-6-18(31-10-12-2-3-13(23)7-15(12)24)19(22)21(29)27(11)20-16(25)8-14(9-17(20)26)30-5-4-28/h2-3,6-9,28H,4-5,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347110

(CHEMBL1797213)Show SMILES CN(C)Cc1cc(OCc2ccc(F)cc2F)c(Cl)c(=O)n1-c1c(F)cccc1F |(29.59,-20.31,;28.25,-21.07,;26.92,-20.3,;28.25,-22.61,;26.91,-23.38,;25.58,-22.6,;24.25,-23.38,;22.92,-22.61,;21.58,-23.39,;20.25,-22.62,;18.93,-23.4,;17.59,-22.64,;17.58,-21.09,;16.25,-20.33,;18.92,-20.32,;20.25,-21.08,;21.58,-20.31,;24.25,-24.92,;22.92,-25.69,;25.58,-25.68,;25.58,-27.22,;26.91,-24.92,;28.24,-25.69,;29.57,-24.92,;29.56,-23.38,;30.9,-25.69,;30.9,-27.24,;29.56,-28,;28.23,-27.23,;26.9,-28,)| Show InChI InChI=1S/C21H17ClF4N2O2/c1-27(2)10-14-9-18(30-11-12-6-7-13(23)8-17(12)26)19(22)21(29)28(14)20-15(24)4-3-5-16(20)25/h3-9H,10-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347108
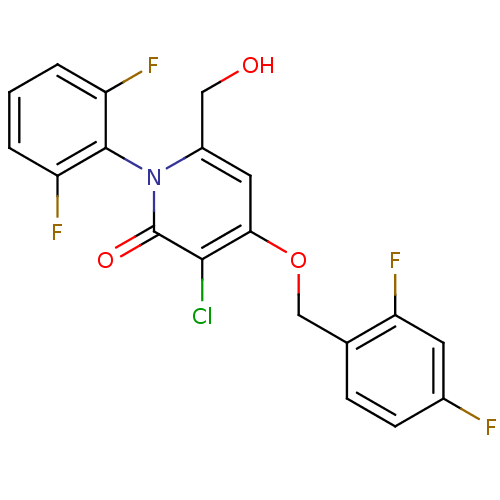
(CHEMBL1797211)Show SMILES OCc1cc(OCc2ccc(F)cc2F)c(Cl)c(=O)n1-c1c(F)cccc1F |(28.8,-8.57,;28.8,-10.11,;27.46,-10.87,;26.13,-10.1,;24.8,-10.87,;23.47,-10.11,;22.14,-10.88,;20.8,-10.12,;19.48,-10.89,;18.14,-10.13,;18.14,-8.58,;16.8,-7.82,;19.47,-7.81,;20.8,-8.58,;22.14,-7.81,;24.8,-12.41,;23.47,-13.19,;26.13,-13.18,;26.13,-14.72,;27.46,-12.41,;28.79,-13.19,;30.12,-12.42,;30.11,-10.88,;31.45,-13.18,;31.45,-14.73,;30.12,-15.5,;28.79,-14.73,;27.45,-15.49,)| Show InChI InChI=1S/C19H12ClF4NO3/c20-17-16(28-9-10-4-5-11(21)6-15(10)24)7-12(8-26)25(19(17)27)18-13(22)2-1-3-14(18)23/h1-7,26H,8-9H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347103
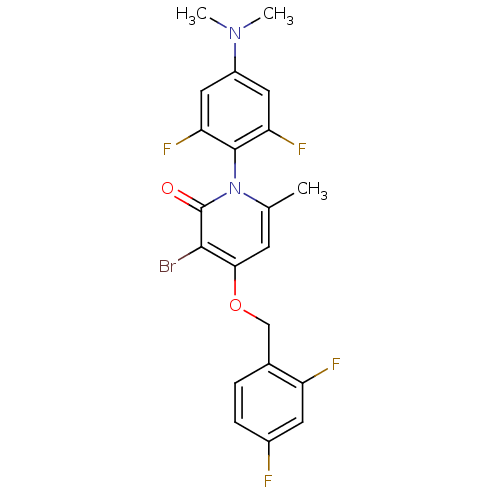
(CHEMBL1797206)Show SMILES CN(C)c1cc(F)c(c(F)c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(6.77,-50.31,;6.78,-48.77,;8.1,-47.99,;5.44,-48,;5.44,-46.45,;4.11,-45.69,;4.11,-44.15,;2.79,-46.46,;2.78,-48,;1.44,-48.76,;4.11,-48.77,;1.45,-45.69,;1.45,-44.15,;2.79,-43.38,;.12,-43.37,;-1.21,-44.15,;-2.54,-43.38,;-3.87,-44.15,;-5.21,-43.39,;-6.53,-44.17,;-7.86,-43.41,;-7.87,-41.86,;-9.21,-41.09,;-6.54,-41.08,;-5.21,-41.85,;-3.87,-41.08,;-1.21,-45.69,;-2.54,-46.46,;.12,-46.45,;.12,-47.99,)| Show InChI InChI=1S/C21H17BrF4N2O2/c1-11-6-18(30-10-12-4-5-13(23)7-15(12)24)19(22)21(29)28(11)20-16(25)8-14(27(2)3)9-17(20)26/h4-9H,10H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347933
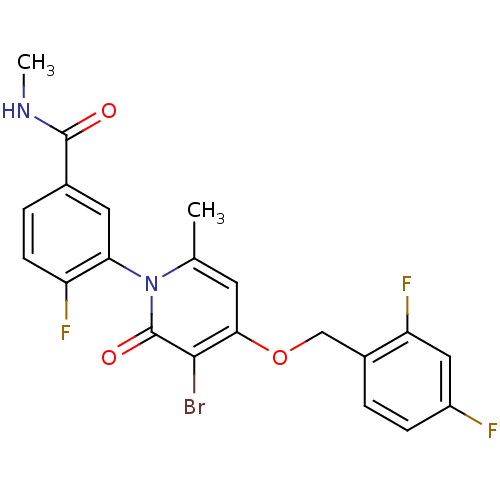
