

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079375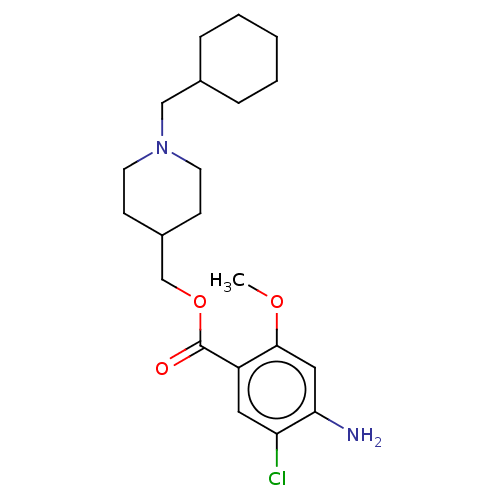 (CHEMBL3417008 | US9663465, 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079375 (CHEMBL3417008 | US9663465, 20) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079377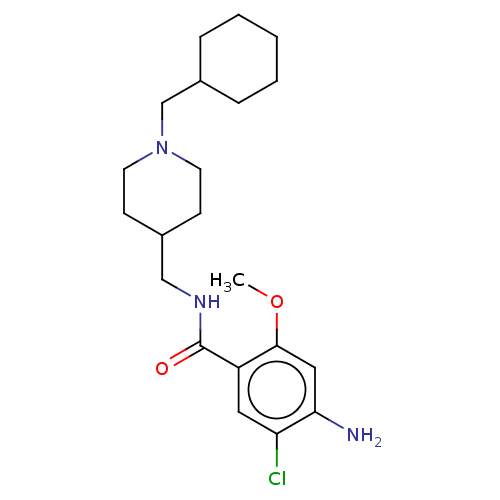 (CHEMBL3417007) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327460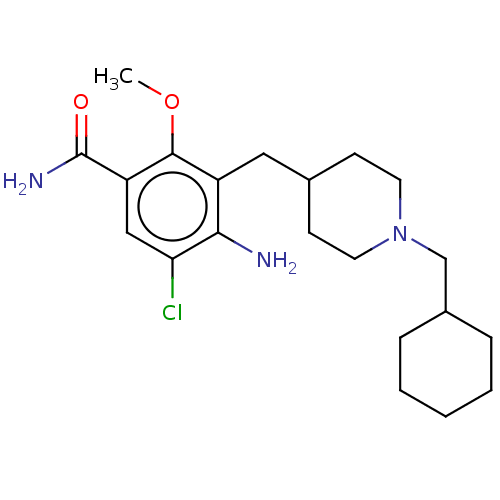 (4-amino-5-chloro-[[1-(cyclohexylmethyl)-4-piperidy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327466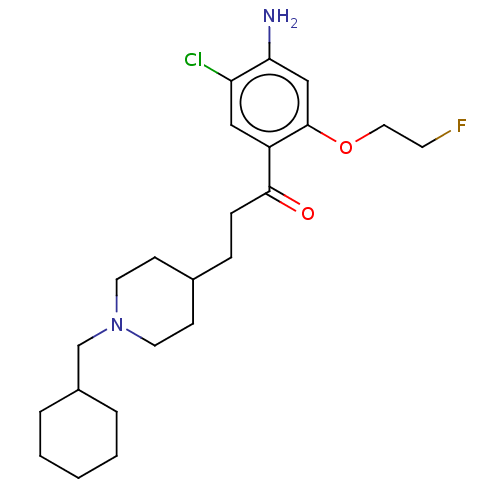 (1-[4-amino-5-chloro-2-(2-fluoroéthoxy)phenyl]-3-[1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327456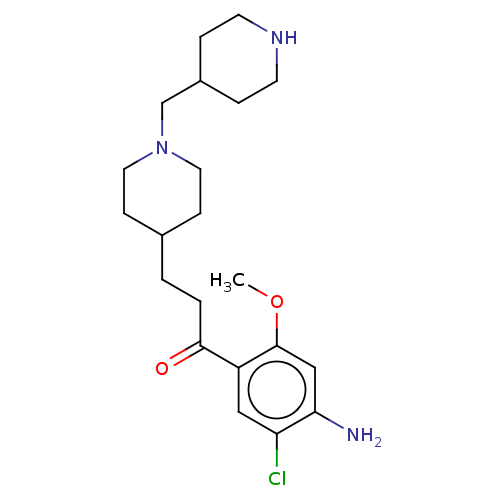 (1-(4-amino-5-chloro-2-methoxyphenyl)-3-[1-[(piperi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079367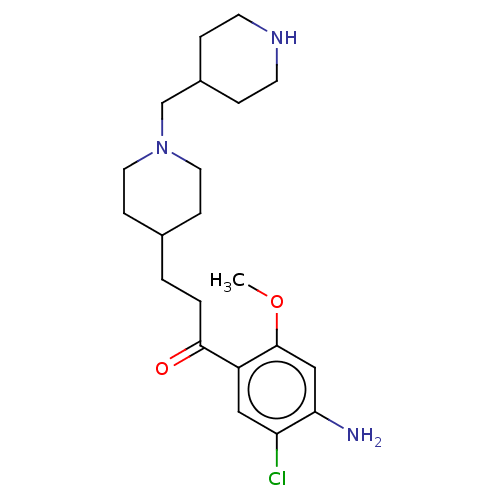 (CHEMBL3416998) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079365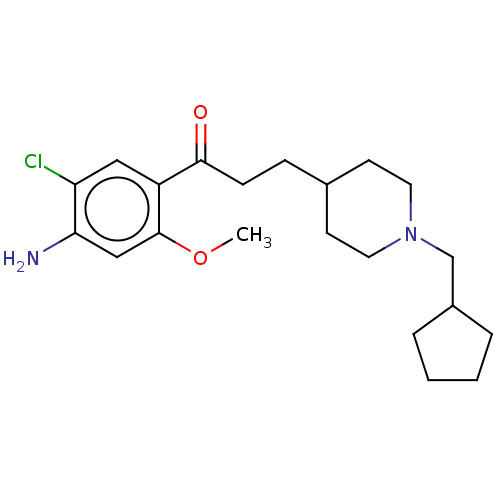 (CHEMBL3416996 | US9663465, 8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM28583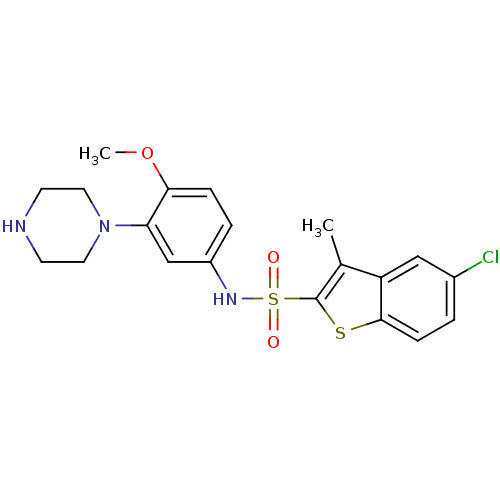 (5-chloro-N-[4-methoxy-3-(piperazin-1-yl)phenyl]-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in BHK cell membrane measured after 60 mins by scintillation counter method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079365 (CHEMBL3416996 | US9663465, 8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507212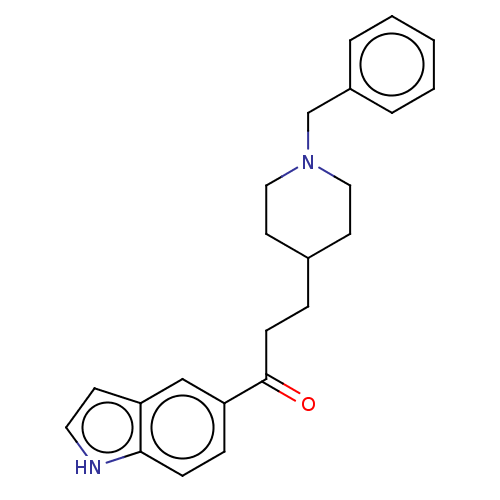 (CHEMBL4585990) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327448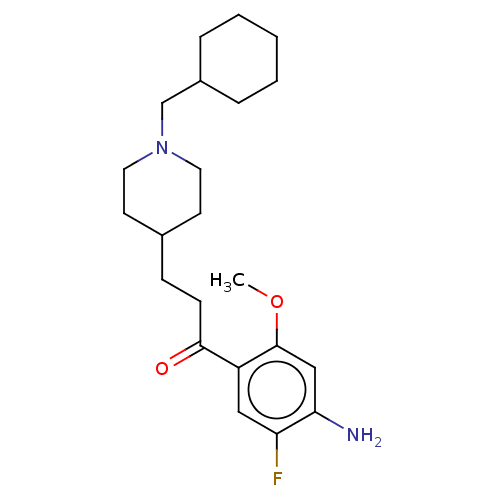 (1-(4-amino-5-fluoro-2-methoxyphenyl)-3-[1-(cyclohe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079366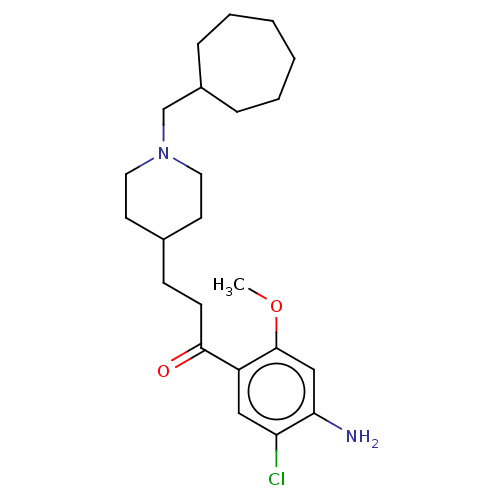 (CHEMBL3416997 | US9663465, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079366 (CHEMBL3416997 | US9663465, 12) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562651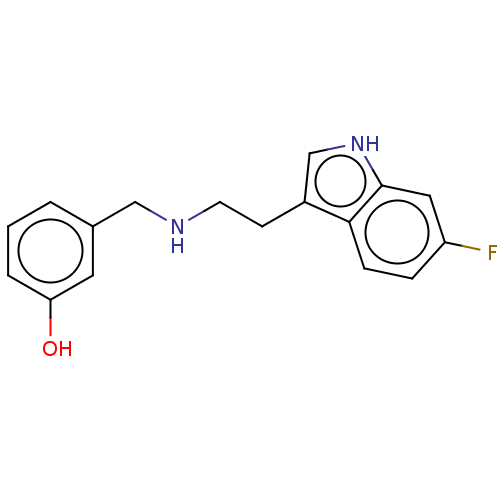 (CHEMBL4788250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507209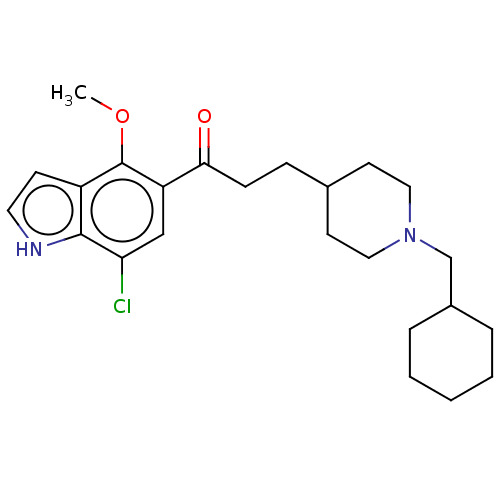 (CHEMBL4537101) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507211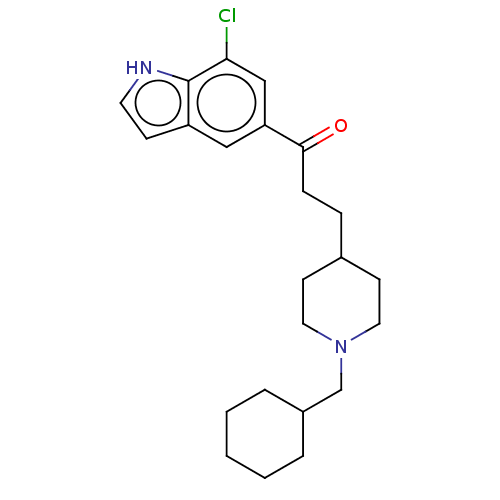 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327461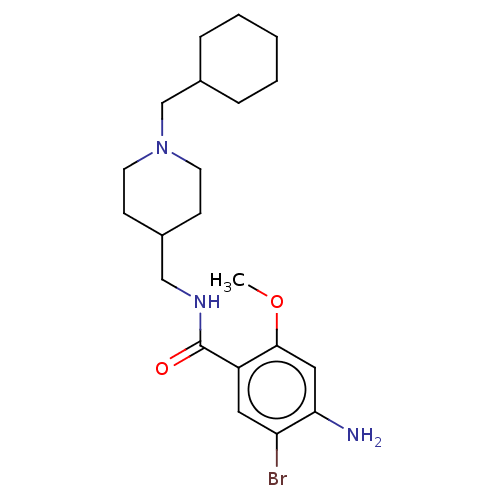 (4-amino-5-bromo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50507208 (CHEMBL4526050) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma-1 receptor in human Jurkat cell membranes after 2 hrs by liquid scintillation counting method | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515598 (CHEMBL4526049) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50019754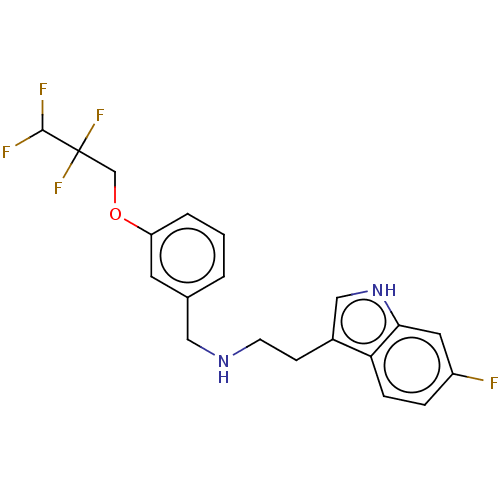 (IDALOPIRDINE | LU-AE58054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079369 (CHEMBL3417000 | US9663465, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079368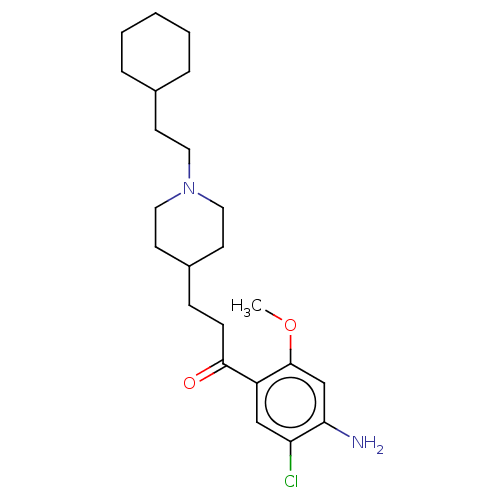 (CHEMBL3416999 | US9663465, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079368 (CHEMBL3416999 | US9663465, 16) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079364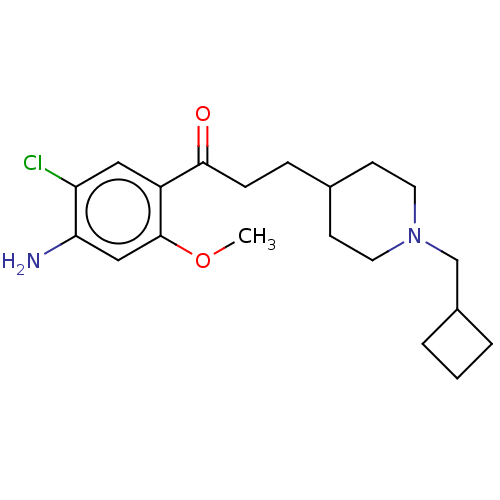 (CHEMBL3416995 | US9663465, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079364 (CHEMBL3416995 | US9663465, 7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327445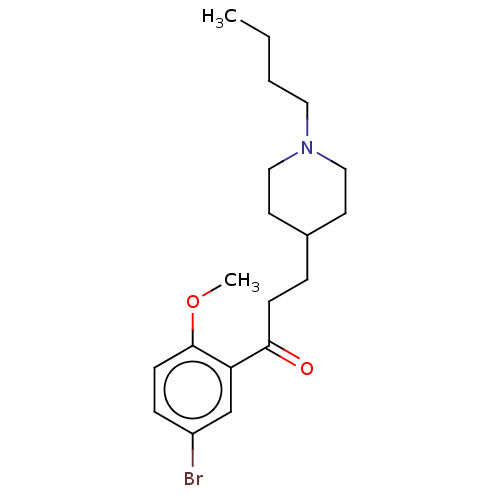 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-butyl-4-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM84950 (CAS_183782 | NSC_183782 | RS 67333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Agonist activity at 5-HT4R (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50562651 (CHEMBL4788250) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-LSD from human 5HT6 receptor expressed in HEK293 cell membrane measured after 60 mins | Citation and Details Article DOI: 10.1016/j.ejmech.2020.113059 BindingDB Entry DOI: 10.7270/Q2KP85V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327465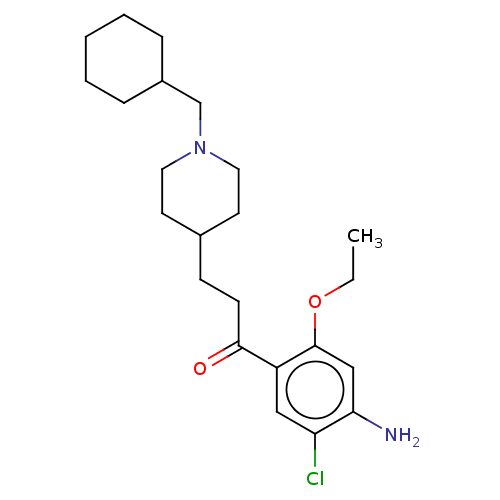 (1-(4-amino-5-chloro-2-éthoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 12.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50515600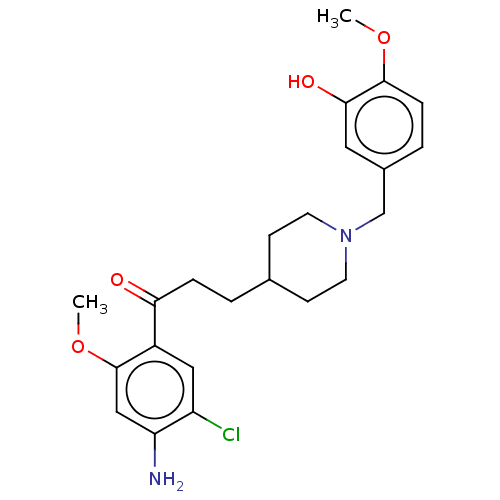 (CHEMBL4580044) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Binding affinity to recombinant human brain 5HT4 receptor by radio ligand binding assay | Eur J Med Chem 182: (2019) Article DOI: 10.1016/j.ejmech.2019.111596 BindingDB Entry DOI: 10.7270/Q2988BC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327467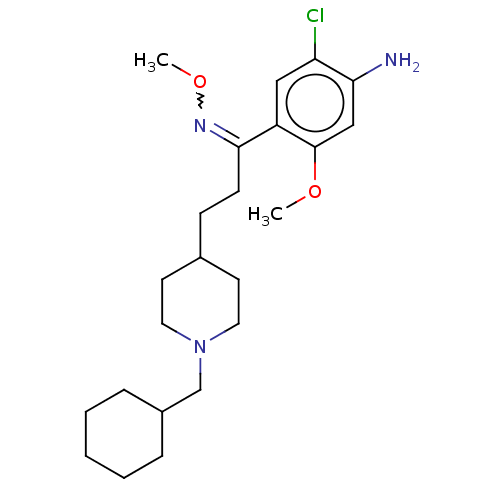 ( 