 Found 476 hits with Last Name = 'bardelle' and Initial = 'c'
Found 476 hits with Last Name = 'bardelle' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079
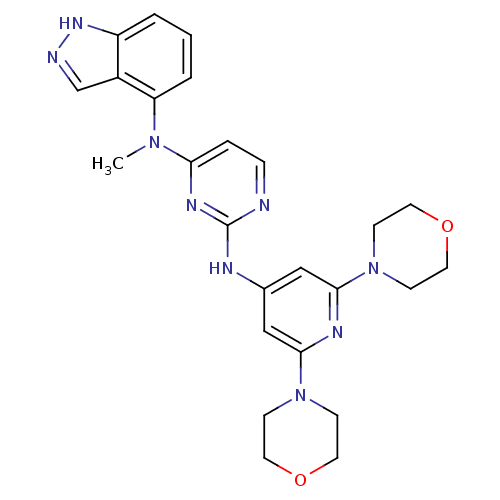
(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293243
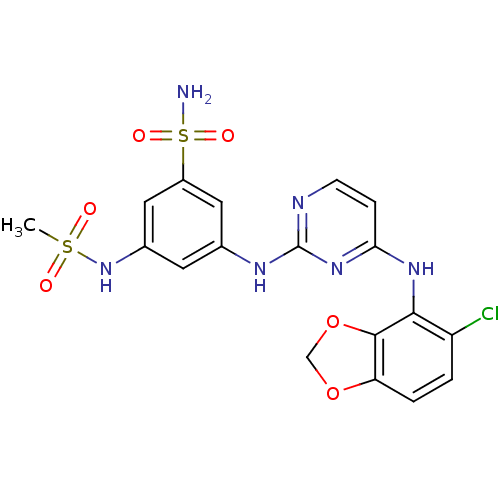
(3-(4-(5-chlorobenzo[d][1,3]dioxol-4-ylamino)pyrimi...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)S(N)(=O)=O Show InChI InChI=1S/C18H17ClN6O6S2/c1-32(26,27)25-11-6-10(7-12(8-11)33(20,28)29)22-18-21-5-4-15(24-18)23-16-13(19)2-3-14-17(16)31-9-30-14/h2-8,25H,9H2,1H3,(H2,20,28,29)(H2,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081185
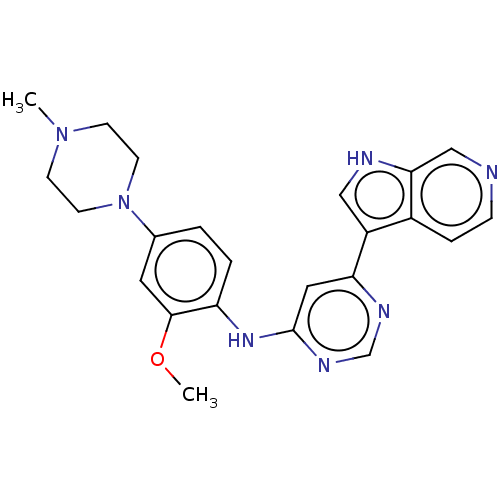
(CHEMBL3421963)Show SMILES COc1cc(ccc1Nc1cc(ncn1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-7-9-30(10-8-29)16-3-4-19(22(11-16)31-2)28-23-12-20(26-15-27-23)18-13-25-21-14-24-6-5-17(18)21/h3-6,11-15,25H,7-10H2,1-2H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070
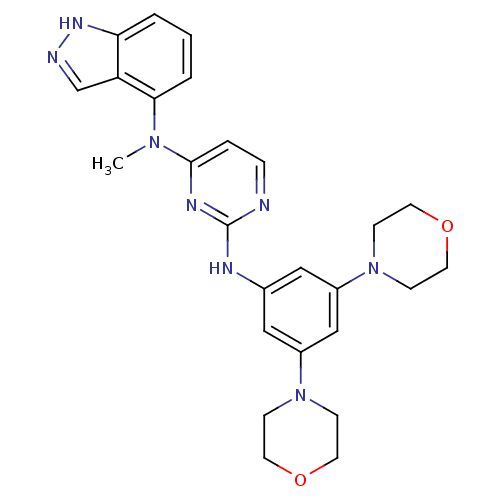
(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293244

(CHEMBL523744 | N-(3-(4-(5-chlorobenzo[d][1,3]dioxo...)Show SMILES CS(=O)(=O)Nc1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H23ClN6O5S/c1-35(30,31)28-15-10-14(11-16(12-15)29-6-8-32-9-7-29)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)34-13-33-18/h2-5,10-12,28H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340570