(CHEMBL1802636)Show SMILES CNC(=O)c1ccc(F)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(21.06,-26.62,;21.02,-25.08,;22.34,-24.28,;23.69,-25.02,;22.31,-22.75,;23.64,-21.98,;23.63,-20.43,;22.3,-19.67,;22.29,-18.13,;20.98,-20.44,;20.97,-21.98,;19.65,-19.67,;19.64,-18.12,;20.97,-17.35,;18.31,-17.36,;16.98,-18.13,;15.64,-17.36,;14.31,-18.13,;12.98,-17.36,;11.65,-18.13,;10.32,-17.37,;10.31,-15.82,;8.98,-15.05,;11.65,-15.05,;12.98,-15.82,;14.32,-15.05,;16.97,-19.67,;15.64,-20.44,;18.31,-20.45,;18.31,-21.99,)| Show InChI InChI=1S/C21H16BrF3N2O3/c1-11-7-18(30-10-13-3-5-14(23)9-16(13)25)19(22)21(29)27(11)17-8-12(20(28)26-2)4-6-15(17)24/h3-9H,10H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347111
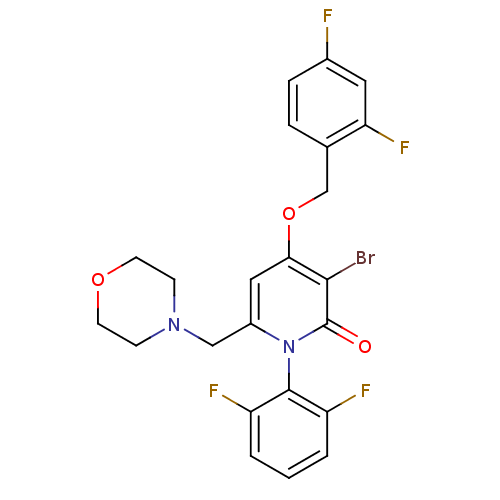
(CHEMBL1797214)Show SMILES Fc1ccc(COc2cc(CN3CCOCC3)n(-c3c(F)cccc3F)c(=O)c2Br)c(F)c1 |(-9.72,-33.39,;-8.38,-34.16,;-8.37,-35.71,;-7.03,-36.47,;-5.71,-35.69,;-4.38,-36.45,;-3.05,-35.68,;-1.71,-36.45,;-.38,-35.67,;.95,-36.45,;2.28,-35.68,;2.29,-34.14,;3.62,-33.38,;3.63,-31.85,;2.3,-31.07,;.96,-31.83,;.95,-33.37,;.95,-37.99,;2.28,-38.76,;3.6,-37.99,;3.6,-36.45,;4.94,-38.75,;4.94,-40.3,;3.6,-41.07,;2.27,-40.3,;.94,-41.06,;-.38,-38.75,;-.38,-40.29,;-1.71,-37.99,;-3.04,-38.76,;-5.71,-34.15,;-4.38,-33.38,;-7.05,-33.38,)| Show InChI InChI=1S/C23H19BrF4N2O3/c24-21-20(33-13-14-4-5-15(25)10-19(14)28)11-16(12-29-6-8-32-9-7-29)30(23(21)31)22-17(26)2-1-3-18(22)27/h1-5,10-11H,6-9,12-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347930
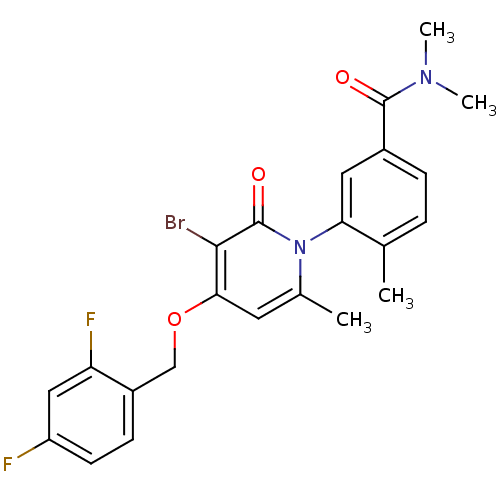
(CHEMBL1802633)Show SMILES CN(C)C(=O)c1ccc(C)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(19.71,-15.18,;19.71,-13.64,;21.04,-12.87,;18.37,-12.87,;17.04,-13.64,;18.37,-11.33,;19.71,-10.56,;19.7,-9.01,;18.36,-8.25,;18.36,-6.71,;17.05,-9.02,;17.04,-10.56,;15.71,-8.26,;15.7,-6.71,;17.04,-5.93,;14.37,-5.94,;13.04,-6.71,;11.71,-5.94,;10.37,-6.71,;9.04,-5.94,;7.72,-6.71,;6.38,-5.95,;6.38,-4.4,;5.04,-3.63,;7.71,-3.63,;9.05,-4.4,;10.38,-3.63,;13.04,-8.26,;11.7,-9.03,;14.37,-9.03,;14.37,-10.57,)| Show InChI InChI=1S/C23H21BrF2N2O3/c1-13-5-6-15(22(29)27(3)4)10-19(13)28-14(2)9-20(21(24)23(28)30)31-12-16-7-8-17(25)11-18(16)26/h5-11H,12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347121
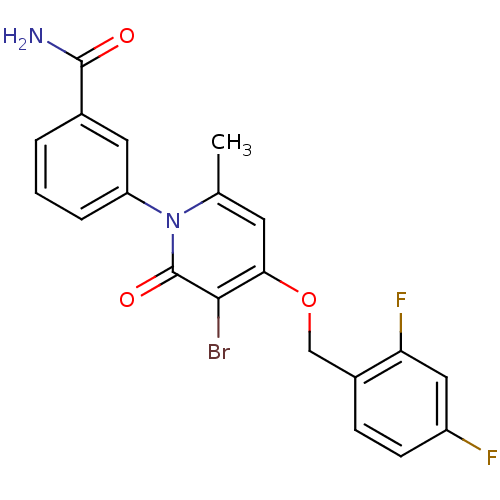
(CHEMBL1797224)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1cccc(c1)C(N)=O Show InChI InChI=1S/C20H15BrF2N2O3/c1-11-7-17(28-10-13-5-6-14(22)9-16(13)23)18(21)20(27)25(11)15-4-2-3-12(8-15)19(24)26/h2-9H,10H2,1H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347121

(CHEMBL1797224)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1cccc(c1)C(N)=O Show InChI InChI=1S/C20H15BrF2N2O3/c1-11-7-17(28-10-13-5-6-14(22)9-16(13)23)18(21)20(27)25(11)15-4-2-3-12(8-15)19(24)26/h2-9H,10H2,1H3,(H2,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347101
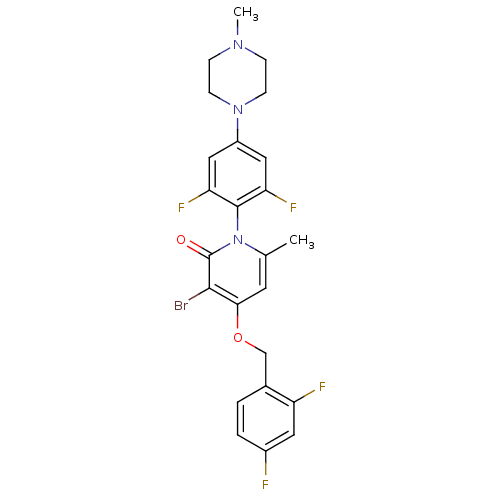
(CHEMBL1797204)Show SMILES CN1CCN(CC1)c1cc(F)c(c(F)c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(9.91,-37.66,;8.58,-36.88,;7.24,-37.65,;5.91,-36.88,;5.92,-35.34,;7.25,-34.56,;8.58,-35.34,;4.59,-34.57,;4.59,-33.02,;3.25,-32.26,;3.25,-30.72,;1.93,-33.03,;1.92,-34.57,;.59,-35.33,;3.25,-35.34,;.6,-32.25,;.6,-30.71,;1.93,-29.95,;-.73,-29.94,;-2.06,-30.71,;-3.4,-29.95,;-4.73,-30.72,;-6.06,-29.96,;-7.38,-30.73,;-8.72,-29.97,;-8.73,-28.43,;-10.07,-27.66,;-7.4,-27.65,;-6.06,-28.42,;-4.73,-27.65,;-2.06,-32.25,;-3.39,-33.03,;-.73,-33.02,;-.73,-34.56,)| Show InChI InChI=1S/C24H22BrF4N3O2/c1-14-9-21(34-13-15-3-4-16(26)10-18(15)27)22(25)24(33)32(14)23-19(28)11-17(12-20(23)29)31-7-5-30(2)6-8-31/h3-4,9-12H,5-8,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347104
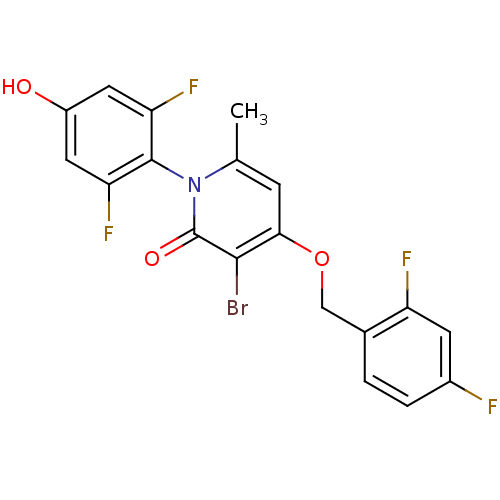
(CHEMBL1797207)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1c(F)cc(O)cc1F |(26.27,-43.79,;24.93,-44.55,;23.6,-43.77,;22.28,-44.55,;20.94,-43.79,;19.61,-44.56,;18.27,-43.79,;16.95,-44.57,;15.62,-43.81,;15.61,-42.26,;14.27,-41.5,;16.94,-41.49,;18.28,-42.25,;19.61,-41.48,;22.28,-46.09,;20.94,-46.86,;23.6,-46.85,;23.6,-48.39,;24.93,-46.09,;26.27,-46.86,;27.59,-46.09,;27.59,-44.55,;28.92,-46.86,;28.93,-48.41,;30.26,-49.18,;27.59,-49.17,;26.26,-48.4,;24.92,-49.17,)| Show InChI InChI=1S/C19H12BrF4NO3/c1-9-4-16(28-8-10-2-3-11(21)5-13(10)22)17(20)19(27)25(9)18-14(23)6-12(26)7-15(18)24/h2-7,26H,8H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347116
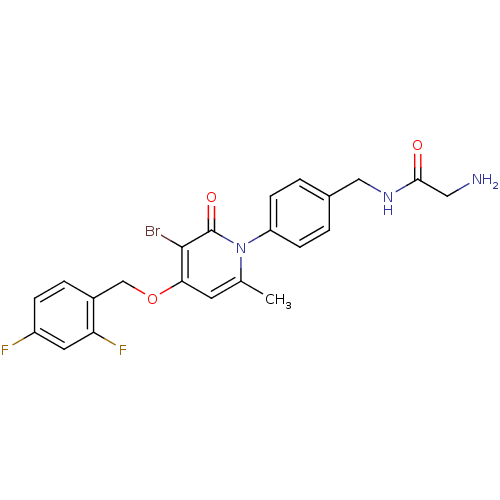
(CHEMBL1797219)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1ccc(CNC(=O)CN)cc1 Show InChI InChI=1S/C22H20BrF2N3O3/c1-13-8-19(31-12-15-4-5-16(24)9-18(15)25)21(23)22(30)28(13)17-6-2-14(3-7-17)11-27-20(29)10-26/h2-9H,10-12,26H2,1H3,(H,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347123
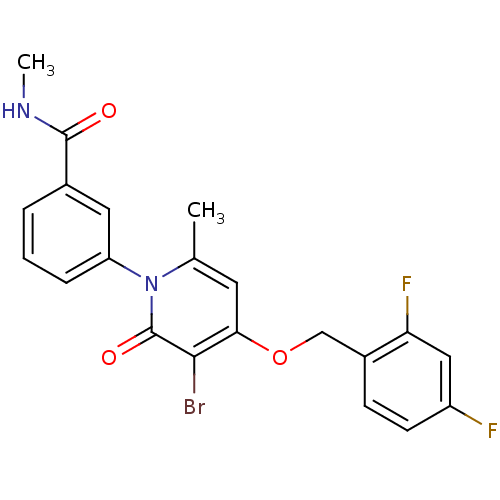
(CHEMBL1797226)Show SMILES CNC(=O)c1cccc(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O Show InChI InChI=1S/C21H17BrF2N2O3/c1-12-8-18(29-11-14-6-7-15(23)10-17(14)24)19(22)21(28)26(12)16-5-3-4-13(9-16)20(27)25-2/h3-10H,11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50094519
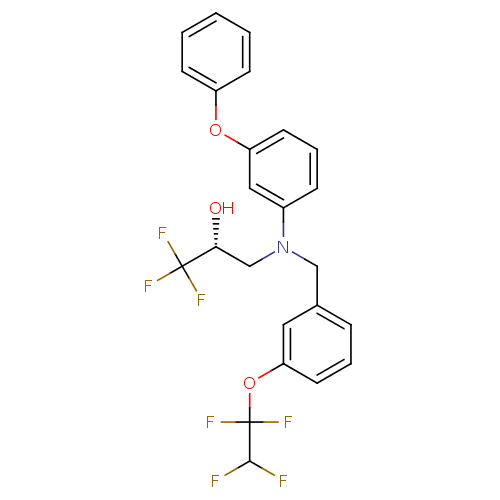
((R)-1,1,1-Trifluoro-3-{(3-phenoxy-phenyl)-[3-(1,1,...)Show SMILES O[C@H](CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccccc2)c1)C(F)(F)F Show InChI InChI=1S/C24H20F7NO3/c25-22(26)24(30,31)35-20-11-4-6-16(12-20)14-32(15-21(33)23(27,28)29)17-7-5-10-19(13-17)34-18-8-2-1-3-9-18/h1-13,21-22,33H,14-15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia
Curated by ChEMBL
| Assay Description
Concentration required for 50% inhibition of cholesteryl ester transfer protein in presence of buffer. |
J Med Chem 45: 3891-904 (2002)
BindingDB Entry DOI: 10.7270/Q2JW8D7P |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128175

(1,1,1-Trifluoro-3-{[3-(3-isopropyl-phenoxy)-phenyl...)Show SMILES CC(C)c1cccc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)c1 Show InChI InChI=1S/C27H26F7NO3/c1-17(2)19-7-4-9-21(13-19)37-22-10-5-8-20(14-22)35(16-24(36)26(30,31)32)15-18-6-3-11-23(12-18)38-27(33,34)25(28)29/h3-14,17,24-25,36H,15-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347114
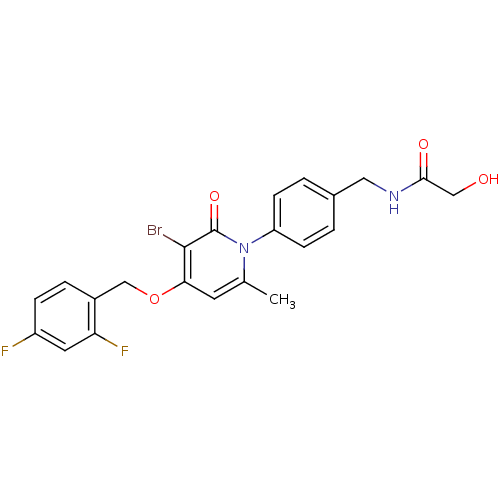
(CHEMBL1797217)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1ccc(CNC(=O)CO)cc1 Show InChI InChI=1S/C22H19BrF2N2O4/c1-13-8-19(31-12-15-4-5-16(24)9-18(15)25)21(23)22(30)27(13)17-6-2-14(3-7-17)10-26-20(29)11-28/h2-9,28H,10-12H2,1H3,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347934

(CHEMBL1802638)Show SMILES CNC(=O)c1ccc(OC)c(c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(2.35,-36.25,;2.32,-34.71,;3.63,-33.91,;4.98,-34.65,;3.6,-32.37,;4.94,-31.6,;4.93,-30.05,;3.59,-29.29,;3.59,-27.75,;4.91,-26.97,;2.28,-30.06,;2.27,-31.6,;.94,-29.29,;.93,-27.74,;2.27,-26.97,;-.4,-26.98,;-1.73,-27.75,;-3.06,-26.98,;-4.4,-27.75,;-5.73,-26.98,;-7.05,-27.75,;-8.39,-26.99,;-8.39,-25.44,;-9.73,-24.67,;-7.06,-24.67,;-5.72,-25.44,;-4.39,-24.67,;-1.73,-29.3,;-3.07,-30.06,;-.4,-30.07,;-.4,-31.61,)| Show InChI InChI=1S/C22H19BrF2N2O4/c1-12-8-19(31-11-14-4-6-15(24)10-16(14)25)20(23)22(29)27(12)17-9-13(21(28)26-2)5-7-18(17)30-3/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50310966
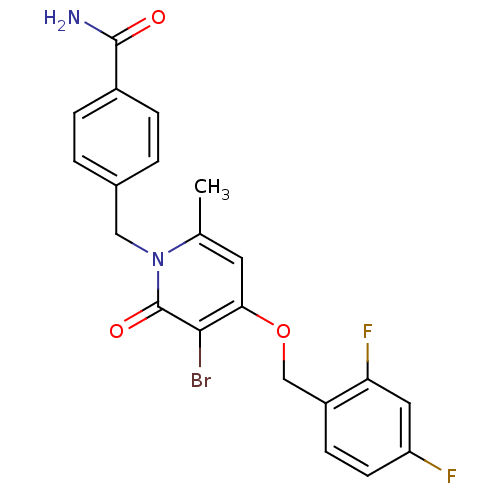
(4-((3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-2-o...)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1Cc1ccc(cc1)C(N)=O Show InChI InChI=1S/C21H17BrF2N2O3/c1-12-8-18(29-11-15-6-7-16(23)9-17(15)24)19(22)21(28)26(12)10-13-2-4-14(5-3-13)20(25)27/h2-9H,10-11H2,1H3,(H2,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347102
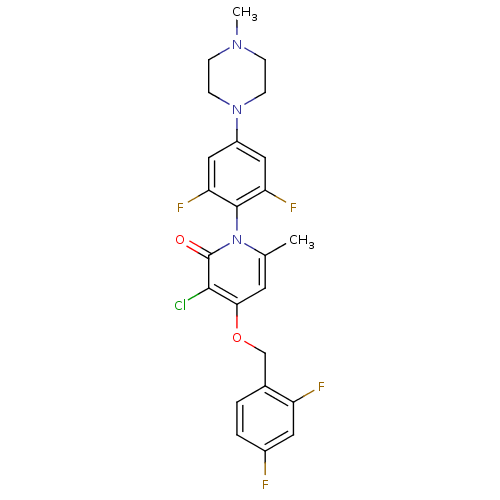
(CHEMBL1797205)Show SMILES CN1CCN(CC1)c1cc(F)c(c(F)c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Cl)c1=O |(32.8,-38.3,;31.47,-37.53,;30.13,-38.29,;28.8,-37.52,;28.81,-35.99,;30.14,-35.21,;31.47,-35.99,;27.48,-35.22,;27.48,-33.67,;26.14,-32.9,;26.14,-31.36,;24.82,-33.67,;24.81,-35.21,;23.48,-35.98,;26.14,-35.98,;23.49,-32.9,;23.49,-31.36,;24.82,-30.59,;22.16,-30.58,;20.83,-31.36,;19.49,-30.59,;18.16,-31.37,;16.83,-30.6,;15.51,-31.38,;14.17,-30.62,;14.16,-29.07,;12.82,-28.31,;15.49,-28.3,;16.83,-29.06,;18.16,-28.29,;20.83,-32.9,;19.5,-33.67,;22.16,-33.66,;22.16,-35.2,)| Show InChI InChI=1S/C24H22ClF4N3O2/c1-14-9-21(34-13-15-3-4-16(26)10-18(15)27)22(25)24(33)32(14)23-19(28)11-17(12-20(23)29)31-7-5-30(2)6-8-31/h3-4,9-12H,5-8,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50310971
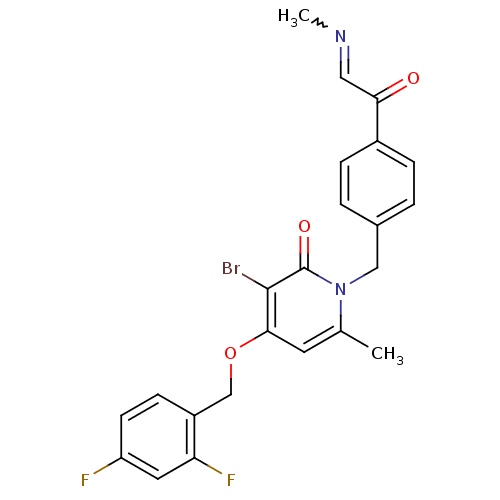
(3-bromo-4-(2,4-difluorobenzyloxy)-6-methyl-1-(4-(2...)Show SMILES CN=CC(=O)c1ccc(Cn2c(C)cc(OCc3ccc(F)cc3F)c(Br)c2=O)cc1 |w:1.0| Show InChI InChI=1S/C23H19BrF2N2O3/c1-14-9-21(31-13-17-7-8-18(25)10-19(17)26)22(24)23(30)28(14)12-15-3-5-16(6-4-15)20(29)11-27-2/h3-11H,12-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128154
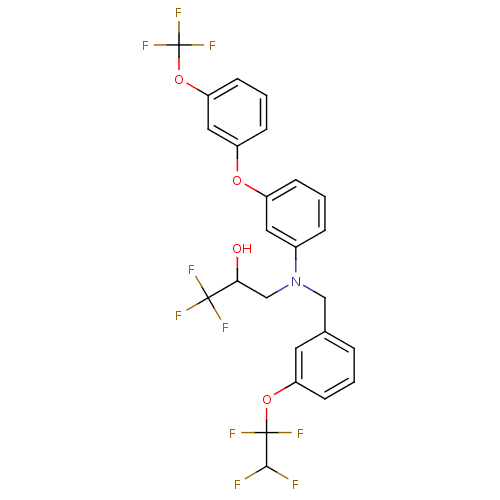
(1,1,1-Trifluoro-3-{[3-(1,1,2,2-tetrafluoro-ethoxy)...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2cccc(OC(F)(F)F)c2)c1)C(F)(F)F Show InChI InChI=1S/C25H19F10NO4/c26-22(27)24(31,32)39-19-8-1-4-15(10-19)13-36(14-21(37)23(28,29)30)16-5-2-6-17(11-16)38-18-7-3-9-20(12-18)40-25(33,34)35/h1-12,21-22,37H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128141

(1,1,1-Trifluoro-3-{[3-(4-fluoro-phenoxy)-phenyl]-[...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2ccc(F)cc2)c1)C(F)(F)F Show InChI InChI=1S/C24H19F8NO3/c25-16-7-9-18(10-8-16)35-19-5-2-4-17(12-19)33(14-21(34)23(28,29)30)13-15-3-1-6-20(11-15)36-24(31,32)22(26)27/h1-12,21-22,34H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128164
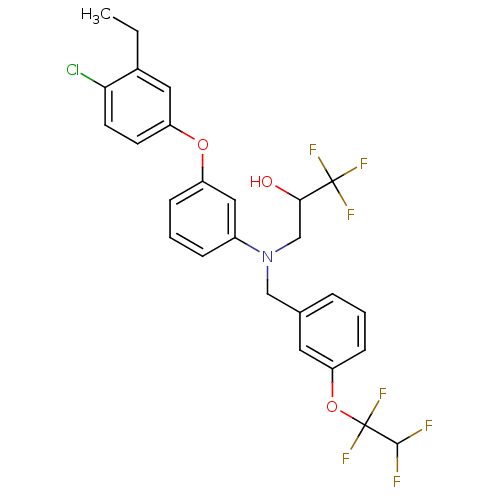
(3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(4-chlo...)Show SMILES CCc1cc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)ccc1Cl Show InChI InChI=1S/C26H23ClF7NO3/c1-2-17-12-20(9-10-22(17)27)37-19-7-4-6-18(13-19)35(15-23(36)25(30,31)32)14-16-5-3-8-21(11-16)38-26(33,34)24(28)29/h3-13,23-24,36H,2,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347097
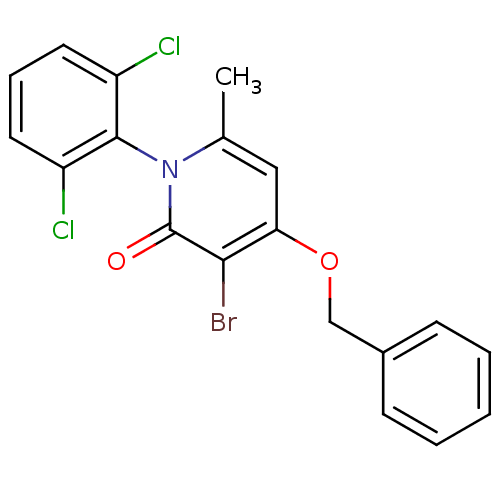
(CHEMBL1797125)Show SMILES Cc1cc(OCc2ccccc2)c(Br)c(=O)n1-c1c(Cl)cccc1Cl |(2.07,-8.4,;.74,-9.16,;-.59,-8.39,;-1.92,-9.16,;-3.26,-8.4,;-4.59,-9.17,;-5.92,-8.4,;-5.92,-6.87,;-7.26,-6.1,;-8.59,-6.87,;-8.58,-8.42,;-7.24,-9.18,;-1.92,-10.7,;-3.25,-11.48,;-.59,-11.47,;-.59,-13.01,;.74,-10.7,;2.07,-11.48,;3.39,-10.71,;3.39,-9.17,;4.73,-11.47,;4.73,-13.02,;3.39,-13.79,;2.06,-13.02,;.73,-13.78,)| Show InChI InChI=1S/C19H14BrCl2NO2/c1-12-10-16(25-11-13-6-3-2-4-7-13)17(20)19(24)23(12)18-14(21)8-5-9-15(18)22/h2-10H,11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128135

(3-((3-(2,3-dichlorophenoxy)phenyl)(3-(1,1,2,2-tetr...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(Oc2cccc(Cl)c2Cl)c1)C(F)(F)F Show InChI InChI=1S/C24H18Cl2F7NO3/c25-18-8-3-9-19(21(18)26)36-16-6-2-5-15(11-16)34(13-20(35)23(29,30)31)12-14-4-1-7-17(10-14)37-24(32,33)22(27)28/h1-11,20,22,35H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347931
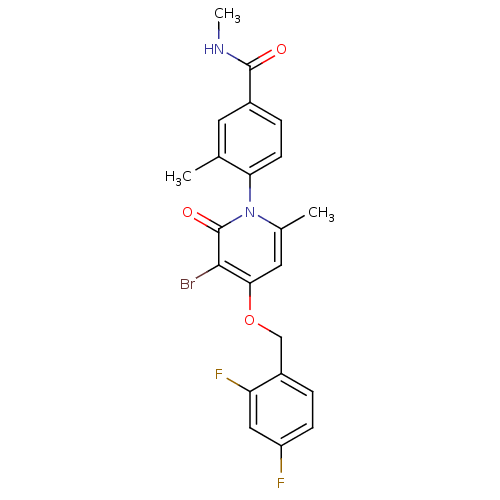
(CHEMBL1802634)Show SMILES CNC(=O)c1ccc(c(C)c1)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(41.54,-12.31,;40.21,-11.54,;38.88,-12.31,;38.88,-13.85,;37.54,-11.55,;36.21,-12.32,;34.87,-11.55,;34.88,-10.01,;36.2,-9.24,;36.19,-7.7,;37.53,-10,;33.54,-9.24,;33.54,-7.69,;34.87,-6.92,;32.2,-6.93,;30.88,-7.7,;29.54,-6.93,;28.21,-7.7,;26.87,-6.93,;25.55,-7.7,;24.22,-6.94,;24.21,-5.39,;22.88,-4.62,;25.55,-4.62,;26.88,-5.39,;28.22,-4.62,;30.87,-9.24,;29.54,-10.01,;32.21,-10.02,;32.21,-11.56,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-8-14(21(28)26-3)5-7-18(12)27-13(2)9-19(20(23)22(27)29)30-11-15-4-6-16(24)10-17(15)25/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50310985
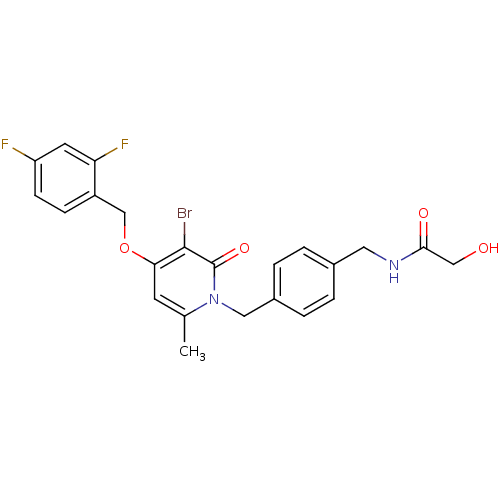
(CHEMBL1078666 | N-(4-((3-bromo-4-(2,4-difluorobenz...)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1Cc1ccc(CNC(=O)CO)cc1 Show InChI InChI=1S/C23H21BrF2N2O4/c1-14-8-20(32-13-17-6-7-18(25)9-19(17)26)22(24)23(31)28(14)11-16-4-2-15(3-5-16)10-27-21(30)12-29/h2-9,29H,10-13H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50347932