2-chloro-4-[[2-[1-(cyclohexylmethyl)-4-piperidyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327462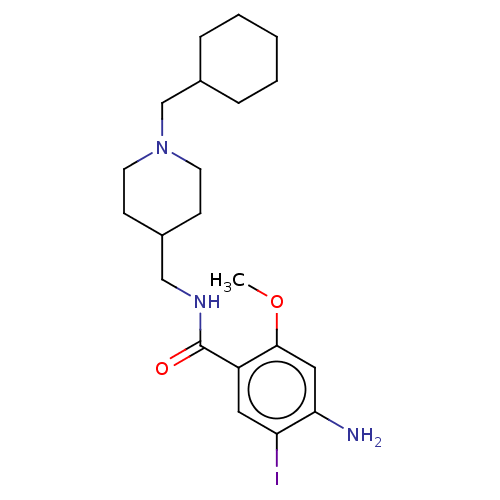 ( 4-amino-5-iodo-N-[[1-(cyclohexylmethyl)-4-piperid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079370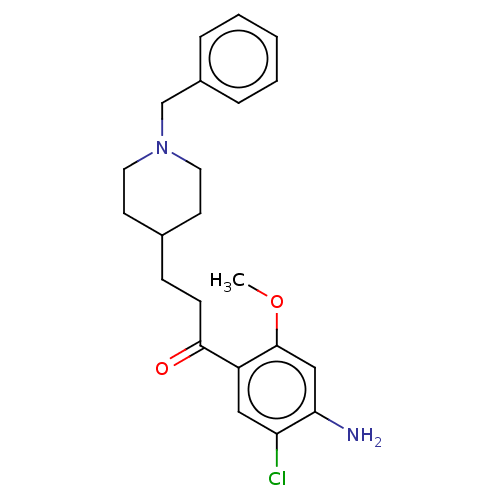 (CHEMBL3417001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50079363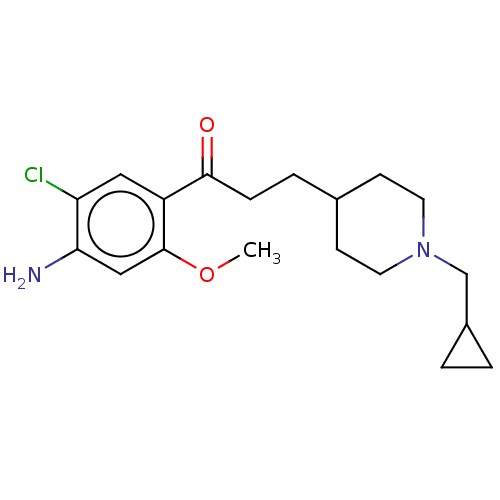 (CHEMBL3414597 | US9663465, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 14.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079363 (CHEMBL3414597 | US9663465, 6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50079362 (CHEMBL3417009 | US9663465, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Inhibition of AChE (unknown origin) | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327457 (1-(4-amino-5-bromo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM327458 ( 1-(4-amino-5-iodo-2-methoxyphenyl)-3-[1-(cyclohex...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITE DE CAEN US Patent | Assay Description Prior to these competition studies, a series of saturation curves were performed in order to check whether the