((2-chloro-5-((2-(3,5-dimorpholinophenylamino)pyrim...)Show SMILES CN(c1ccc(Cl)c(CO)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H31ClN6O3/c1-31(21-2-3-24(27)19(14-21)18-34)25-4-5-28-26(30-25)29-20-15-22(32-6-10-35-11-7-32)17-23(16-20)33-8-12-36-13-9-33/h2-5,14-17,34H,6-13,18H2,1H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247
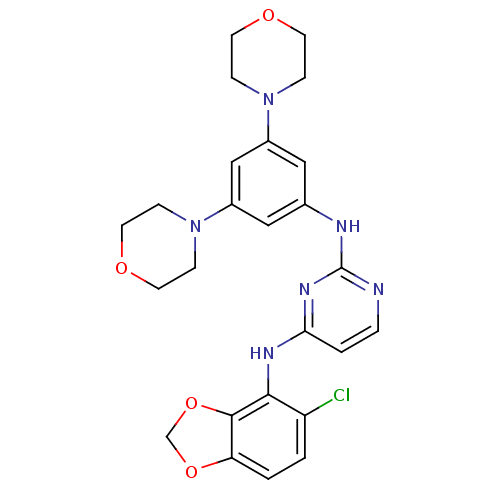
(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340551
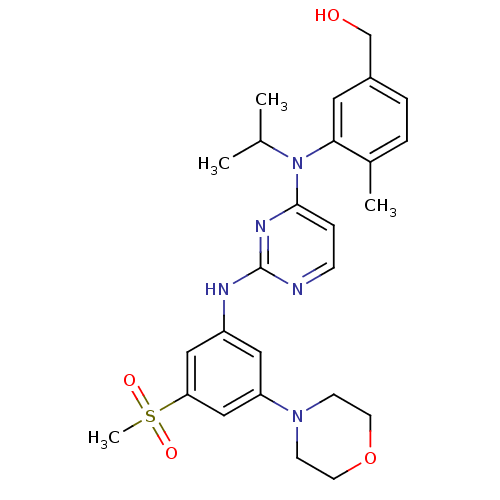
((3-(isopropyl(2-(3-(methylsulfonyl)-5-morpholinoph...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N5O4S/c1-18(2)31(24-13-20(17-32)6-5-19(24)3)25-7-8-27-26(29-25)28-21-14-22(30-9-11-35-12-10-30)16-23(15-21)36(4,33)34/h5-8,13-16,18,32H,9-12,17H2,1-4H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340566

(CHEMBL1762535 | N2-(3,5-dimorpholinophenyl)-N4-(5-...)Show SMILES COc1cncc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H31N7O3/c1-30(22-16-23(33-2)18-26-17-22)24-3-4-27-25(29-24)28-19-13-20(31-5-9-34-10-6-31)15-21(14-19)32-7-11-35-12-8-32/h3-4,13-18H,5-12H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293242
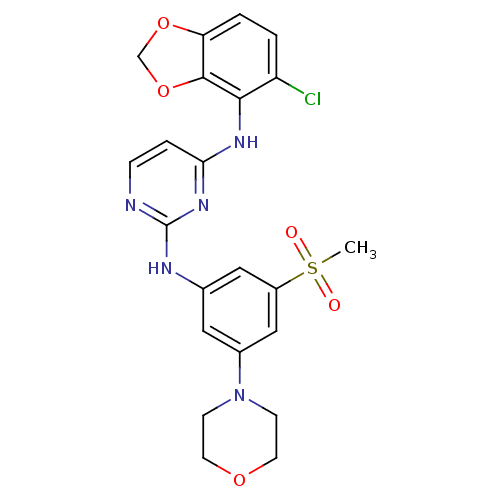
(CHEMBL497198 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES CS(=O)(=O)c1cc(Nc2nccc(Nc3c4OCOc4ccc3Cl)n2)cc(c1)N1CCOCC1 Show InChI InChI=1S/C22H22ClN5O5S/c1-34(29,30)16-11-14(10-15(12-16)28-6-8-31-9-7-28)25-22-24-5-4-19(27-22)26-20-17(23)2-3-18-21(20)33-13-32-18/h2-5,10-12H,6-9,13H2,1H3,(H2,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 |
Bioorg Med Chem Lett 18: 5717-21 (2009)
Article DOI: 10.1016/j.bmcl.2008.09.087
BindingDB Entry DOI: 10.7270/Q2N016JJ |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340572
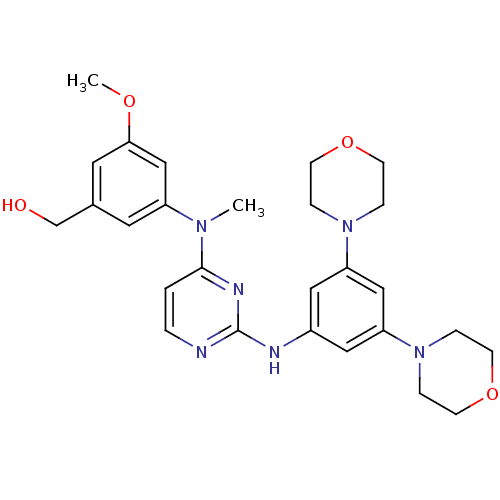
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES COc1cc(CO)cc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O4/c1-31(22-13-20(19-34)14-25(18-22)35-2)26-3-4-28-27(30-26)29-21-15-23(32-5-9-36-10-6-32)17-24(16-21)33-7-11-37-12-8-33/h3-4,13-18,34H,5-12,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340576

(CHEMBL1762525 | N2-(3,5-dimorpholinophenyl)-N4-(3-...)Show SMILES COc1cccc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(21-4-3-5-24(19-21)33-2)25-6-7-27-26(29-25)28-20-16-22(31-8-12-34-13-9-31)18-23(17-20)32-10-14-35-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081186
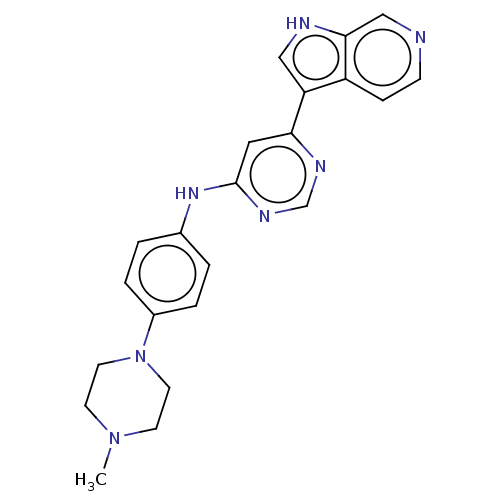
(CHEMBL3421962)Show SMILES CN1CCN(CC1)c1ccc(Nc2cc(ncn2)-c2c[nH]c3cnccc23)cc1 Show InChI InChI=1S/C22H23N7/c1-28-8-10-29(11-9-28)17-4-2-16(3-5-17)27-22-12-20(25-15-26-22)19-13-24-21-14-23-7-6-18(19)21/h2-7,12-15,24H,8-11H2,1H3,(H,25,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329080