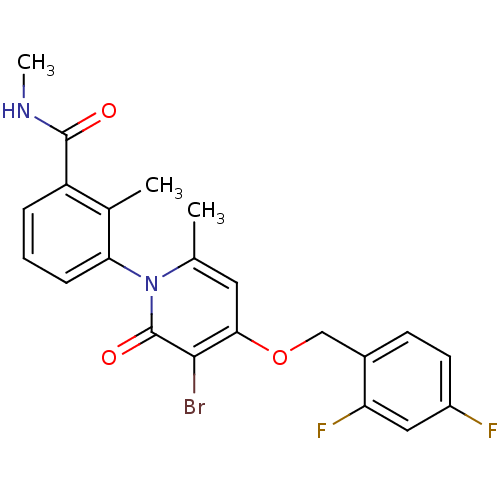
(CHEMBL1802635)Show SMILES CNC(=O)c1cccc(c1C)-n1c(C)cc(OCc2ccc(F)cc2F)c(Br)c1=O |(7.91,-18.61,;6.58,-19.39,;5.24,-18.63,;5.23,-17.09,;3.91,-19.4,;3.92,-20.95,;2.58,-21.72,;1.25,-20.96,;1.26,-19.41,;2.58,-18.64,;2.57,-17.1,;-.08,-18.65,;-.08,-17.1,;1.25,-16.32,;-1.42,-16.33,;-2.75,-17.1,;-4.08,-16.33,;-5.42,-17.1,;-6.75,-16.33,;-8.07,-17.1,;-9.41,-16.34,;-9.41,-14.79,;-10.75,-14.03,;-8.08,-14.02,;-6.74,-14.79,;-5.41,-14.03,;-2.75,-18.65,;-4.08,-19.42,;-1.42,-19.42,;-1.42,-20.96,)| Show InChI InChI=1S/C22H19BrF2N2O3/c1-12-9-19(30-11-14-7-8-15(24)10-17(14)25)20(23)22(29)27(12)18-6-4-5-16(13(18)2)21(28)26-3/h4-10H,11H2,1-3H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation FITC-labeled Hsp27 after 60 mins by fluorescence based cascade assay |
Bioorg Med Chem Lett 21: 4066-71 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.121
BindingDB Entry DOI: 10.7270/Q2SB46R2 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
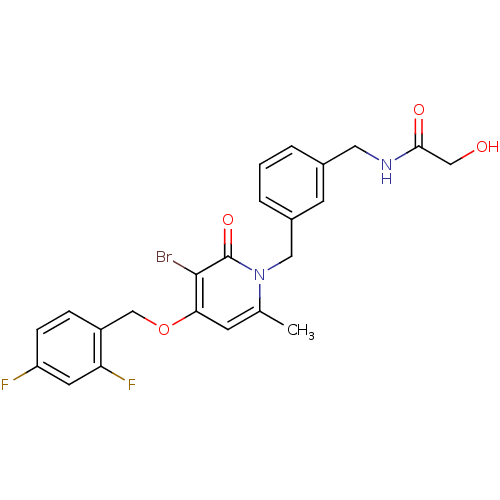
(Homo sapiens (Human)) | BDBM50310987
(CHEMBL1078594 | N-(3-((3-bromo-4-(2,4-difluorobenz...)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1Cc1cccc(CNC(=O)CO)c1 Show InChI InChI=1S/C23H21BrF2N2O4/c1-14-7-20(32-13-17-5-6-18(25)9-19(17)26)22(24)23(31)28(14)11-16-4-2-3-15(8-16)10-27-21(30)12-29/h2-9,29H,10-13H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
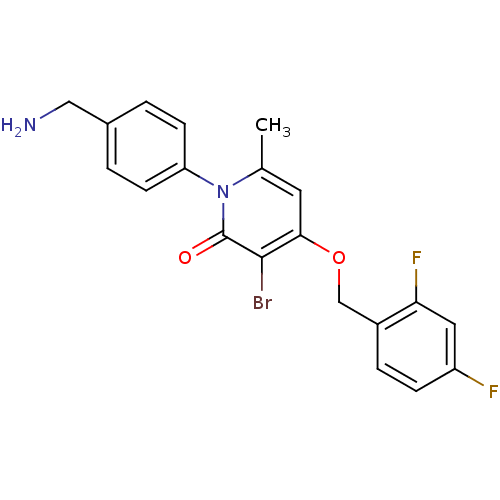
(Homo sapiens (Human)) | BDBM50347112
(CHEMBL1797215)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Br)c(=O)n1-c1ccc(CN)cc1 Show InChI InChI=1S/C20H17BrF2N2O2/c1-12-8-18(27-11-14-4-5-15(22)9-17(14)23)19(21)20(26)25(12)16-6-2-13(10-24)3-7-16/h2-9H,10-11,24H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha assessed as phosphorylation of fluorescently-labelled MK2 using Hsp27 peptide as substrate after 60 mins by fluorescence assay |
Bioorg Med Chem Lett 21: 4059-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.120
BindingDB Entry DOI: 10.7270/Q25Q4WFH |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50310986
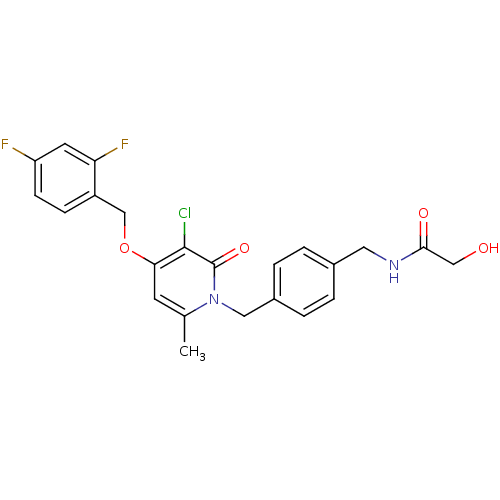
