

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458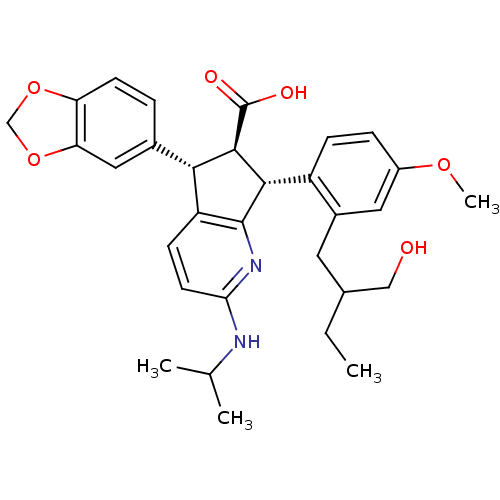 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141458 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472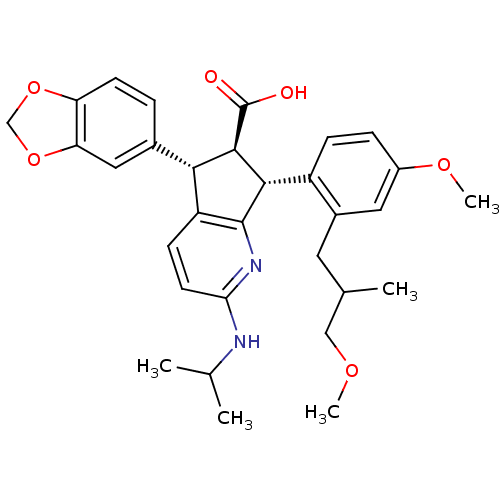 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141472 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141475 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141465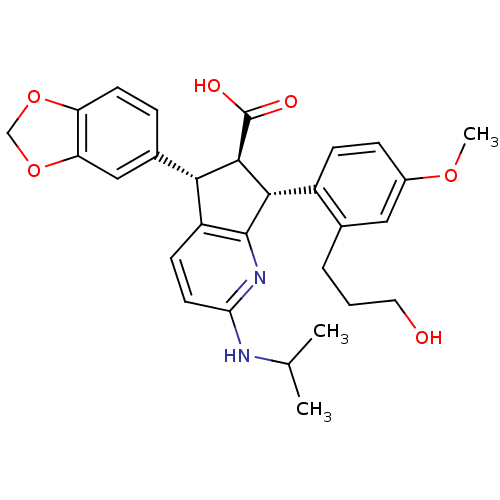 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141468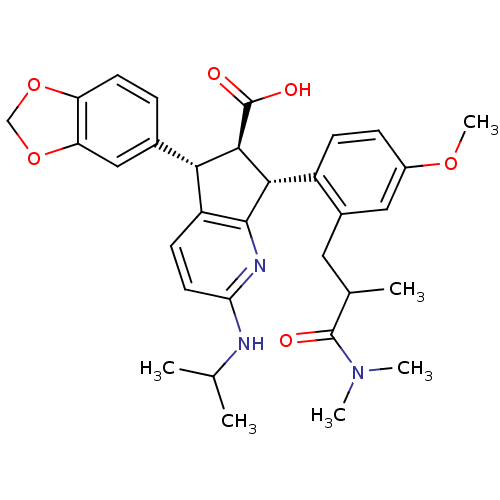 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141468 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-dimethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141471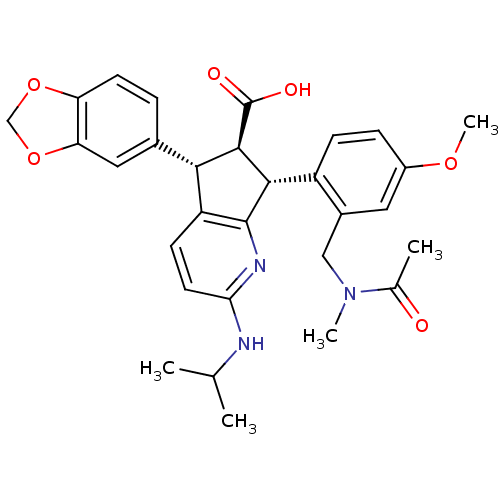 ((5S,6R,7R)-7-{2-[(Acetyl-methyl-amino)-methyl]-4-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141463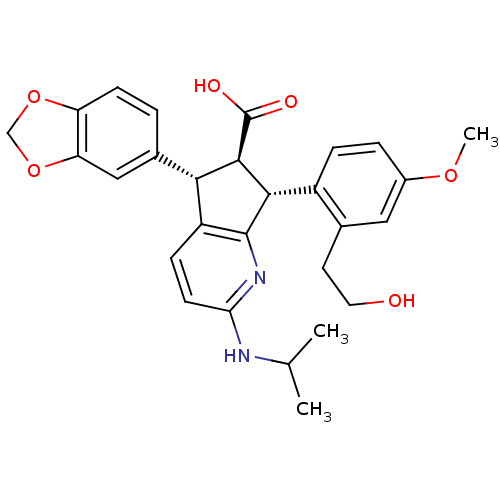 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141470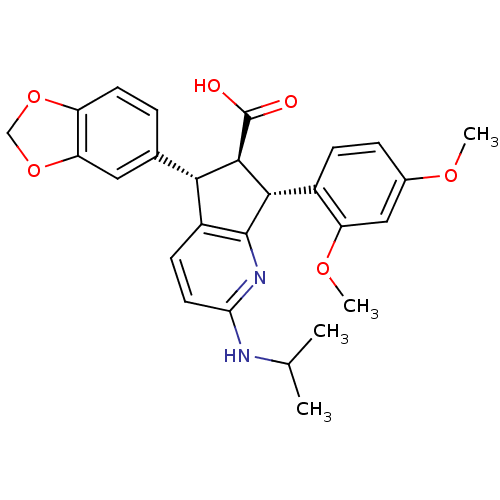 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-(2,4-dimethox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141461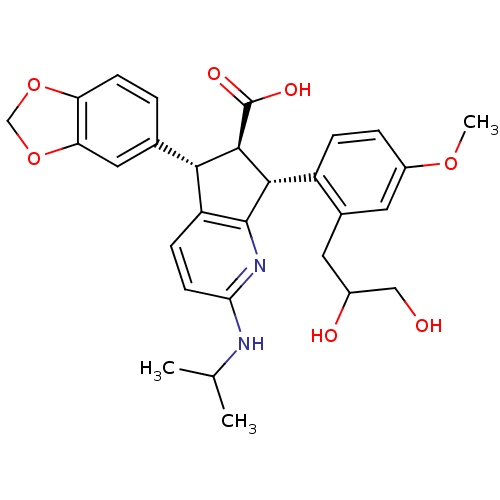 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2,3-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141461 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2,3-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141467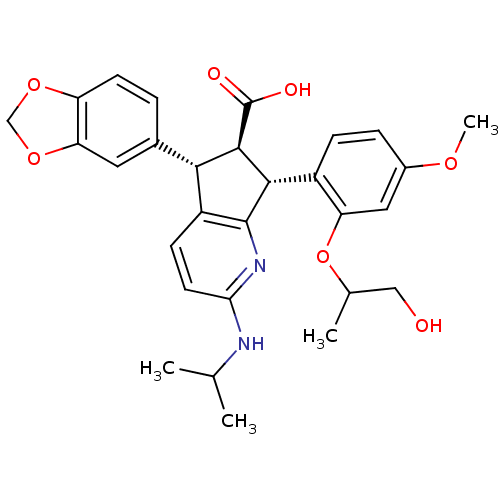 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141467 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(2-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141460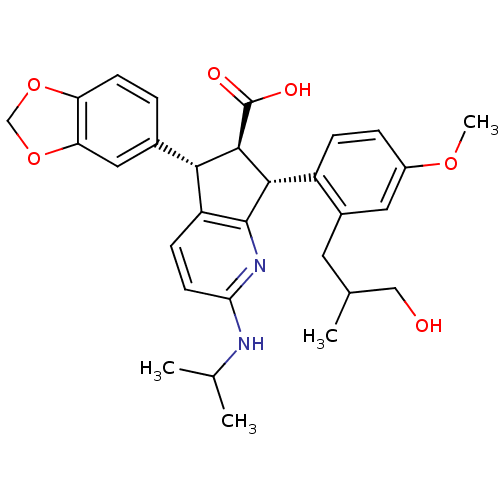 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin B receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141460 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0590 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141473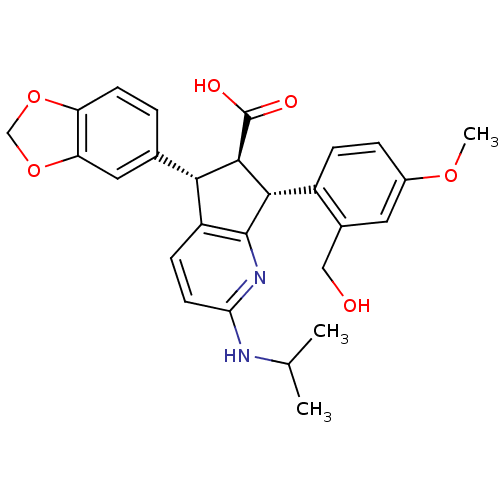 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-(2-hydroxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141457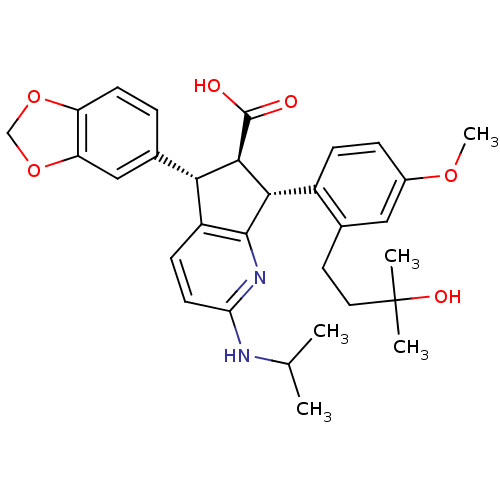 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-[2-(3-hydroxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0650 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119678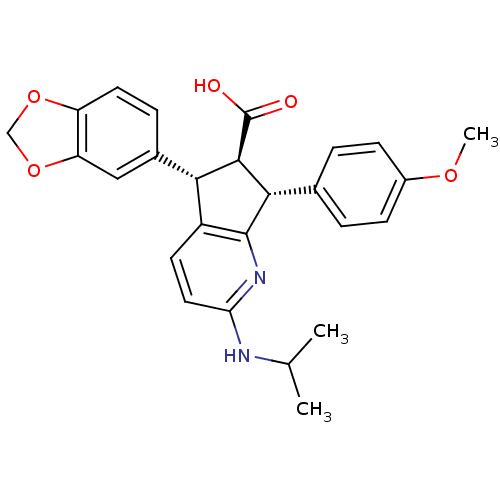 ((R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamino-7-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141459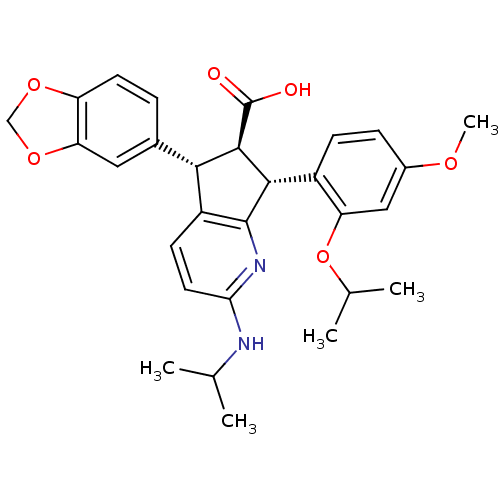 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-(2-isopropoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119676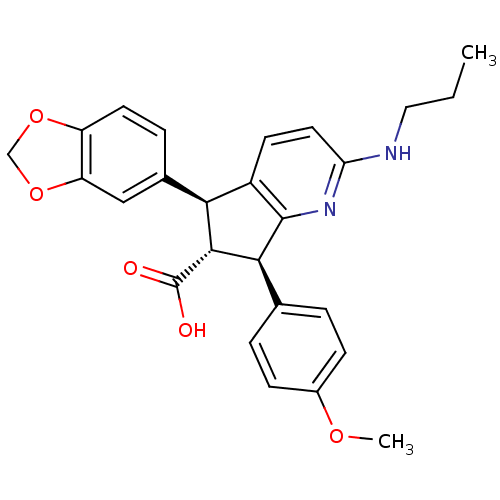 ((R)-5-Benzo[1,3]dioxol-5-yl-7-(4-methoxy-phenyl)-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119665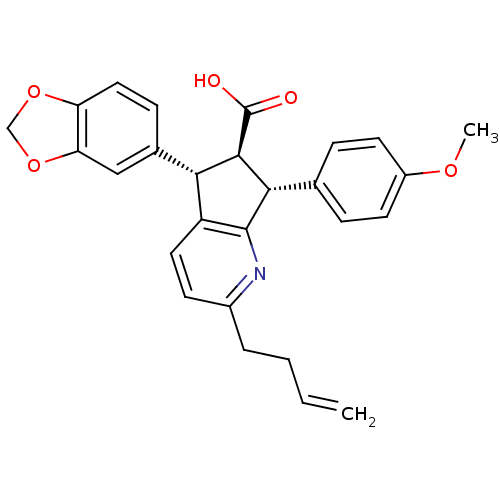 (5-Benzo[1,3]dioxol-5-yl-2-but-3-enyl-7-(4-methoxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119671 (5-Benzo[1,3]dioxol-5-yl-2-butyl-7-(4-methoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141469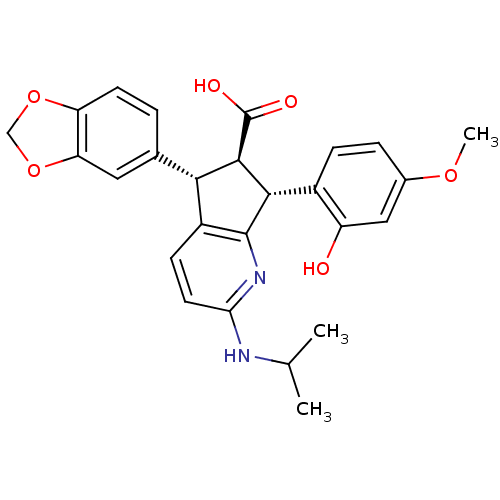 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-7-(2-hydroxy-4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126609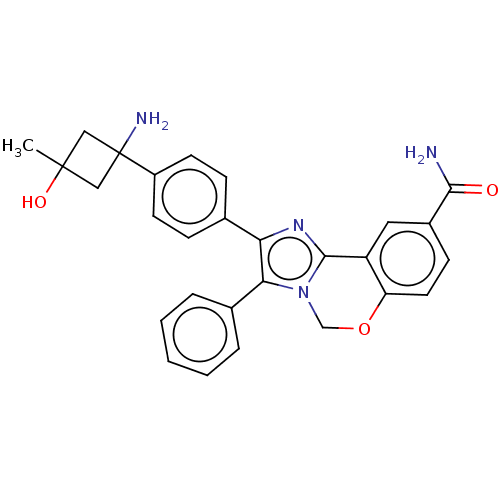 (US8772283, 53) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126581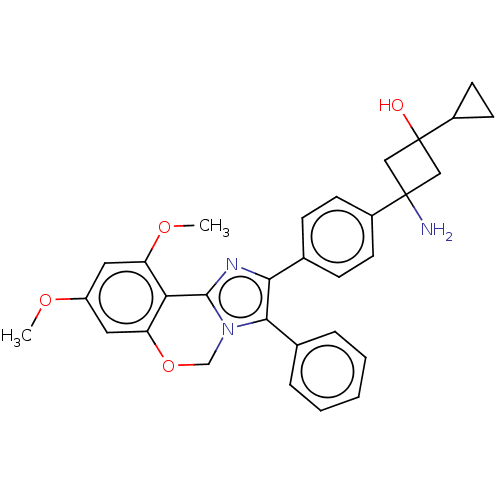 (US8772283, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126608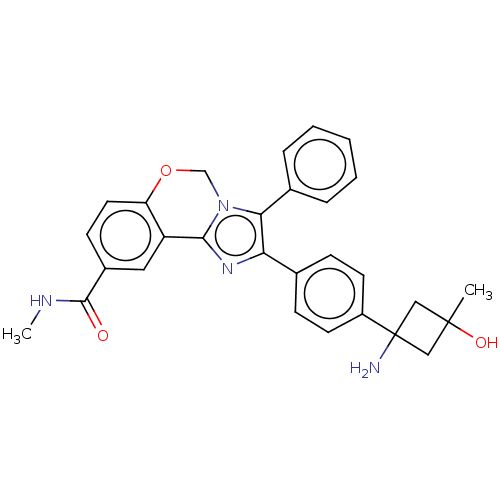 (US8772283, 52) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126557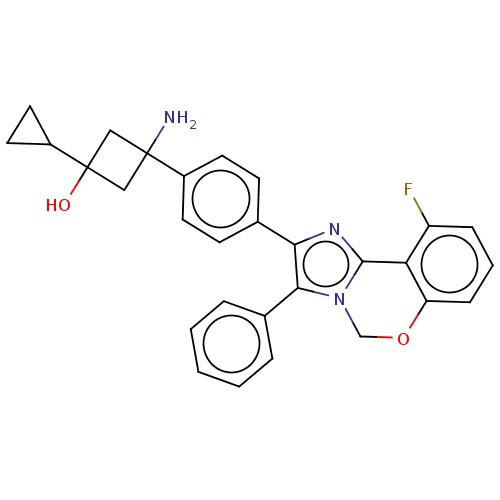 (US8772283, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126578 (US8772283, 22) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126611 (US8772283, 55) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119663 (5-Benzo[1,3]dioxol-5-yl-2-isobutyl-7-(4-methoxy-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126604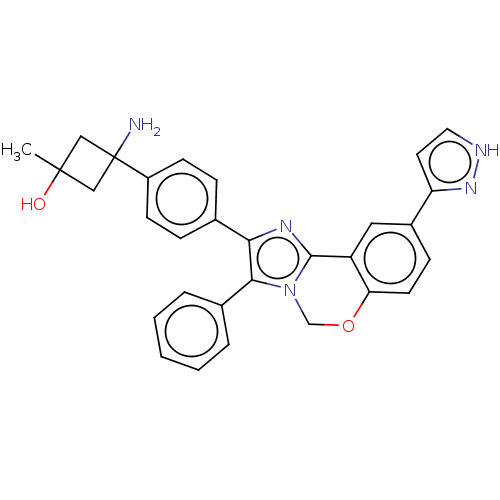 (US8772283, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126572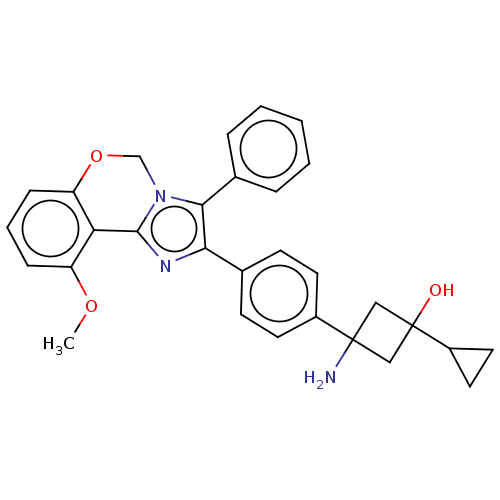 (US8772283, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119670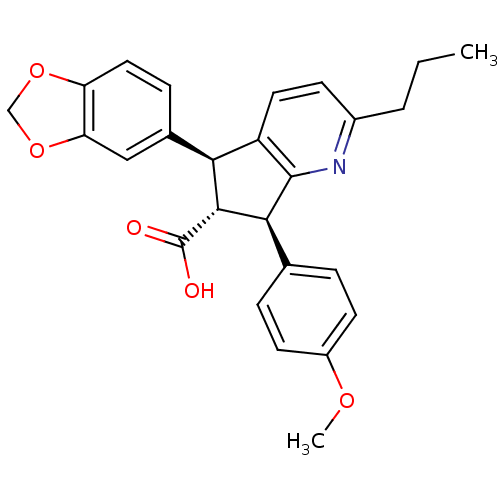 (5-Benzo[1,3]dioxol-5-yl-7-(4-methoxy-phenyl)-2-pro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126618 (US8772283, 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126617 (US8772283, 61) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50141462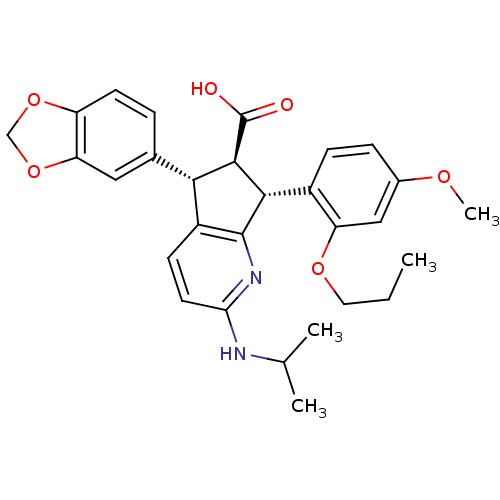 ((5S,6R,7R)-5-Benzo[1,3]dioxol-5-yl-2-isopropylamin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute in collaboration with Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against [125I]-ET-1 binding to human endothelin A receptor | Bioorg Med Chem Lett 14: 1503-7 (2004) Article DOI: 10.1016/j.bmcl.2004.01.008 