pharmacological parameters Kd, Bmax an... | US Patent US9663465 (2017) BindingDB Entry DOI: 10.7270/Q23J3G2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079371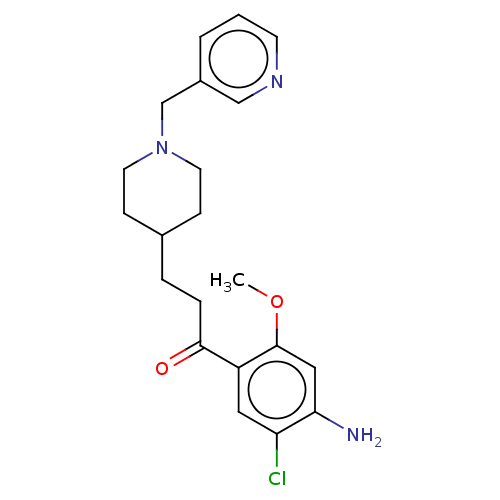 (CHEMBL3417002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (GUINEA PIG) | BDBM50079372 (CHEMBL3417003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CERMN Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from 5-HT4R in guinea pig brain membranes incubated for 30 mins by radioligand binding assay | J Med Chem 58: 3172-87 (2015) Article DOI: 10.1021/acs.jmedchem.5b00115 BindingDB Entry DOI: 10.7270/Q2HQ41MH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507215 (CHEMBL4562793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50507211 (CHEMBL4444843) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Normandie Univ Curated by ChEMBL | Assay Description Displacement of [3H]-GR113808 from recombinant human 5-HT4BR expressed in membranes after 60 mins | Eur J Med Chem 162: 234-248 (2019) Article DOI: 10.1016/j.ejmech.2018.10.064 BindingDB Entry DOI: 10.7270/Q2T72MRJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |