

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201010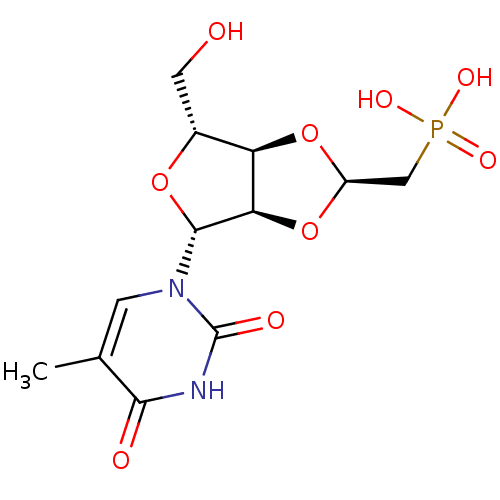 (((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201013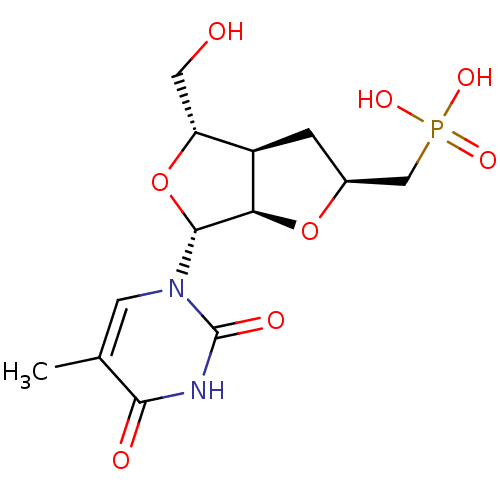 (((2S,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201015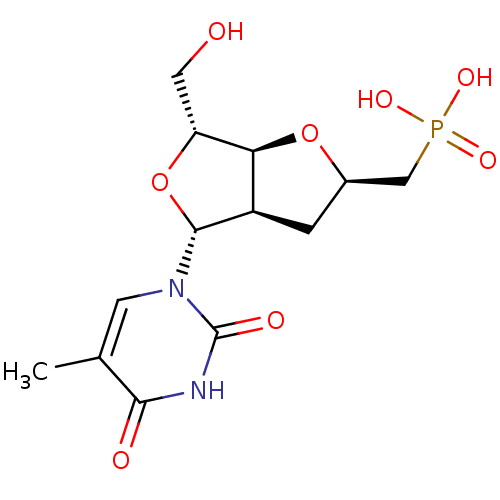 (((2R,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201012 (((2R,3aR,4S,6R,6aR)-4-(hydroxymethyl)-6-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201011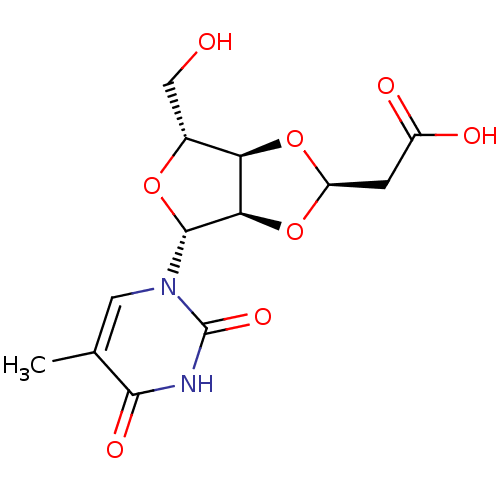 (2-((2R,3aR,4R,6R,6aR)-4-(hydroxymethyl)-6-(5-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50201014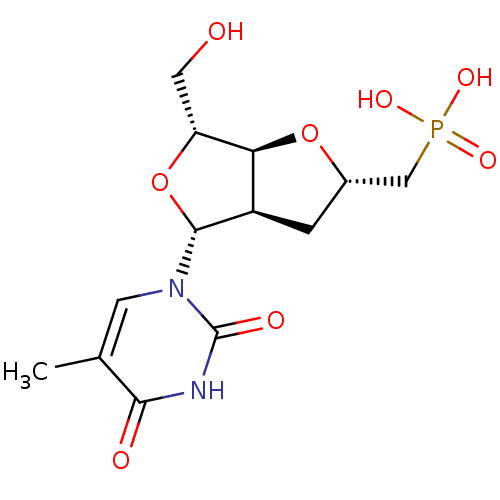 (((2S,3aR,4R,6R,6aS)-6-(hydroxymethyl)-4-(5-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Attenuon, LLC Curated by ChEMBL | Assay Description Inhibition of human thymidine phosphorylase by continuous spectrophotometric assay | J Med Chem 49: 7807-15 (2006) Article DOI: 10.1021/jm060428u BindingDB Entry DOI: 10.7270/Q2FB52KF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191945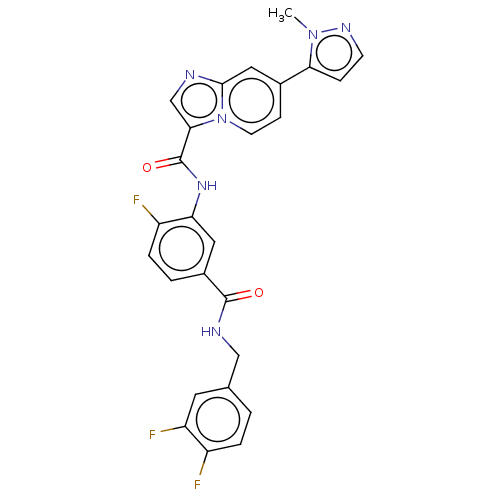 (CHEMBL3904768) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191977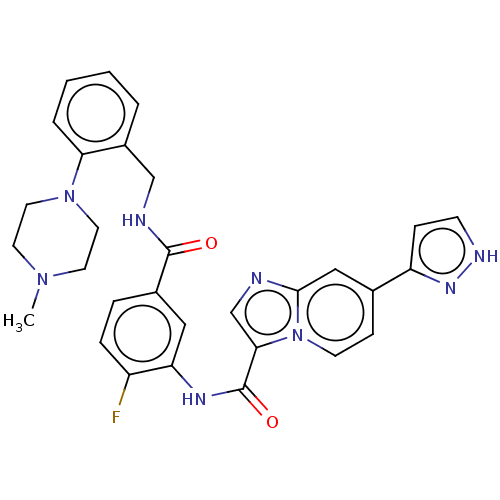 (CHEMBL3983564) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099511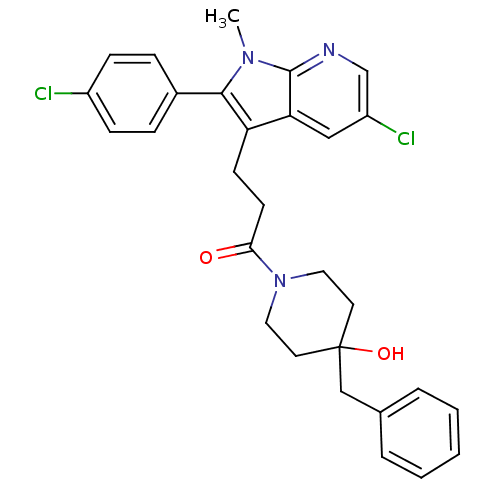 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099516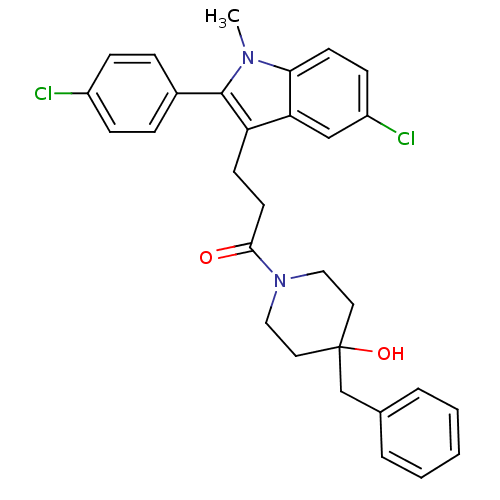 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191981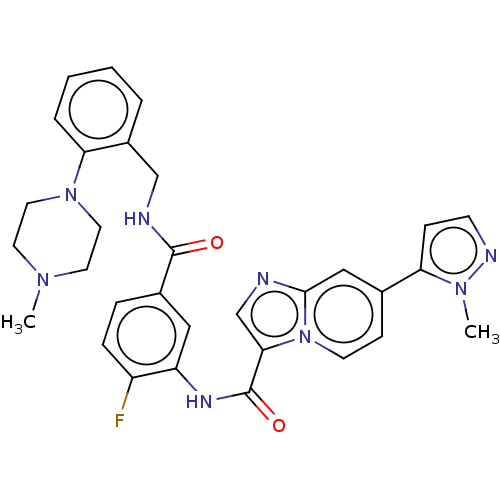 (CHEMBL3979322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099514 (3-[5-Acetyl-2-(4-chloro-phenyl)-1-methyl-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191973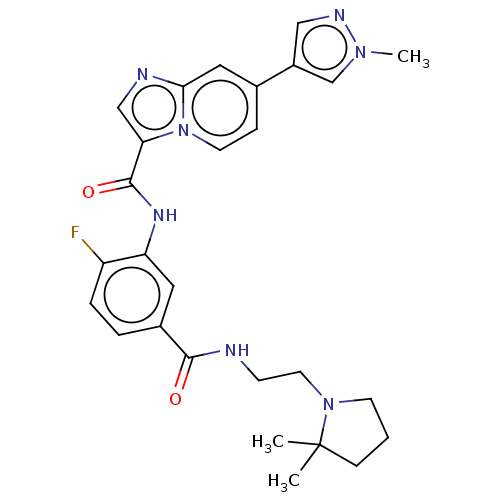 (CHEMBL3940697) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191978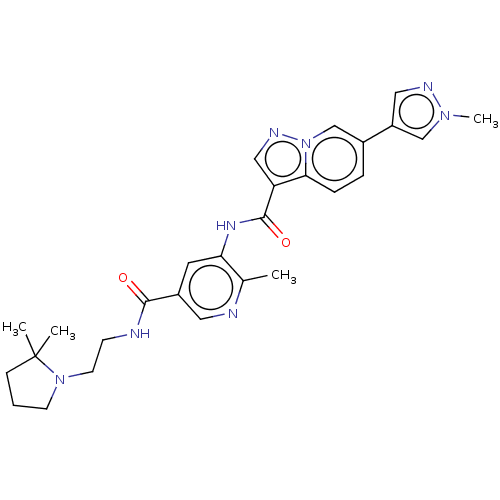 (CHEMBL3915941) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114943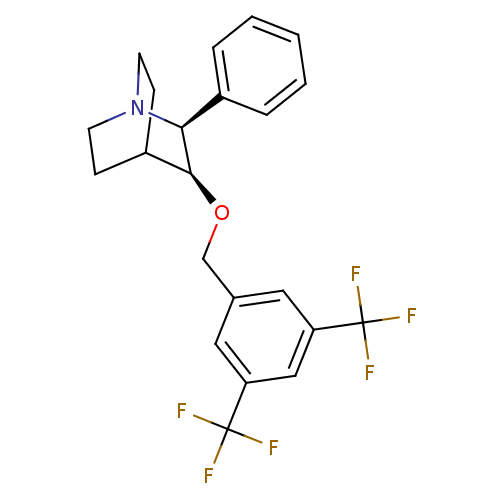 ((2S,3S)-3-(3,5-Bis-trifluoromethyl-benzyloxy)-2-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibitory concentration expressed as displacement of [125I]-labeled substance P from the cloned human Tachykinin receptor 1 expressed in ... | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099506 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099528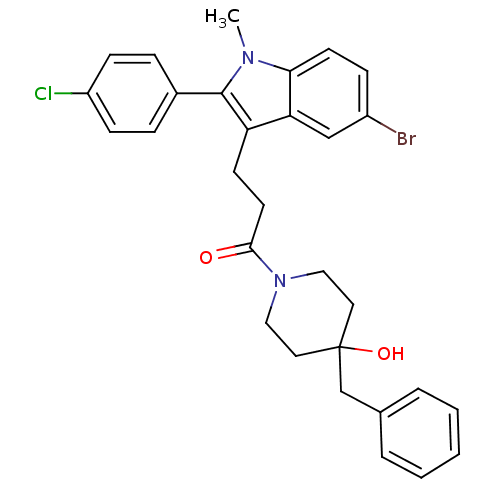 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[5-bromo-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099510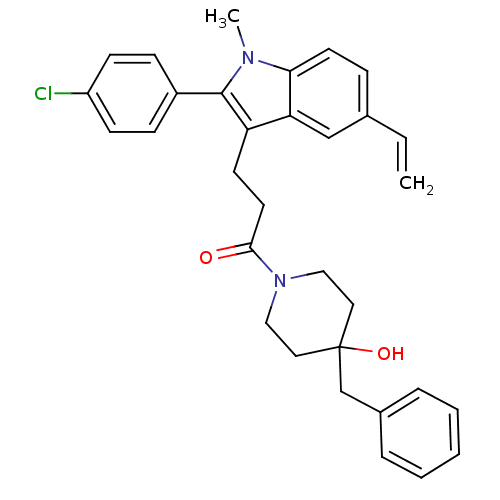 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[2-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114944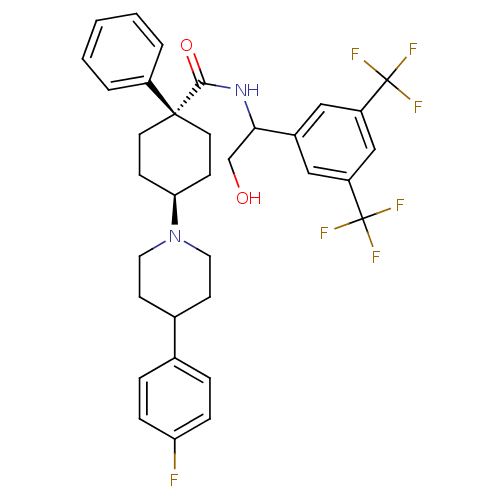 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191974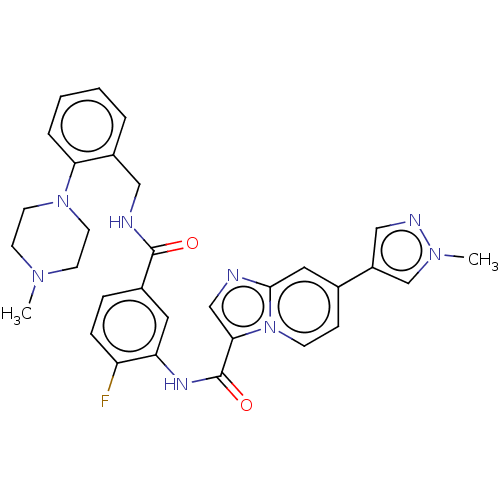 (CHEMBL3913766) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114933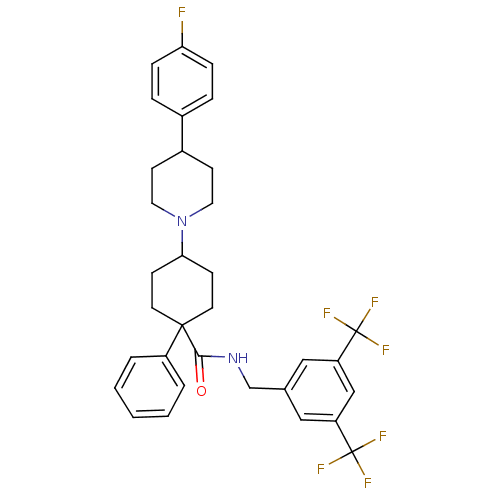 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114937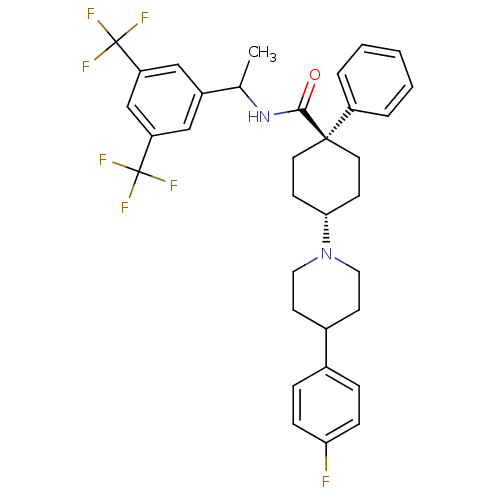 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114939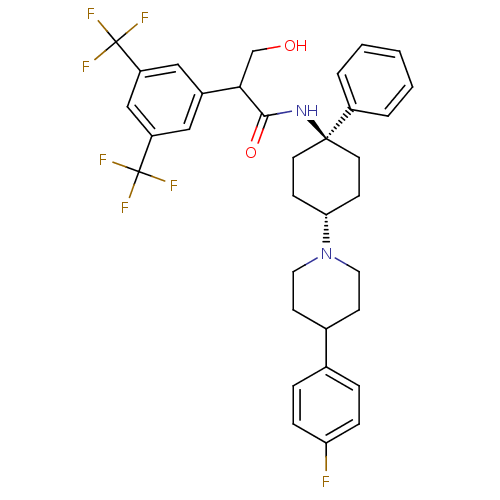 (2-(3,5-Bis-trifluoromethyl-phenyl)-N-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114949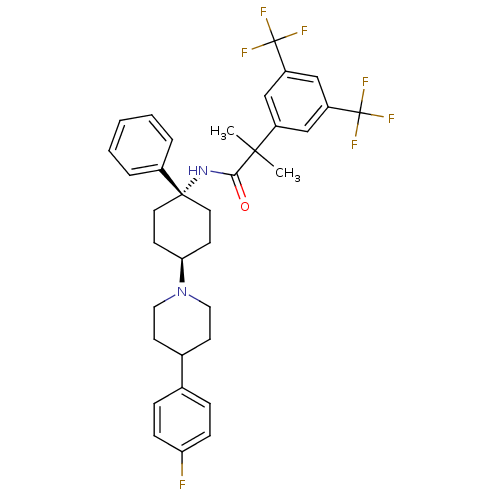 (2-(3,5-Bis-trifluoromethyl-phenyl)-N-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191975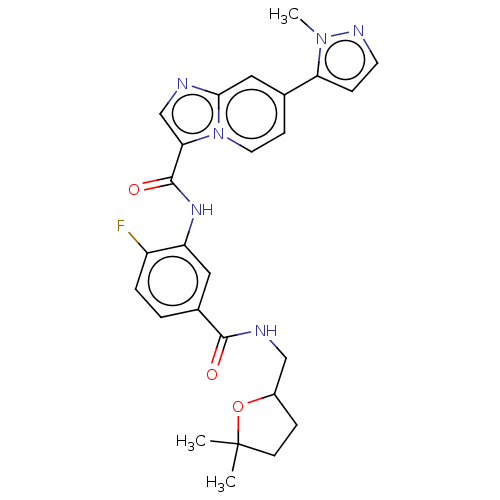 (CHEMBL3975580) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099512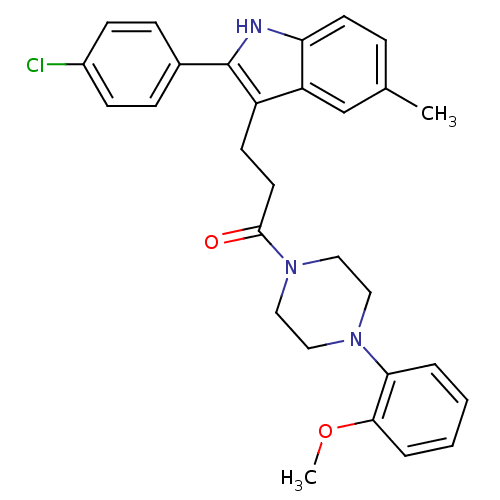 (3-[2-(4-Chloro-phenyl)-5-methyl-1H-indol-3-yl]-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114940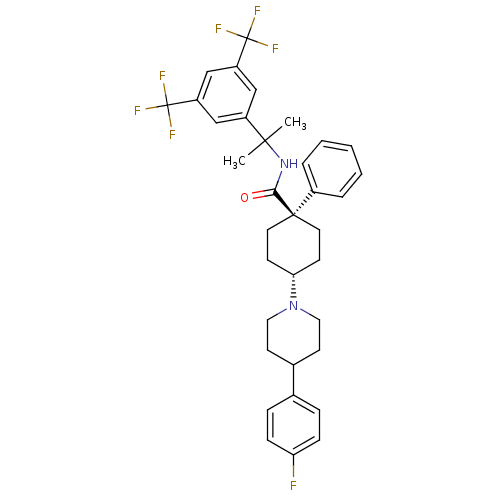 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114942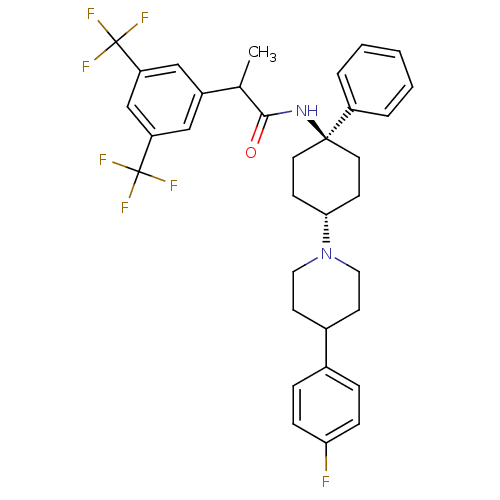 (2-(3,5-Bis-trifluoromethyl-phenyl)-N-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114938 (2-(3,5-Bis-trifluoromethyl-phenyl)-N-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114934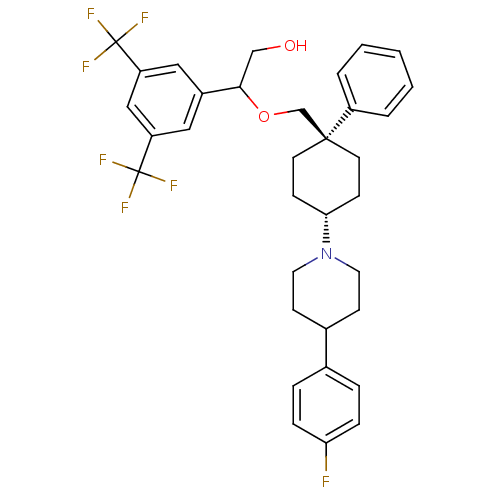 (2-(3,5-Bis-trifluoromethyl-phenyl)-2-{4-[4-(4-fluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191946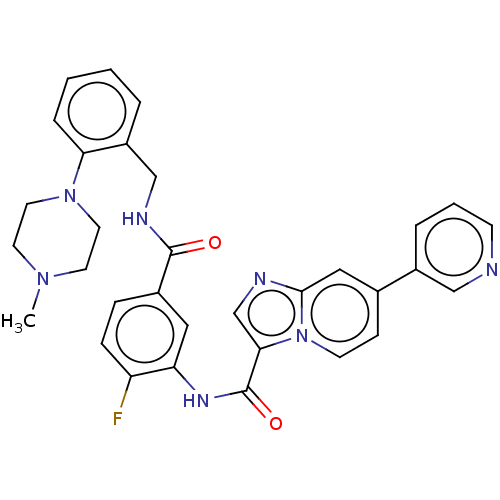 (CHEMBL3950278) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114937 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114937 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099519 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[6-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099507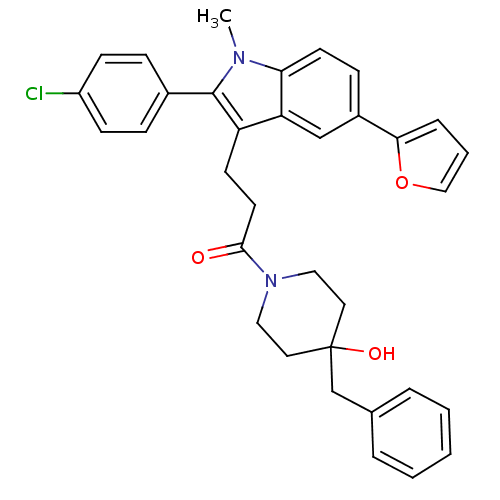 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[2-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099524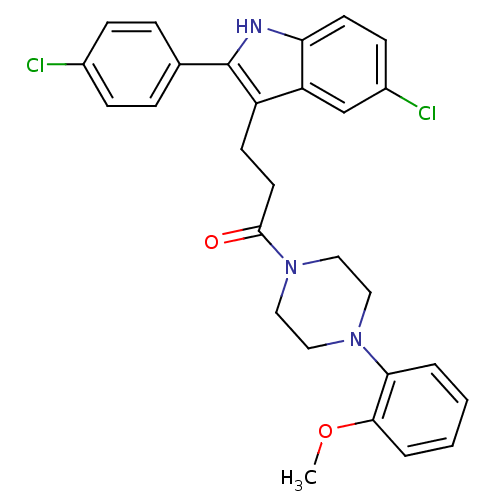 (3-[5-Chloro-2-(4-chloro-phenyl)-1H-indol-3-yl]-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50034271 (4-(3,5-Bis-trifluoromethyl-benzyloxymethyl)-4-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191980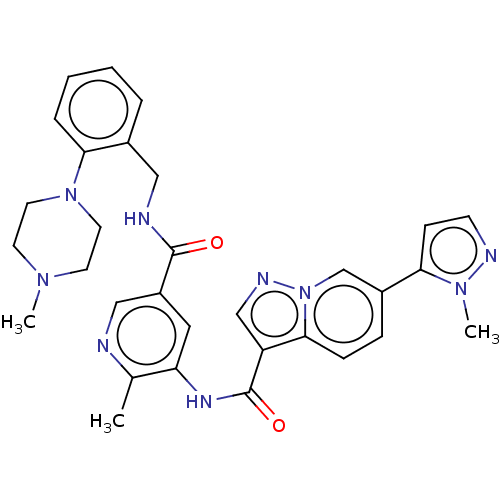 (CHEMBL3985689) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099509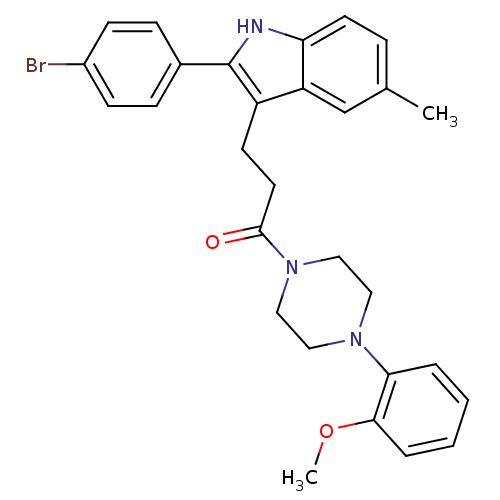 (3-[2-(4-Bromo-phenyl)-5-methyl-1H-indol-3-yl]-1-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192005 (CHEMBL3947262) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50191972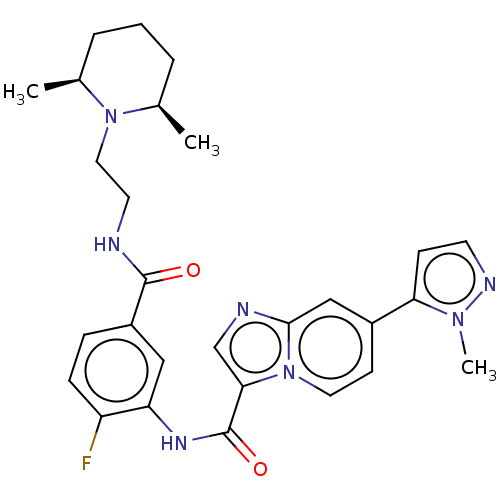 (CHEMBL3895824) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192006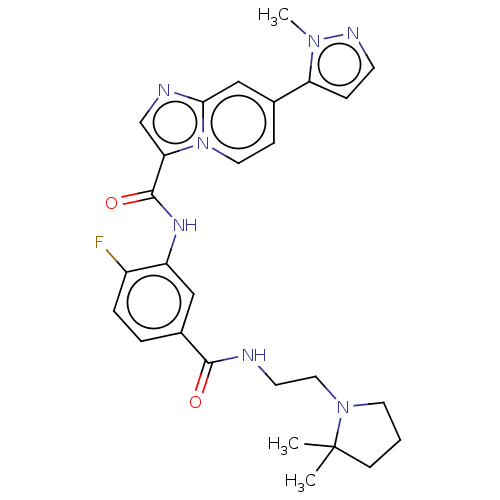 (CHEMBL3955987) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50192007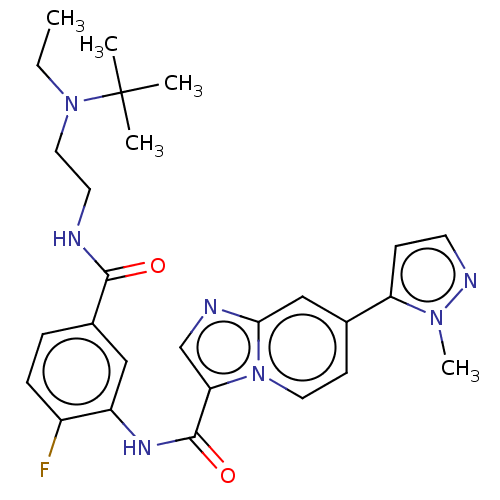 (CHEMBL3902237) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191973 (CHEMBL3940697) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114941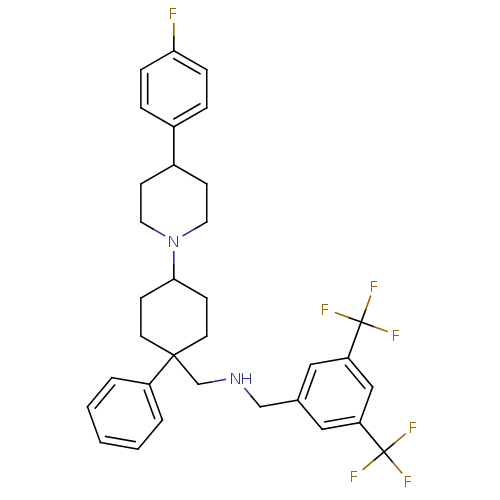 ((3,5-Bis-trifluoromethyl-benzyl)-{4-[4-(4-fluoro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099513 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[2-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099521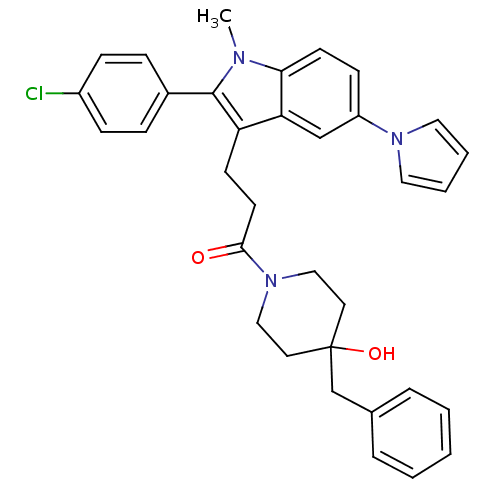 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[2-(4-chlo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50114946 (4-[4-(4-Fluoro-phenyl)-piperidin-1-yl]-1-phenyl-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]-labeled substance P from the cloned Tachykinin receptor 1 | Bioorg Med Chem Lett 12: 1755-8 (2002) BindingDB Entry DOI: 10.7270/Q2SX6CKH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50099522 (1-(4-Benzyl-4-hydroxy-piperidin-1-yl)-3-[5-chloro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The Neuroscience Research Centre Curated by ChEMBL | Assay Description Concentration required for displacement of [125I]-labeled substance P from cloned hNK1 receptor expressed in CHO cells | Bioorg Med Chem Lett 11: 1233-6 (2001) BindingDB Entry DOI: 10.7270/Q2PK0FDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Rattus norvegicus) | BDBM50191975 (CHEMBL3975580) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR) Curated by ChEMBL | Assay Description Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... | J Med Chem 59: 7901-14 (2016) Article DOI: 10.1021/acs.jmedchem.6b00703 BindingDB Entry DOI: 10.7270/Q25X2BW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 143 total ) | Next | Last >> |