BindingDB Entry DOI: 10.7270/Q23R0SB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126605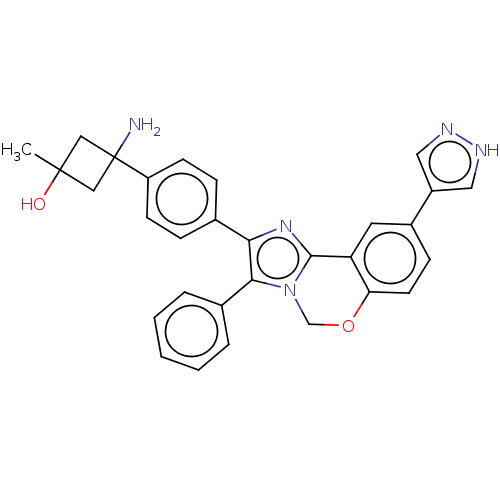 (US8772283, 49) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126591 (US8772283, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126612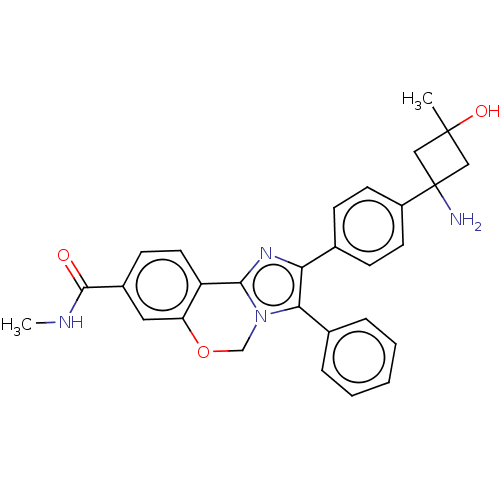 (US8772283, 56) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126558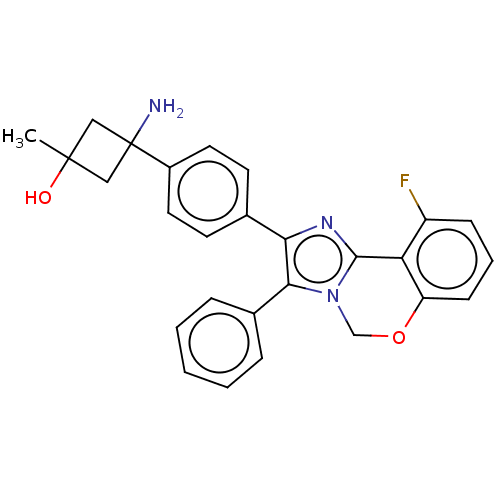 (US8772283, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119673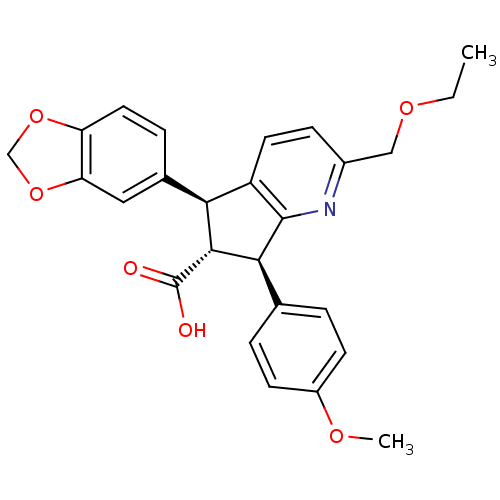 ((R)-5-Benzo[1,3]dioxol-5-yl-2-ethoxymethyl-7-(4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126615 (US8772283, 59) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126600 (US8772283, 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50119662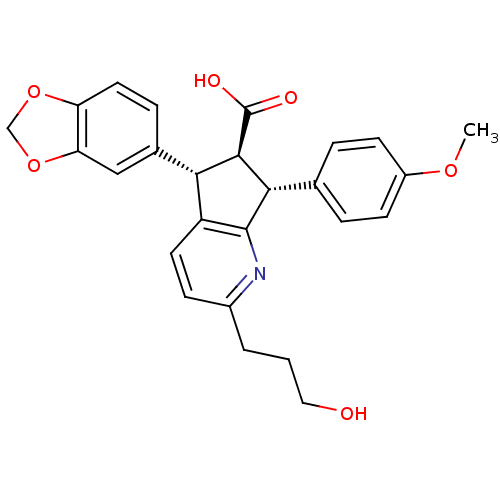 ((R)-5-Benzo[1,3]dioxol-5-yl-2-(3-hydroxy-propyl)-7...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Tsukuba Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against I-labeled ET-1 binding to Endothelin A receptor | Bioorg Med Chem Lett 12: 3041-5 (2002) BindingDB Entry DOI: 10.7270/Q2KK9B3N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126616 (US8772283, 60) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126574 (US8772283, 18) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126614 (US8772283, 58) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-beta serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM126560 (US8772283, 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.710 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description Preparation of AKT1 and AKT2 and measurement of in vitro inhibitory activity of the above-mentioned compounds against AKT1 and AKT2 kinase activity w... | US Patent US8772283 (2014) BindingDB Entry DOI: 10.7270/Q21J98F0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 444 total ) | Next | Last >> |