(CHEMBL1270378 | N2-(2,6-dimorpholinopyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(nc(n2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N10O2/c1-32(19-4-2-3-18-17(19)16-26-31-18)21-5-6-25-23(29-21)27-20-15-22(33-7-11-35-12-8-33)30-24(28-20)34-9-13-36-14-10-34/h2-6,15-16H,7-14H2,1H3,(H,26,31)(H,25,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081174
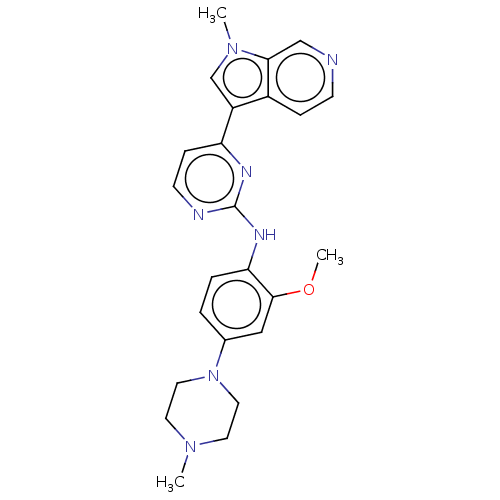
(CHEMBL3421968)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-29-10-12-31(13-11-29)17-4-5-21(23(14-17)32-3)28-24-26-9-7-20(27-24)19-16-30(2)22-15-25-8-6-18(19)22/h4-9,14-16H,10-13H2,1-3H3,(H,26,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340555
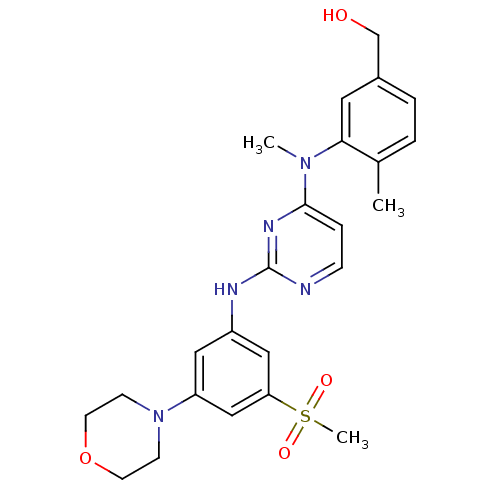
((4-methyl-3-(methyl(2-(3-(methylsulfonyl)-5-morpho...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C24H29N5O4S/c1-17-4-5-18(16-30)12-22(17)28(2)23-6-7-25-24(27-23)26-19-13-20(29-8-10-33-11-9-29)15-21(14-19)34(3,31)32/h4-7,12-15,30H,8-11,16H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081188
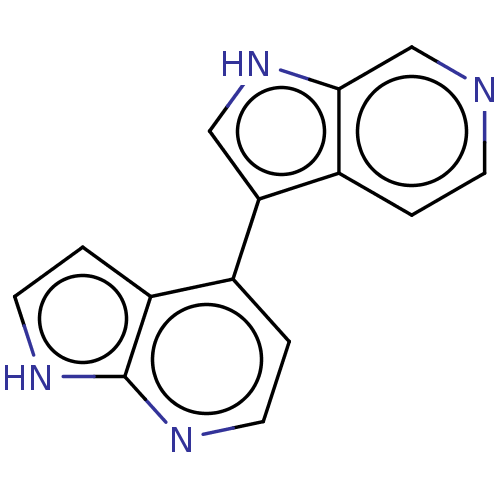
(CHEMBL3421981)Show InChI InChI=1S/C14H10N4/c1-4-15-8-13-10(1)12(7-18-13)9-2-5-16-14-11(9)3-6-17-14/h1-8,18H,(H,16,17) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340562
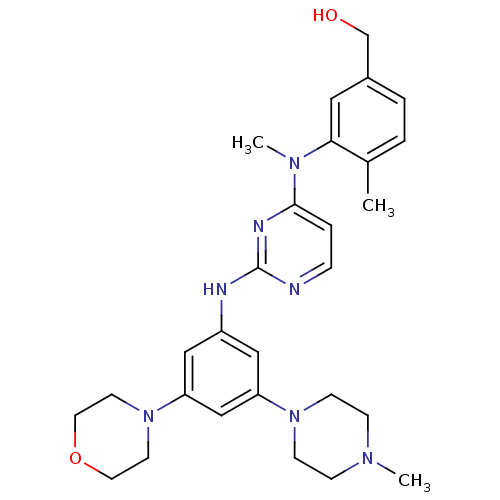
((4-methyl-3-(methyl(2-(3-(4-methylpiperazin-1-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCN(C)CC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H37N7O2/c1-21-4-5-22(20-36)16-26(21)33(3)27-6-7-29-28(31-27)30-23-17-24(34-10-8-32(2)9-11-34)19-25(18-23)35-12-14-37-15-13-35/h4-7,16-19,36H,8-15,20H2,1-3H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340554
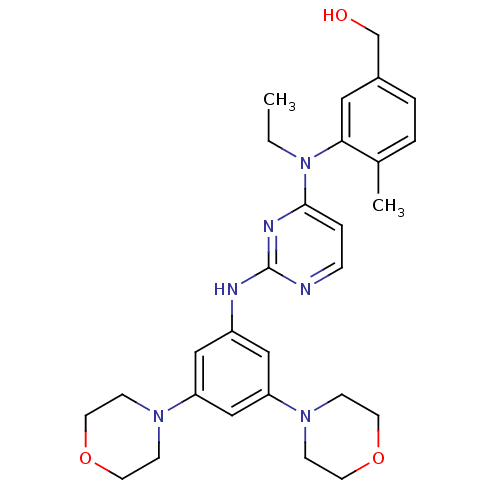
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CCN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H36N6O3/c1-3-34(26-16-22(20-35)5-4-21(26)2)27-6-7-29-28(31-27)30-23-17-24(32-8-12-36-13-9-32)19-25(18-23)33-10-14-37-15-11-33/h4-7,16-19,35H,3,8-15,20H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340571
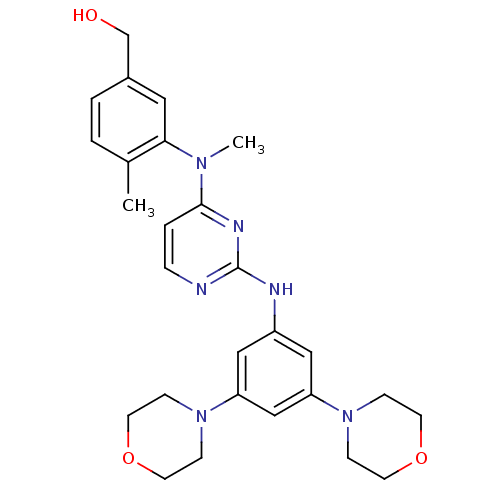
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C27H34N6O3/c1-20-3-4-21(19-34)15-25(20)31(2)26-5-6-28-27(30-26)29-22-16-23(32-7-11-35-12-8-32)18-24(17-22)33-9-13-36-14-10-33/h3-6,15-18,34H,7-14,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50293247

(CHEMBL468970 | N4-(5-chlorobenzo[d][1,3]dioxol-4-y...)Show SMILES Clc1ccc2OCOc2c1Nc1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H27ClN6O4/c26-20-1-2-21-24(36-16-35-21)23(20)29-22-3-4-27-25(30-22)28-17-13-18(31-5-9-33-10-6-31)15-19(14-17)32-7-11-34-12-8-32/h1-4,13-15H,5-12,16H2,(H2,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 by acoustic dispensing assay |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081181

(CHEMBL3421967)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnccc12)N1CCN(C)CC1 Show InChI InChI=1S/C23H25N7O/c1-29-9-11-30(12-10-29)16-3-4-20(22(13-16)31-2)28-23-25-8-6-19(27-23)18-14-26-21-15-24-7-5-17(18)21/h3-8,13-15,26H,9-12H2,1-2H3,(H,25,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081180
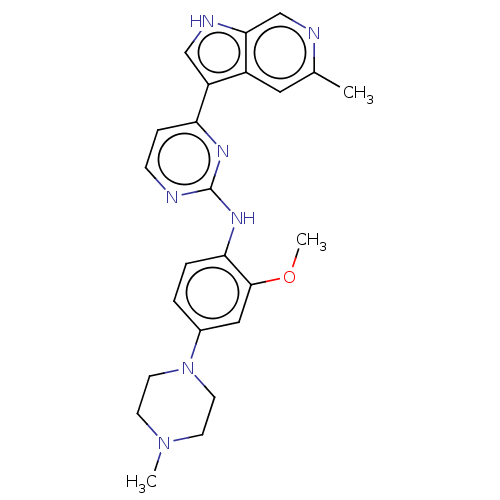
(CHEMBL3421969)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnc(C)cc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-16-12-18-19(14-27-22(18)15-26-16)20-6-7-25-24(28-20)29-21-5-4-17(13-23(21)32-3)31-10-8-30(2)9-11-31/h4-7,12-15,27H,8-11H2,1-3H3,(H,25,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340553

((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C29H38N6O3/c1-21(2)35(27-16-23(20-36)5-4-22(27)3)28-6-7-30-29(32-28)31-24-17-25(33-8-12-37-13-9-33)19-26(18-24)34-10-14-38-15-11-34/h4-7,16-19,21,36H,8-15,20H2,1-3H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329079

(CHEMBL1270280 | N2-(2,6-dimorpholinopyridin-4-yl)-...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C25H29N9O2/c1-32(21-4-2-3-20-19(21)17-27-31-20)22-5-6-26-25(30-22)28-18-15-23(33-7-11-35-12-8-33)29-24(16-18)34-9-13-36-14-10-34/h2-6,15-17H,7-14H2,1H3,(H,27,31)(H,26,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081179

