 Found 1135 hits with Last Name = 'garcia-calvo' and Initial = 'm'
Found 1135 hits with Last Name = 'garcia-calvo' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-3
(Homo sapiens (Human)) | BDBM10246
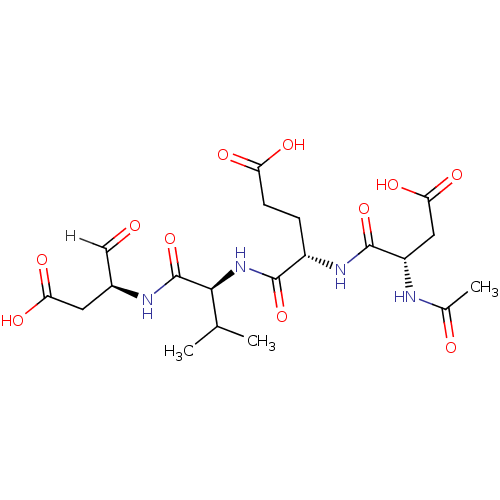
((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50174298
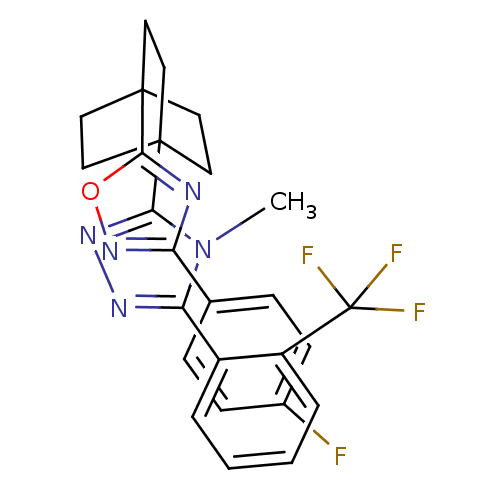
(3-(4-(3-(4-fluorophenyl)-1,2,4-oxadiazol-5-yl)bicy...)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1nc(no1)-c1ccc(F)cc1)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C26H23F4N5O/c1-35-21(18-4-2-3-5-19(18)26(28,29)30)32-33-22(35)24-10-13-25(14-11-24,15-12-24)23-31-20(34-36-23)16-6-8-17(27)9-7-16/h2-9H,10-15H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50089636
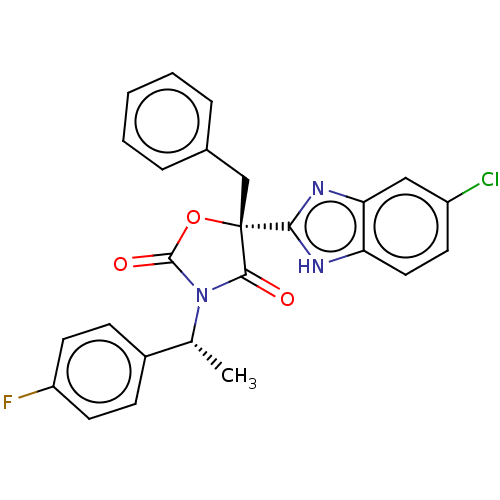
(CHEMBL3578271)Show SMILES C[C@@H](N1C(=O)O[C@@](Cc2ccccc2)(C1=O)c1nc2cc(Cl)ccc2[nH]1)c1ccc(F)cc1 |r| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to mineralocorticoid receptor (unknown origin) |
ACS Med Chem Lett 6: 461-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00010
BindingDB Entry DOI: 10.7270/Q21J9CH4 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50340378
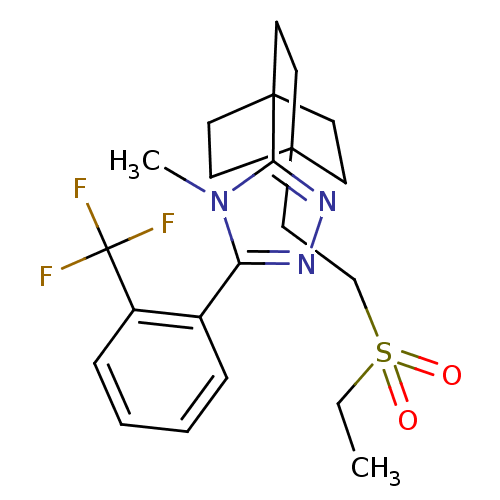
(3-(4-(2-(ethylsulfonyl)ethyl)bicyclo[2.2.2]octan-1...)Show SMILES CCS(=O)(=O)CCC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C22H28F3N3O2S/c1-3-31(29,30)15-14-20-8-11-21(12-9-20,13-10-20)19-27-26-18(28(19)2)16-6-4-5-7-17(16)22(23,24)25/h4-7H,3,8-15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50435691
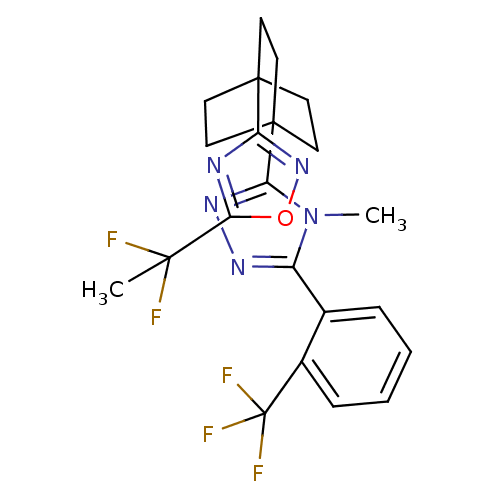
(CHEMBL2391968)Show SMILES Cn1c(nnc1C12CCC(CC1)(CC2)c1noc(n1)C(C)(F)F)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C22H22F5N5O/c1-19(23,24)18-28-16(31-33-18)20-7-10-21(11-8-20,12-9-20)17-30-29-15(32(17)2)13-5-3-4-6-14(13)22(25,26)27/h3-6H,7-12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116262
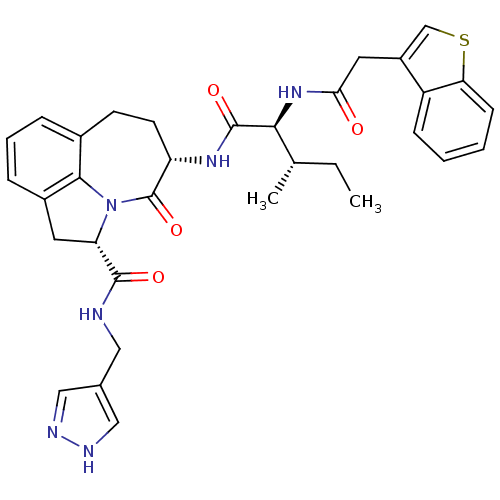
((2S,5S)-5-[(S)-2-(2-Benzo[b]thiophen-3-yl-acetylam...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1csc2ccccc12)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C33H36N6O4S/c1-3-19(2)29(38-28(40)14-23-18-44-27-10-5-4-9-24(23)27)32(42)37-25-12-11-21-7-6-8-22-13-26(39(30(21)22)33(25)43)31(41)34-15-20-16-35-36-17-20/h4-10,16-19,25-26,29H,3,11-15H2,1-2H3,(H,34,41)(H,35,36)(H,37,42)(H,38,40)/t19-,25-,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116260
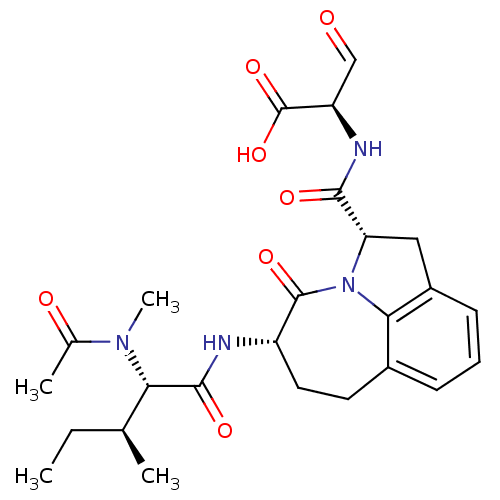
((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116256
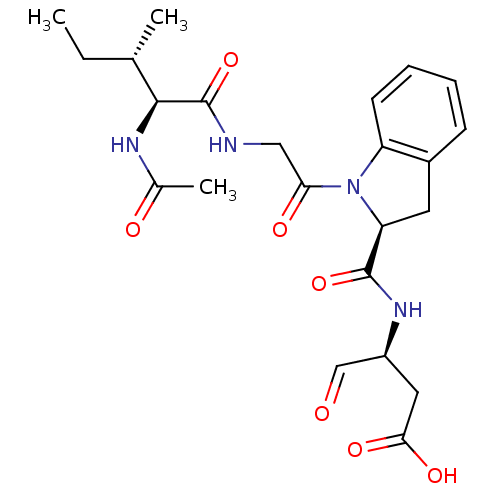
((S)-3-({1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-p...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1[C@@H](Cc2ccccc12)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C23H30N4O7/c1-4-13(2)21(25-14(3)29)23(34)24-11-19(30)27-17-8-6-5-7-15(17)9-18(27)22(33)26-16(12-28)10-20(31)32/h5-8,12-13,16,18,21H,4,9-11H2,1-3H3,(H,24,34)(H,25,29)(H,26,33)(H,31,32)/t13-,16-,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Rattus norvegicus (rat)) | BDBM50340386
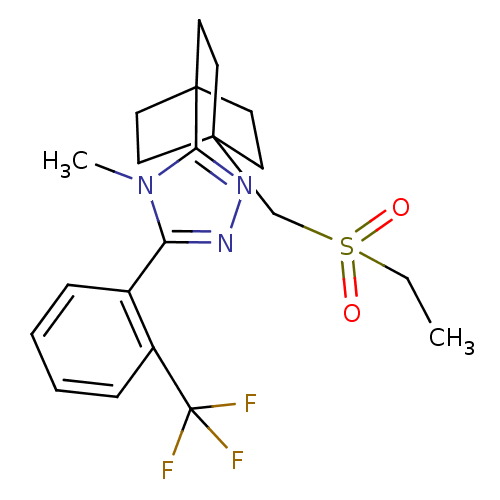
(3-(4-(ethylsulfonylmethyl)bicyclo[2.2.2]octan-1-yl...)Show SMILES CCS(=O)(=O)CC12CCC(CC1)(CC2)c1nnc(-c2ccccc2C(F)(F)F)n1C Show InChI InChI=1S/C21H26F3N3O2S/c1-3-30(28,29)14-19-8-11-20(12-9-19,13-10-19)18-26-25-17(27(18)2)15-6-4-5-7-16(15)21(22,23)24/h4-7H,3,8-14H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Inhibition of rat 11beta-HSD1 |
Bioorg Med Chem Lett 23: 3650-3 (2013)
Article DOI: 10.1016/j.bmcl.2013.03.011
BindingDB Entry DOI: 10.7270/Q29S1SDM |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116255
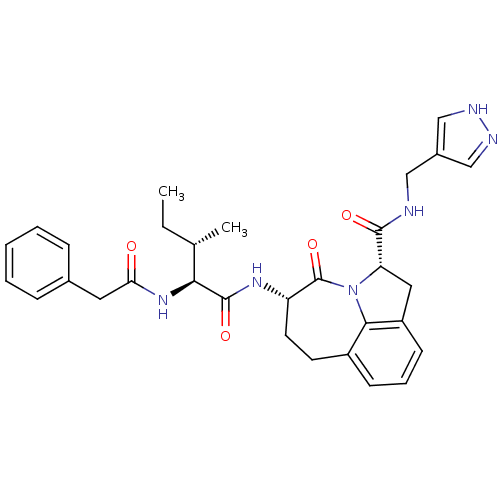
((2S,5S)-5-((S)-3-(S)-Methyl-2-phenylacetylamino-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1ccccc1)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C31H36N6O4/c1-3-19(2)27(36-26(38)14-20-8-5-4-6-9-20)30(40)35-24-13-12-22-10-7-11-23-15-25(37(28(22)23)31(24)41)29(39)32-16-21-17-33-34-18-21/h4-11,17-19,24-25,27H,3,12-16H2,1-2H3,(H,32,39)(H,33,34)(H,35,40)(H,36,38)/t19-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116267
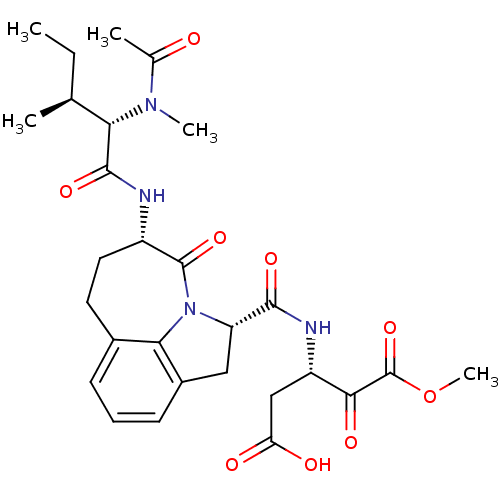
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM10246

((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116268
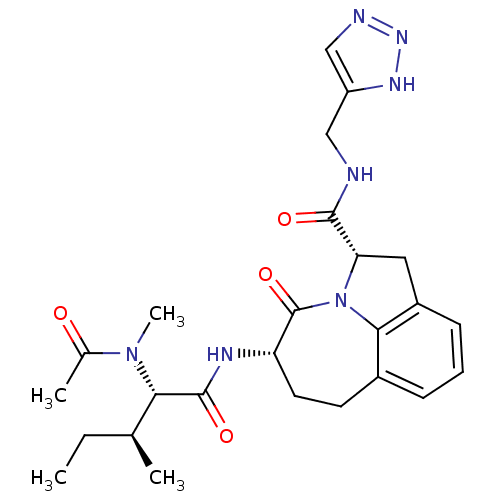
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1 Show InChI InChI=1S/C25H33N7O4/c1-5-14(2)21(31(4)15(3)33)24(35)28-19-10-9-16-7-6-8-17-11-20(32(22(16)17)25(19)36)23(34)26-12-18-13-27-30-29-18/h6-8,13-14,19-21H,5,9-12H2,1-4H3,(H,26,34)(H,28,35)(H,27,29,30)/t14-,19-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116269
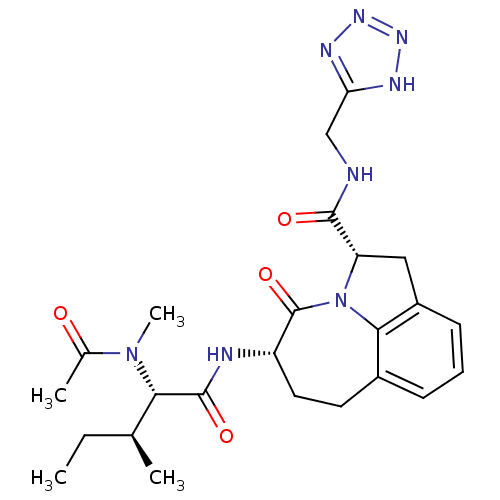
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C24H32N8O4/c1-5-13(2)20(31(4)14(3)33)23(35)26-17-10-9-15-7-6-8-16-11-18(32(21(15)16)24(17)36)22(34)25-12-19-27-29-30-28-19/h6-8,13,17-18,20H,5,9-12H2,1-4H3,(H,25,34)(H,26,35)(H,27,28,29,30)/t13-,17-,18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116261
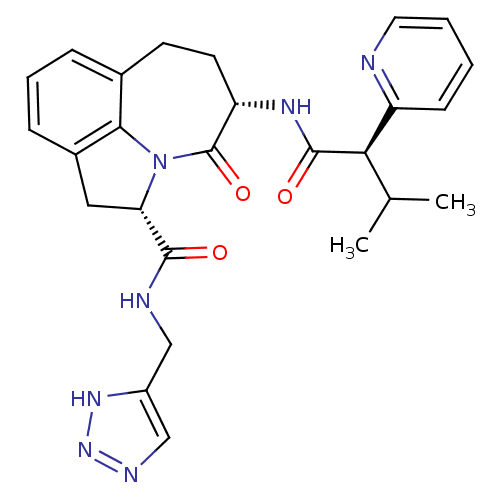
((2S,5S)-5-((R)-3-Methyl-2-pyridin-2-yl-butyrylamin...)Show SMILES CC(C)[C@@H](C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1)c1ccccn1 Show InChI InChI=1S/C26H29N7O3/c1-15(2)22(19-8-3-4-11-27-19)25(35)30-20-10-9-16-6-5-7-17-12-21(33(23(16)17)26(20)36)24(34)28-13-18-14-29-32-31-18/h3-8,11,14-15,20-22H,9-10,12-13H2,1-2H3,(H,28,34)(H,30,35)(H,29,31,32)/t20-,21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116259
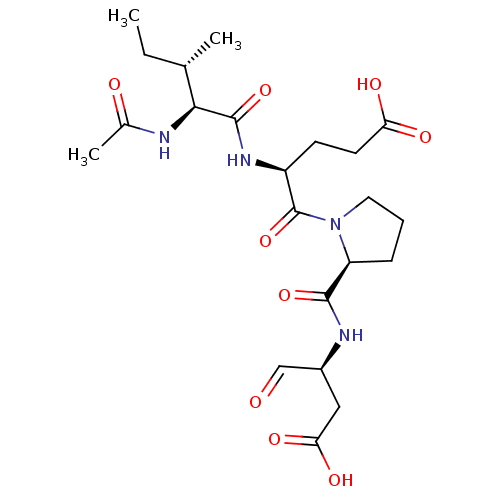
((S)-4-((3S,4S)-2-Acetylamino-3-methyl-pentanoylami...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C22H34N4O9/c1-4-12(2)19(23-13(3)28)21(34)25-15(7-8-17(29)30)22(35)26-9-5-6-16(26)20(33)24-14(11-27)10-18(31)32/h11-12,14-16,19H,4-10H2,1-3H3,(H,23,28)(H,24,33)(H,25,34)(H,29,30)(H,31,32)/t12-,14-,15-,16-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116263

((S)-3-{[(S)-(5S,7S)-6-((3S,4S)-2-Acetylamino-3-met...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CC[C@H]2CC[C@H](N2C1=O)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C21H32N4O7/c1-4-11(2)18(22-12(3)27)20(31)24-15-7-5-14-6-8-16(25(14)21(15)32)19(30)23-13(10-26)9-17(28)29/h10-11,13-16,18H,4-9H2,1-3H3,(H,22,27)(H,23,30)(H,24,31)(H,28,29)/t11-,13-,14-,15-,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116258
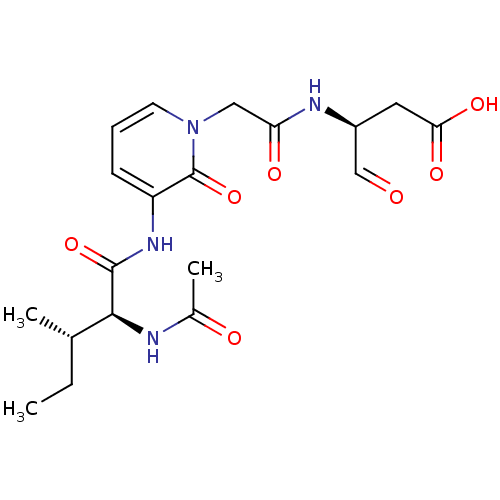
((S)-3-{2-[3-((2S,3S)-2-Acetylamino-3-methyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)Nc1cccn(CC(=O)N[C@@H](CC(O)=O)C=O)c1=O Show InChI InChI=1S/C19H26N4O7/c1-4-11(2)17(20-12(3)25)18(29)22-14-6-5-7-23(19(14)30)9-15(26)21-13(10-24)8-16(27)28/h5-7,10-11,13,17H,4,8-9H2,1-3H3,(H,20,25)(H,21,26)(H,22,29)(H,27,28)/t11-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116267

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116257
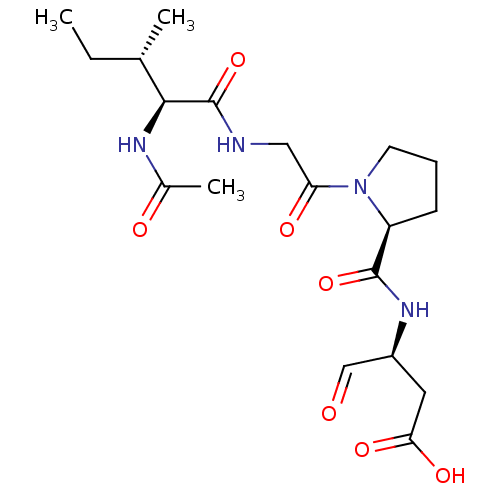
(3-[((S)-{1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C19H30N4O7/c1-4-11(2)17(21-12(3)25)19(30)20-9-15(26)23-7-5-6-14(23)18(29)22-13(10-24)8-16(27)28/h10-11,13-14,17H,4-9H2,1-3H3,(H,20,30)(H,21,25)(H,22,29)(H,27,28)/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-8 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116270

((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cc(=O)[nH]o1 Show InChI InChI=1S/C26H33N5O6/c1-5-14(2)22(30(4)15(3)32)25(35)28-19-10-9-16-7-6-8-17-11-20(31(23(16)17)26(19)36)24(34)27-13-18-12-21(33)29-37-18/h6-8,12,14,19-20,22H,5,9-11,13H2,1-4H3,(H,27,34)(H,28,35)(H,29,33)/t14-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116254

(3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)-3-m...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H34N4O6/c1-5-14(2)21(28(4)15(3)30)24(34)27-18-10-9-16-7-6-8-17-13-19(29(22(16)17)25(18)35)23(33)26-12-11-20(31)32/h6-8,14,18-19,21H,5,9-13H2,1-4H3,(H,26,33)(H,27,34)(H,31,32)/t14-,18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116266
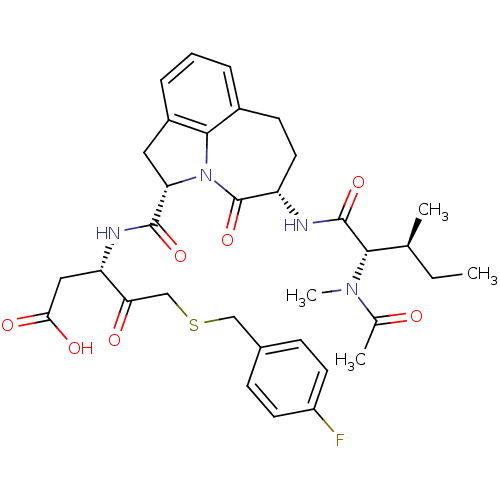
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)CSCc1ccc(F)cc1 Show InChI InChI=1S/C34H41FN4O7S/c1-5-19(2)30(38(4)20(3)40)33(45)36-25-14-11-22-7-6-8-23-15-27(39(31(22)23)34(25)46)32(44)37-26(16-29(42)43)28(41)18-47-17-21-9-12-24(35)13-10-21/h6-10,12-13,19,25-27,30H,5,11,14-18H2,1-4H3,(H,36,45)(H,37,44)(H,42,43)/t19-,25-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364407
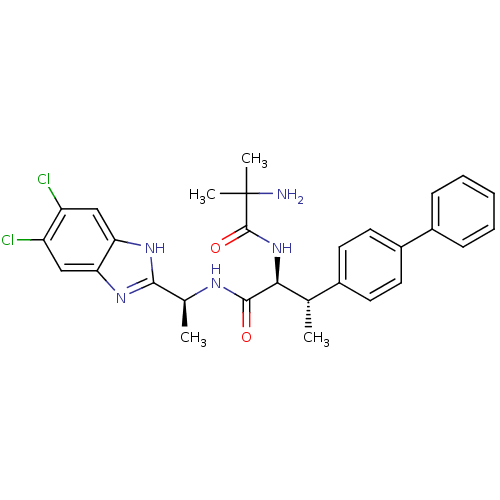
(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443348
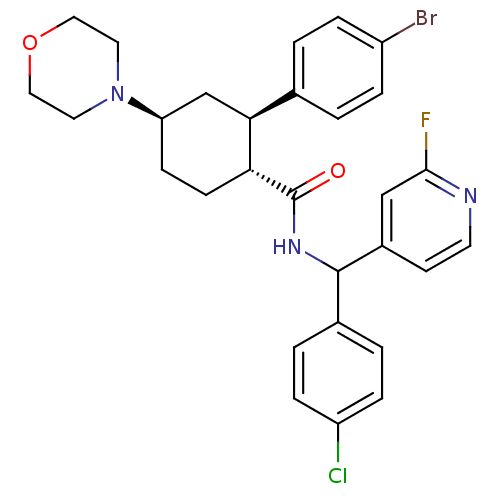
(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0790 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364689

(CHEMBL1951478)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(CC2)S(=O)(=O)c2ccc(cn2)C(F)(F)F)c2ccccc12 Show InChI InChI=1S/C23H19ClF3N3O4S/c24-16-7-10-30(31)19(13-16)21-17-3-1-2-4-18(17)22(34-21)8-11-29(12-9-22)35(32,33)20-6-5-15(14-28-20)23(25,26)27/h1-7,10,13-14,21H,8-9,11-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364386
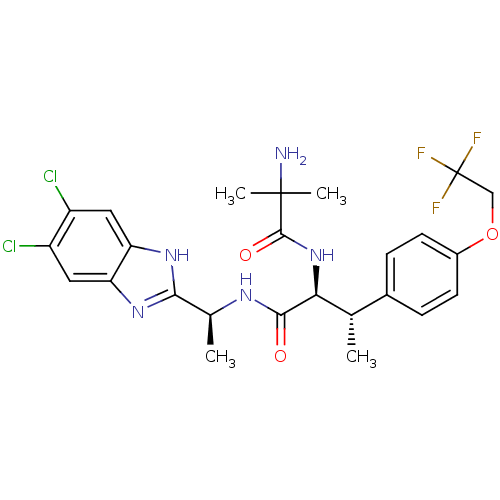
(CHEMBL1950440)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(OCC(F)(F)F)cc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C25H28Cl2F3N5O3/c1-12(14-5-7-15(8-6-14)38-11-25(28,29)30)20(35-23(37)24(3,4)31)22(36)32-13(2)21-33-18-9-16(26)17(27)10-19(18)34-21/h5-10,12-13,20H,11,31H2,1-4H3,(H,32,36)(H,33,34)(H,35,37)/t12-,13-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50443351
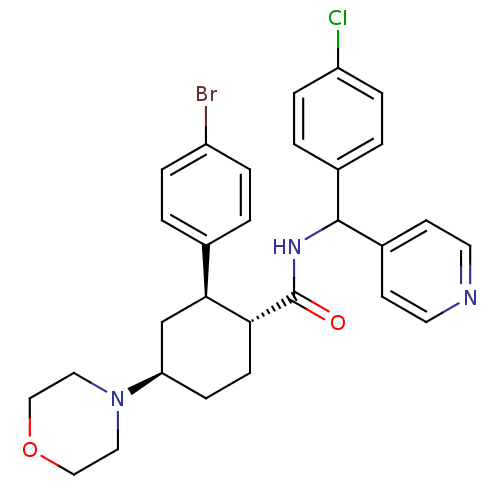
(CHEMBL3086037 | US8669252, 15)Show SMILES Clc1ccc(cc1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccncc1 |r| Show InChI InChI=1S/C29H31BrClN3O2/c30-23-5-1-20(2-6-23)27-19-25(34-15-17-36-18-16-34)9-10-26(27)29(35)33-28(22-11-13-32-14-12-22)21-3-7-24(31)8-4-21/h1-8,11-14,25-28H,9-10,15-19H2,(H,33,35)/t25-,26-,27+,28?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364676
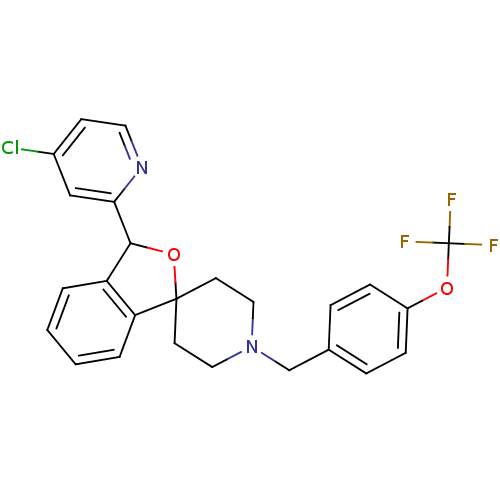
(CHEMBL1951465 | US8785634, 1)Show SMILES FC(F)(F)Oc1ccc(CN2CCC3(CC2)OC(c2ccccc32)c2cc(Cl)ccn2)cc1 Show InChI InChI=1S/C25H22ClF3N2O2/c26-18-9-12-30-22(15-18)23-20-3-1-2-4-21(20)24(33-23)10-13-31(14-11-24)16-17-5-7-19(8-6-17)32-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50383421
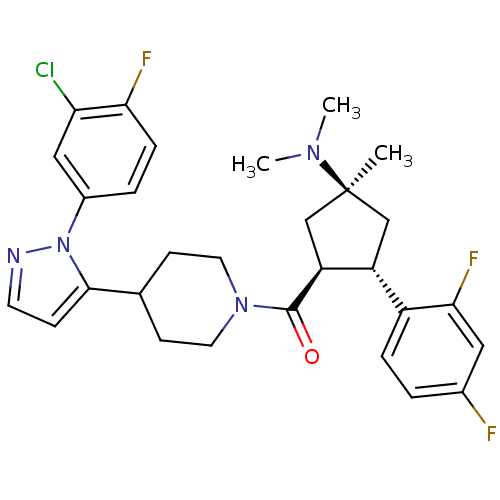
(CHEMBL2031595)Show SMILES CN(C)[C@]1(C)C[C@@H]([C@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(35(2)3)16-22(21-6-4-19(31)14-26(21)33)23(17-29)28(38)36-12-9-18(10-13-36)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+,29+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382913
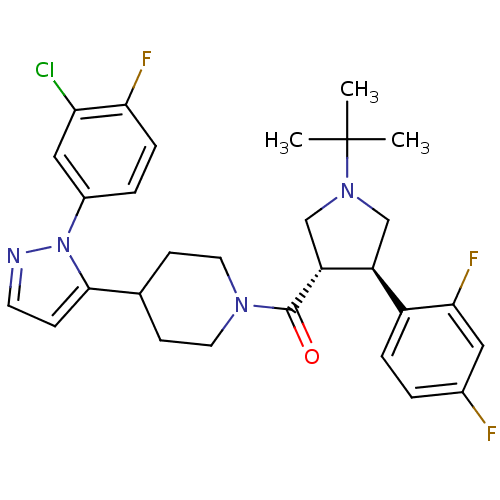
(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382899

(CHEMBL2022787)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(C)c(Cl)c1 |r| Show InChI InChI=1S/C31H37ClF2N4O/c1-19-6-8-23(16-27(19)32)38-29(14-20(2)35-38)21-10-12-36(13-11-21)30(39)26-18-37(31(3,4)5)17-25(26)24-9-7-22(33)15-28(24)34/h6-9,14-16,21,25-26H,10-13,17-18H2,1-5H3/t25-,26+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382904
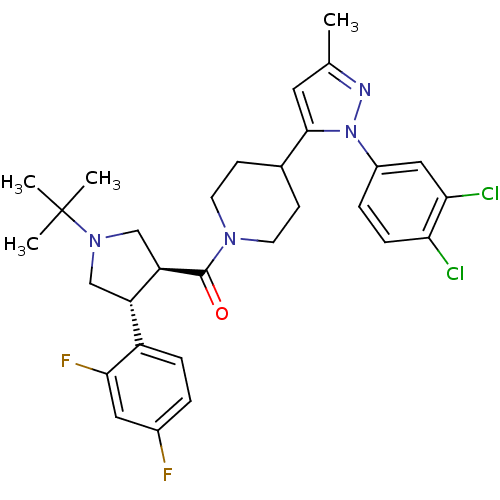
(CHEMBL2022793)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-28(38(35-18)21-6-8-25(31)26(32)15-21)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-5-20(33)14-27(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50443348

(CHEMBL3086040 | US8669252, 12)Show SMILES Fc1cc(ccn1)C(NC(=O)[C@@H]1CC[C@H](C[C@H]1c1ccc(Br)cc1)N1CCOCC1)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C29H30BrClFN3O2/c30-22-5-1-19(2-6-22)26-18-24(35-13-15-37-16-14-35)9-10-25(26)29(36)34-28(20-3-7-23(31)8-4-20)21-11-12-33-27(32)17-21/h1-8,11-12,17,24-26,28H,9-10,13-16,18H2,(H,34,36)/t24-,25-,26+,28?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant mouse PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by fluorescence assay |
Bioorg Med Chem Lett 23: 6228-33 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.094
BindingDB Entry DOI: 10.7270/Q2DZ09Q4 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50361786
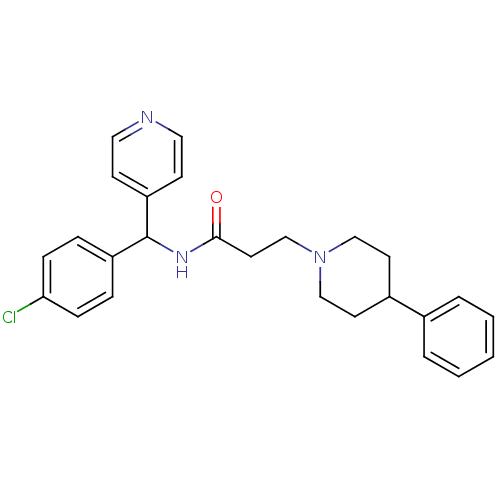
(CHEMBL1938522)Show SMILES Clc1ccc(cc1)C(NC(=O)CCN1CCC(CC1)c1ccccc1)c1ccncc1 Show InChI InChI=1S/C26H28ClN3O/c27-24-8-6-22(7-9-24)26(23-10-15-28-16-11-23)29-25(31)14-19-30-17-12-21(13-18-30)20-4-2-1-3-5-20/h1-11,15-16,21,26H,12-14,17-19H2,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate measured for 30 mins by continuous fluorometric assay |
Bioorg Med Chem Lett 22: 658-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.060
BindingDB Entry DOI: 10.7270/Q2B56K50 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382913

(CHEMBL2023210)Show SMILES CC(C)(C)N1C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1ccnn1-c1ccc(F)c(Cl)c1 |r| Show InChI InChI=1S/C29H32ClF3N4O/c1-29(2,3)36-16-22(21-6-4-19(31)14-26(21)33)23(17-36)28(38)35-12-9-18(10-13-35)27-8-11-34-37(27)20-5-7-25(32)24(30)15-20/h4-8,11,14-15,18,22-23H,9-10,12-13,16-17H2,1-3H3/t22-,23+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2811-7 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.075
BindingDB Entry DOI: 10.7270/Q2T154PX |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50364685
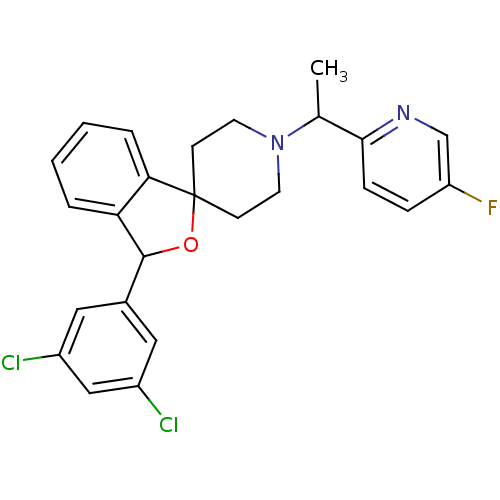
(CHEMBL1951474)Show SMILES CC(N1CCC2(CC1)OC(c1ccccc21)c1cc(Cl)cc(Cl)c1)c1ccc(F)cn1 Show InChI InChI=1S/C25H23Cl2FN2O/c1-16(23-7-6-20(28)15-29-23)30-10-8-25(9-11-30)22-5-3-2-4-21(22)24(31-25)17-12-18(26)14-19(27)13-17/h2-7,12-16,24H,8-11H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Homo sapiens (Human)) | BDBM50364407

(CHEMBL1950444)Show SMILES C[C@H](NC(=O)[C@@H](NC(=O)C(C)(C)N)[C@@H](C)c1ccc(cc1)-c1ccccc1)c1nc2cc(Cl)c(Cl)cc2[nH]1 |r| Show InChI InChI=1S/C29H31Cl2N5O2/c1-16(18-10-12-20(13-11-18)19-8-6-5-7-9-19)25(36-28(38)29(3,4)32)27(37)33-17(2)26-34-23-14-21(30)22(31)15-24(23)35-26/h5-17,25H,32H2,1-4H3,(H,33,37)(H,34,35)(H,36,38)/t16-,17-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PrCP using Mca-Ala-Pro-Lys(Dnp)-OH as substrate for 30 mins by continuous fluorimetric assay |
Bioorg Med Chem Lett 22: 1774-8 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.064