(CHEMBL1078667 | N-(4-((3-chloro-4-(2,4-difluoroben...)Show SMILES Cc1cc(OCc2ccc(F)cc2F)c(Cl)c(=O)n1Cc1ccc(CNC(=O)CO)cc1 Show InChI InChI=1S/C23H21ClF2N2O4/c1-14-8-20(32-13-17-6-7-18(25)9-19(17)26)22(24)23(31)28(14)11-16-4-2-15(3-5-16)10-27-21(30)12-29/h2-9,29H,10-13H2,1H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
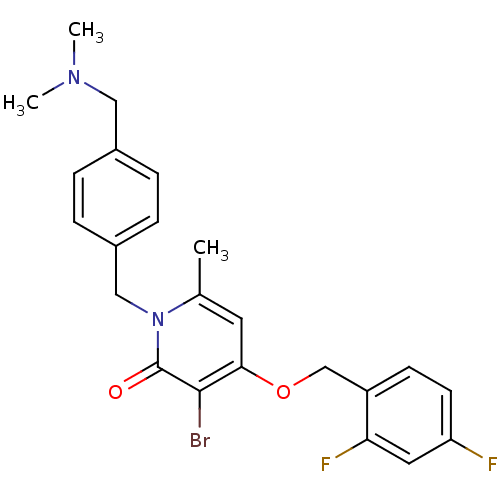
(Homo sapiens (Human)) | BDBM50310984
(3-bromo-4-(2,4-difluorobenzyloxy)-1-(4-((dimethyla...)Show SMILES CN(C)Cc1ccc(Cn2c(C)cc(OCc3ccc(F)cc3F)c(Br)c2=O)cc1 Show InChI InChI=1S/C23H23BrF2N2O2/c1-15-10-21(30-14-18-8-9-19(25)11-20(18)26)22(24)23(29)28(15)13-17-6-4-16(5-7-17)12-27(2)3/h4-11H,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128176
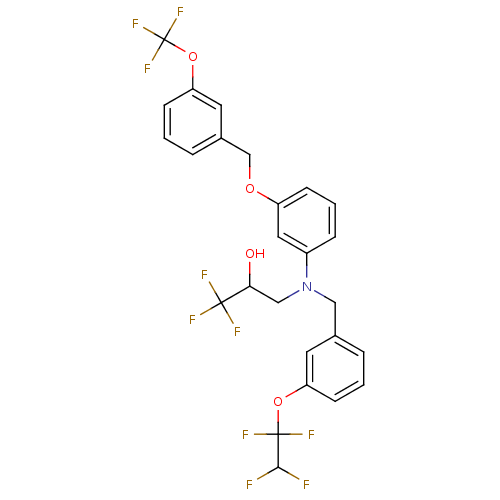
(1,1,1-Trifluoro-3-{[3-(1,1,2,2-tetrafluoro-ethoxy)...)Show SMILES OC(CN(Cc1cccc(OC(F)(F)C(F)F)c1)c1cccc(OCc2cccc(OC(F)(F)F)c2)c1)C(F)(F)F Show InChI InChI=1S/C26H21F10NO4/c27-23(28)25(32,33)40-20-8-1-4-16(10-20)13-37(14-22(38)24(29,30)31)18-6-3-7-19(12-18)39-15-17-5-2-9-21(11-17)41-26(34,35)36/h1-12,22-23,38H,13-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50128146

(3-((3-(1,1,2,2-tetrafluoroethoxy)benzyl)(3-(3-ethy...)Show SMILES CCc1cccc(Oc2cccc(c2)N(CC(O)C(F)(F)F)Cc2cccc(OC(F)(F)C(F)F)c2)c1 Show InChI InChI=1S/C26H24F7NO3/c1-2-17-6-3-9-20(12-17)36-21-10-5-8-19(14-21)34(16-23(35)25(29,30)31)15-18-7-4-11-22(13-18)37-26(32,33)24(27)28/h3-14,23-24,35H,2,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Discovery Research (Pfizer Global Research and Development)
Curated by ChEMBL
| Assay Description
Compound was tested in vitro for inhibitory activity against recombinant human cholesteryl ester transfer protein in buffer |
J Med Chem 46: 2152-68 (2003)
Article DOI: 10.1021/jm020528+
BindingDB Entry DOI: 10.7270/Q2QV3KWK |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50310983
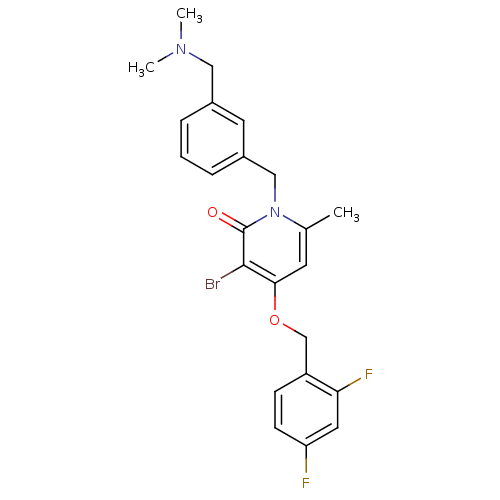
(3-bromo-4-(2,4-difluorobenzyloxy)-1-(3-((dimethyla...)Show SMILES CN(C)Cc1cccc(Cn2c(C)cc(OCc3ccc(F)cc3F)c(Br)c2=O)c1 Show InChI InChI=1S/C23H23BrF2N2O2/c1-15-9-21(30-14-18-7-8-19(25)11-20(18)26)22(24)23(29)28(15)13-17-6-4-5-16(10-17)12-27(2)3/h4-11H,12-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Corporation
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
Bioorg Med Chem Lett 19: 5851-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.082
BindingDB Entry DOI: 10.7270/Q2HM59DG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data