(CHEMBL3421970)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2c(C)nccc12)N1CCN(C)CC1 Show InChI InChI=1S/C24H27N7O/c1-16-23-18(6-8-25-16)19(15-27-23)20-7-9-26-24(28-20)29-21-5-4-17(14-22(21)32-3)31-12-10-30(2)11-13-31/h4-9,14-15,27H,10-13H2,1-3H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340556
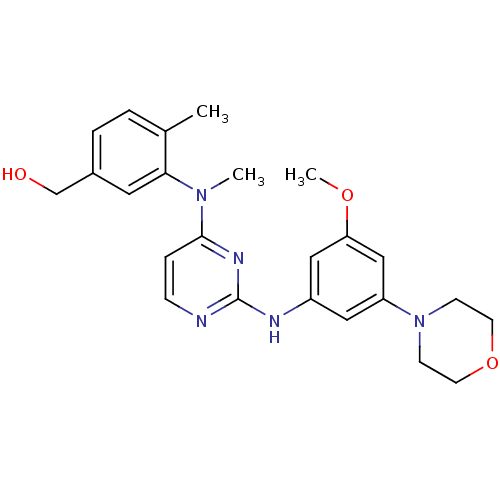
((3-((2-(3-methoxy-5-morpholinophenylamino)pyrimidi...)Show SMILES COc1cc(Nc2nccc(n2)N(C)c2cc(CO)ccc2C)cc(c1)N1CCOCC1 Show InChI InChI=1S/C24H29N5O3/c1-17-4-5-18(16-30)12-22(17)28(2)23-6-7-25-24(27-23)26-19-13-20(15-21(14-19)31-3)29-8-10-32-11-9-29/h4-7,12-15,30H,8-11,16H2,1-3H3,(H,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340560
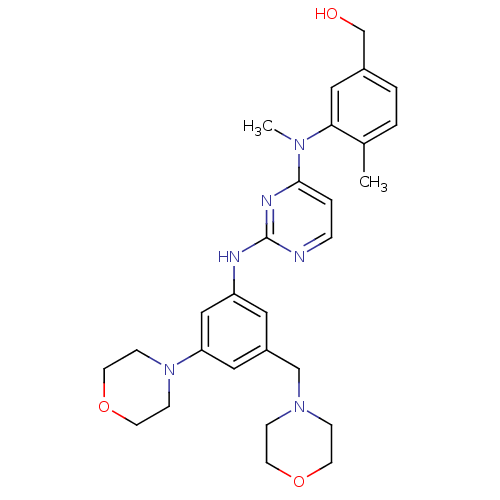
((4-methyl-3-(methyl(2-(3-morpholino-5-(morpholinom...)Show SMILES CN(c1ccnc(Nc2cc(CN3CCOCC3)cc(c2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H36N6O3/c1-21-3-4-22(20-35)17-26(21)32(2)27-5-6-29-28(31-27)30-24-15-23(19-33-7-11-36-12-8-33)16-25(18-24)34-9-13-37-14-10-34/h3-6,15-18,35H,7-14,19-20H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340577

(CHEMBL1762524 | N4-(3-chlorophenyl)-N2-(3,5-dimorp...)Show SMILES CN(c1cccc(Cl)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H29ClN6O2/c1-30(21-4-2-3-19(26)15-21)24-5-6-27-25(29-24)28-20-16-22(31-7-11-33-12-8-31)18-23(17-20)32-9-13-34-14-10-32/h2-6,15-18H,7-14H2,1H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340567
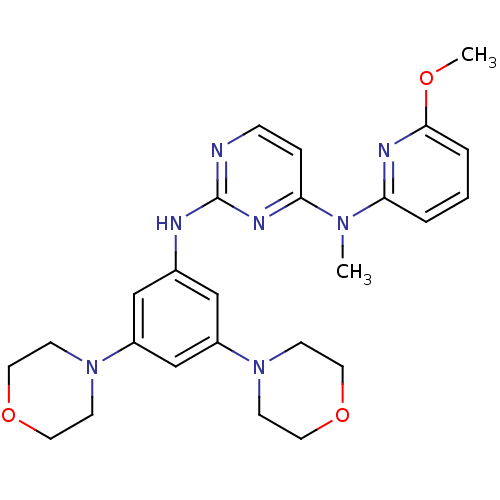
(CHEMBL1762534 | N2-(3,5-dimorpholinophenyl)-N4-(6-...)Show SMILES COc1cccc(n1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C25H31N7O3/c1-30(22-4-3-5-24(28-22)33-2)23-6-7-26-25(29-23)27-19-16-20(31-8-12-34-13-9-31)18-21(17-19)32-10-14-35-15-11-32/h3-7,16-18H,8-15H2,1-2H3,(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584925
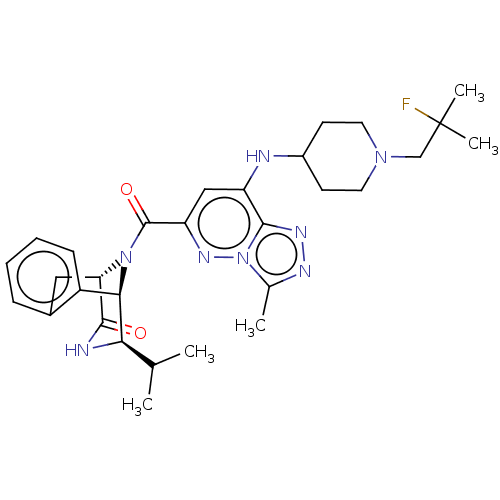
(CHEMBL5079885)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(C)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:3.8.2:13.12.11| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (981 to 1108 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340551

((3-(isopropyl(2-(3-(methylsulfonyl)-5-morpholinoph...)Show SMILES CC(C)N(c1ccnc(Nc2cc(cc(c2)S(C)(=O)=O)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N5O4S/c1-18(2)31(24-13-20(17-32)6-5-19(24)3)25-7-8-27-26(29-25)28-21-14-22(30-9-11-35-12-10-30)16-23(15-21)36(4,33)34/h5-8,13-16,18,32H,9-12,17H2,1-4H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340572

((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES COc1cc(CO)cc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O4/c1-31(22-13-20(19-34)14-25(18-22)35-2)26-3-4-28-27(30-26)29-21-15-23(32-5-9-36-10-6-32)17-24(16-21)33-7-11-37-12-8-33/h3-4,13-18,34H,5-12,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340561

(4-(3-(4-((5-(hydroxymethyl)-2-methylphenyl)(methyl...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCN(O)CC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C27H35N7O3/c1-20-3-4-21(19-35)15-25(20)31(2)26-5-6-28-27(30-26)29-22-16-23(32-7-9-34(36)10-8-32)18-24(17-22)33-11-13-37-14-12-33/h3-6,15-18,35-36H,7-14,19H2,1-2H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
ATPase family AAA domain-containing protein 2
(Homo sapiens (Human)) | BDBM50584925

(CHEMBL5079885)Show SMILES [H][C@]12Cc3ccccc3[C@]([H])([C@H](NC1=O)C(C)C)N2C(=O)c1cc(NC2CCN(CC(C)(C)F)CC2)c2nnc(C)n2n1 |r,THB:19:18:3.8.2:13.12.11| | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of GST-ATAD2 BD (951 to 1132 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) and biotinylated tetra-acetylated histone... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01871
BindingDB Entry DOI: 10.7270/Q2280CH8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081191

(CHEMBL3421978)Show InChI InChI=1S/C19H14N4/c1-2-4-14(5-3-1)6-7-19-21-11-9-17(23-19)16-12-22-18-13-20-10-8-15(16)18/h1-13,22H/b7-6+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081189
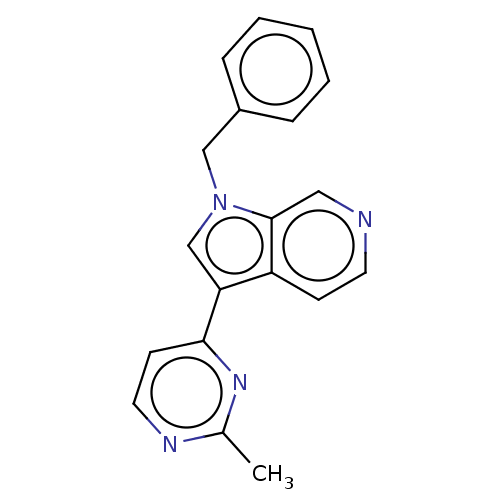
(CHEMBL3421980)Show InChI InChI=1S/C19H16N4/c1-14-21-10-8-18(22-14)17-13-23(12-15-5-3-2-4-6-15)19-11-20-9-7-16(17)19/h2-11,13H,12H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340558
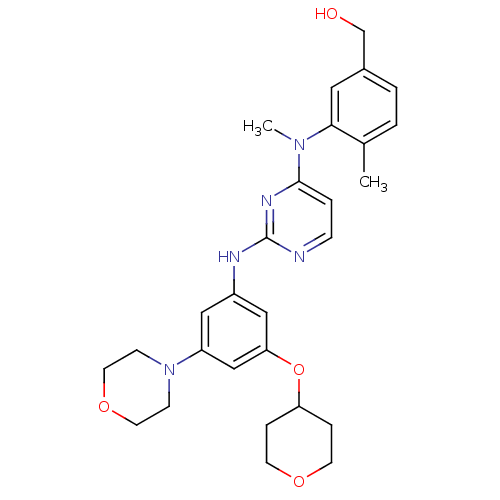
((4-methyl-3-(methyl(2-(3-morpholino-5-(tetrahydro-...)Show SMILES CN(c1ccnc(Nc2cc(OC3CCOCC3)cc(c2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H35N5O4/c1-20-3-4-21(19-34)15-26(20)32(2)27-5-8-29-28(31-27)30-22-16-23(33-9-13-36-14-10-33)18-25(17-22)37-24-6-11-35-12-7-24/h3-5,8,15-18,24,34H,6-7,9-14,19H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340578
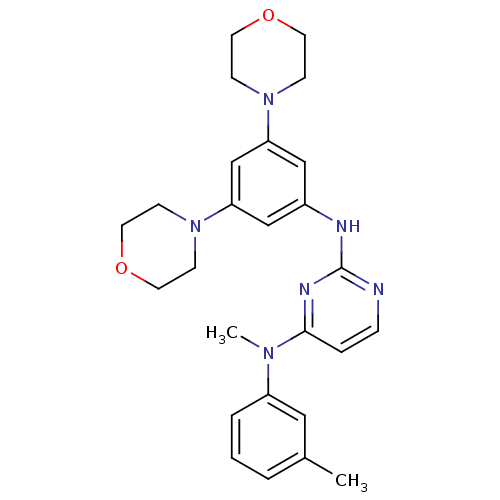
(CHEMBL1762523 | N2-(3,5-dimorpholinophenyl)-N4-met...)Show SMILES CN(c1cccc(C)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O2/c1-20-4-3-5-22(16-20)30(2)25-6-7-27-26(29-25)28-21-17-23(31-8-12-33-13-9-31)19-24(18-21)32-10-14-34-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340557