BindingDB Entry DOI: 10.7270/Q27H1K28 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
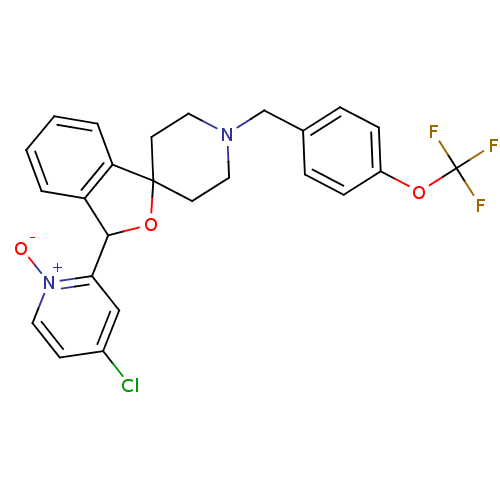
(Mus musculus) | BDBM50364679
(CHEMBL1951468 | US8785634, 2)Show SMILES [O-][n+]1ccc(Cl)cc1C1OC2(CCN(Cc3ccc(OC(F)(F)F)cc3)CC2)c2ccccc12 Show InChI InChI=1S/C25H22ClF3N2O3/c26-18-9-12-31(32)22(15-18)23-20-3-1-2-4-21(20)24(34-23)10-13-30(14-11-24)16-17-5-7-19(8-6-17)33-25(27,28)29/h1-9,12,15,23H,10-11,13-14,16H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
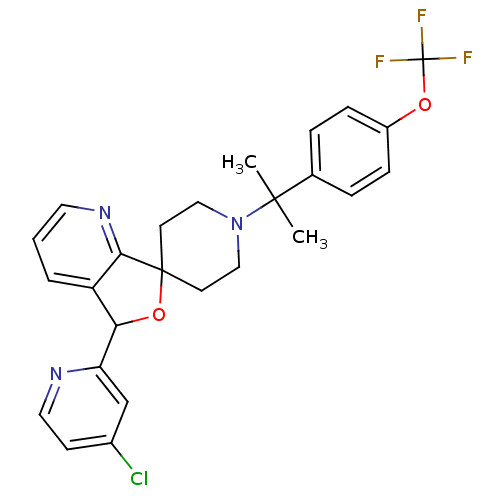
(Mus musculus) | BDBM50364687
(CHEMBL1951476 | US8785634, 8)Show SMILES CC(C)(N1CCC2(CC1)OC(c1cccnc21)c1cc(Cl)ccn1)c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C26H25ClF3N3O2/c1-24(2,17-5-7-19(8-6-17)34-26(28,29)30)33-14-10-25(11-15-33)23-20(4-3-12-32-23)22(35-25)21-16-18(27)9-13-31-21/h3-9,12-13,16,22H,10-11,14-15H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 1550-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.002
BindingDB Entry DOI: 10.7270/Q2JS9QXQ |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
(Mus musculus) | BDBM50382906

(CHEMBL2023202)Show SMILES Cc1cc(C2CCN(CC2)C(=O)[C@@H]2CN(C[C@H]2c2ccc(F)cc2F)C(C)(C)C)n(n1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-36(12-10-19)29(39)24-17-37(30(2,3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |
Lysosomal Pro-X carboxypeptidase
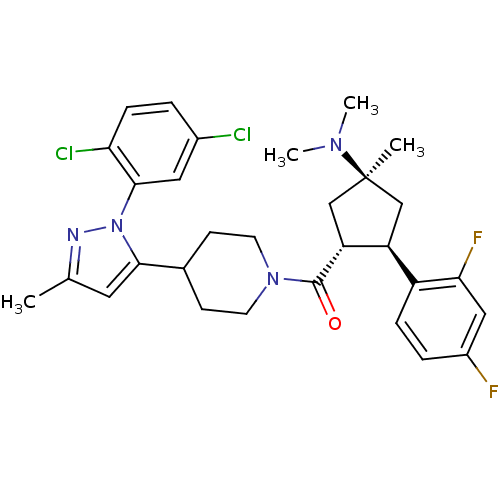
(Mus musculus) | BDBM50383427
(CHEMBL2031588)Show SMILES CN(C)[C@]1(C)C[C@H]([C@@H](C1)c1ccc(F)cc1F)C(=O)N1CCC(CC1)c1cc(C)nn1-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C30H34Cl2F2N4O/c1-18-13-27(38(35-18)28-14-20(31)5-8-25(28)32)19-9-11-37(12-10-19)29(39)24-17-30(2,36(3)4)16-23(24)22-7-6-21(33)15-26(22)34/h5-8,13-15,19,23-24H,9-12,16-17H2,1-4H3/t23-,24+,30-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse PrCP |
Bioorg Med Chem Lett 22: 2818-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.02.077
BindingDB Entry DOI: 10.7270/Q2J967D7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data