((3-((2-(3-(2-methoxyethoxy)-5-morpholinophenylamin...)Show SMILES COCCOc1cc(Nc2nccc(n2)N(C)c2cc(CO)ccc2C)cc(c1)N1CCOCC1 Show InChI InChI=1S/C26H33N5O4/c1-19-4-5-20(18-32)14-24(19)30(2)25-6-7-27-26(29-25)28-21-15-22(31-8-10-34-11-9-31)17-23(16-21)35-13-12-33-3/h4-7,14-17,32H,8-13,18H2,1-3H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340550

((3-((2-(2,6-dimorpholinopyridin-4-ylamino)pyrimidi...)Show SMILES CN(c1ccnc(Nc2cc(nc(c2)N2CCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C26H33N7O3/c1-19-3-4-20(18-34)15-22(19)31(2)23-5-6-27-26(30-23)28-21-16-24(32-7-11-35-12-8-32)29-25(17-21)33-9-13-36-14-10-33/h3-6,15-17,34H,7-14,18H2,1-2H3,(H,27,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340563
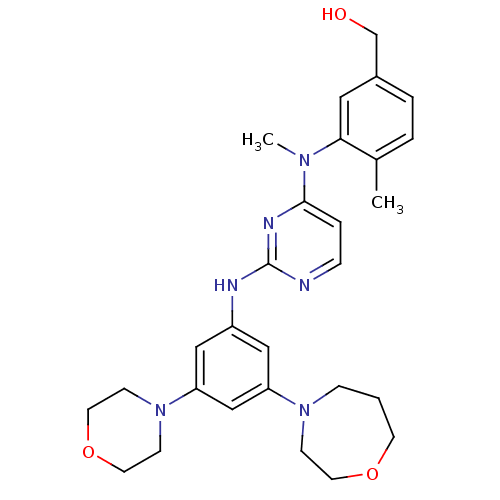
((4-methyl-3-(methyl(2-(3-morpholino-5-(1,4-oxazepa...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCCOCC2)N2CCOCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H36N6O3/c1-21-4-5-22(20-35)16-26(21)32(2)27-6-7-29-28(31-27)30-23-17-24(33-8-3-12-36-13-9-33)19-25(18-23)34-10-14-37-15-11-34/h4-7,16-19,35H,3,8-15,20H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50329070

(CHEMBL1269858 | N2-(3,5-dimorpholinophenyl)-N4-(1H...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1cccc2[nH]ncc12 Show InChI InChI=1S/C26H30N8O2/c1-32(24-4-2-3-23-22(24)18-28-31-23)25-5-6-27-26(30-25)29-19-15-20(33-7-11-35-12-8-33)17-21(16-19)34-9-13-36-14-10-34/h2-6,15-18H,7-14H2,1H3,(H,28,31)(H,27,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 20: 6242-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.100
BindingDB Entry DOI: 10.7270/Q2QR4XCB |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081178
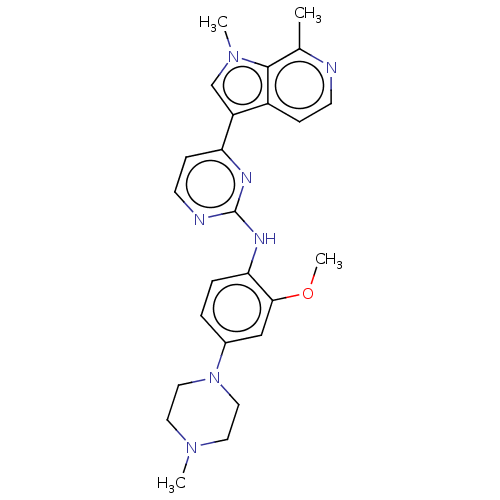
(CHEMBL3421971)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1cn(C)c2c(C)nccc12)N1CCN(C)CC1 Show InChI InChI=1S/C25H29N7O/c1-17-24-19(7-9-26-17)20(16-31(24)3)21-8-10-27-25(28-21)29-22-6-5-18(15-23(22)33-4)32-13-11-30(2)12-14-32/h5-10,15-16H,11-14H2,1-4H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50081182

(CHEMBL3421966)Show SMILES COc1cc(ccc1Nc1nccc(n1)-c1c[nH]c2cnccc12)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C24H25N7O2/c1-16(32)30-9-11-31(12-10-30)17-3-4-21(23(13-17)33-2)29-24-26-8-6-20(28-24)19-14-27-22-15-25-7-5-18(19)22/h3-8,13-15,27H,9-12H2,1-2H3,(H,26,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Dyrk1B (unknown origin) using FITC-Asp-His-Thr-Gly-Phe-Leu-Thr-Glu-Tyr-Val-Ala-Thr-Arg-NH2 as substrate after 60 mins |
J Med Chem 58: 2834-44 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00098
BindingDB Entry DOI: 10.7270/Q23B61VW |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340576

(CHEMBL1762525 | N2-(3,5-dimorpholinophenyl)-N4-(3-...)Show SMILES COc1cccc(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(21-4-3-5-24(19-21)33-2)25-6-7-27-26(29-25)28-20-16-22(31-8-12-34-13-9-31)18-23(17-20)32-10-14-35-15-11-32/h3-7,16-19H,8-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EphB4 autophosphorylation expressed in CHOK1 cells |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340564
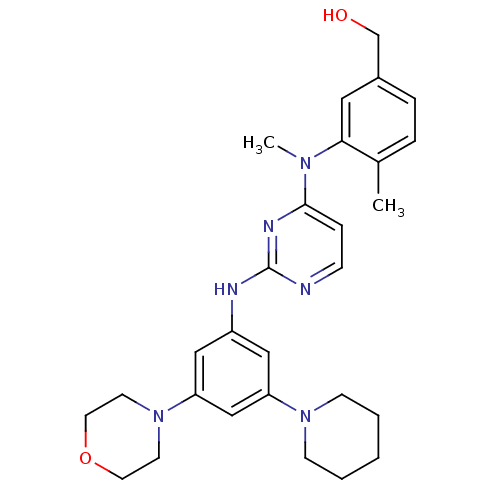
((4-methyl-3-(methyl(2-(3-morpholino-5-(piperidin-1...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCCCC2)n1)c1cc(CO)ccc1C Show InChI InChI=1S/C28H36N6O2/c1-21-6-7-22(20-35)16-26(21)32(2)27-8-9-29-28(31-27)30-23-17-24(33-10-4-3-5-11-33)19-25(18-23)34-12-14-36-15-13-34/h6-9,16-19,35H,3-5,10-15,20H2,1-2H3,(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340573
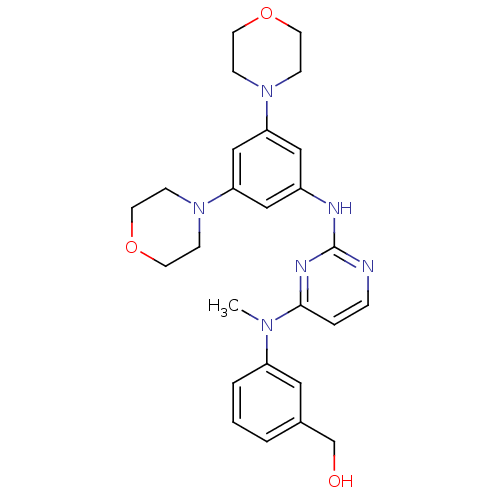
((3-((2-(3,5-dimorpholinophenylamino)pyrimidin-4-yl...)Show SMILES CN(c1cccc(CO)c1)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C26H32N6O3/c1-30(22-4-2-3-20(15-22)19-33)25-5-6-27-26(29-25)28-21-16-23(31-7-11-34-12-8-31)18-24(17-21)32-9-13-35-14-10-32/h2-6,15-18,33H,7-14,19H2,1H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340574
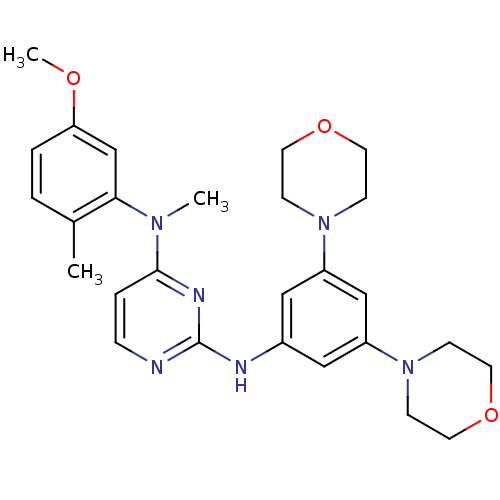
(CHEMBL1762527 | N2-(3,5-dimorpholinophenyl)-N4-(5-...)Show SMILES COc1ccc(C)c(c1)N(C)c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1 Show InChI InChI=1S/C27H34N6O3/c1-20-4-5-24(34-3)19-25(20)31(2)26-6-7-28-27(30-26)29-21-16-22(32-8-12-35-13-9-32)18-23(17-21)33-10-14-36-15-11-33/h4-7,16-19H,8-15H2,1-3H3,(H,28,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |
Ephrin type-B receptor 4
(Homo sapiens (Human)) | BDBM50340579
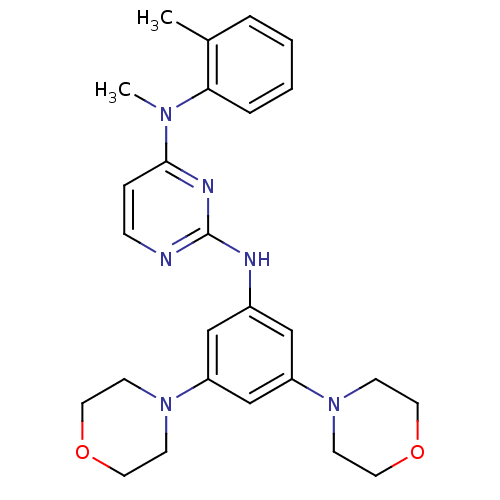
(CHEMBL1762522 | N2-(3,5-dimorpholinophenyl)-N4-met...)Show SMILES CN(c1ccnc(Nc2cc(cc(c2)N2CCOCC2)N2CCOCC2)n1)c1ccccc1C Show InChI InChI=1S/C26H32N6O2/c1-20-5-3-4-6-24(20)30(2)25-7-8-27-26(29-25)28-21-17-22(31-9-13-33-14-10-31)19-23(18-21)32-11-15-34-16-12-32/h3-8,17-19H,9-16H2,1-2H3,(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches
Curated by ChEMBL
| Assay Description
Inhibition of human EPHB4 |
Bioorg Med Chem Lett 21: 2207-11 (2011)
Article DOI: 10.1016/j.bmcl.2011.03.009
BindingDB Entry DOI: 10.7270/Q2PK0GGR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data