 Found 223 hits with Last Name = 'ohmori' and Initial = 'o'
Found 223 hits with Last Name = 'ohmori' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cathepsin K
(Homo sapiens (Human)) | BDBM50223939
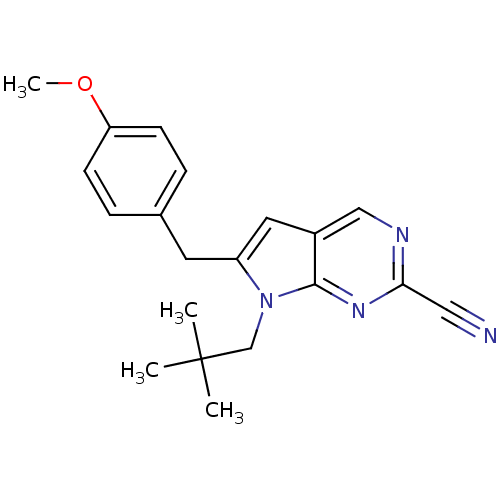
(6-(4-methoxybenzyl)-7-neopentyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H22N4O/c1-20(2,3)13-24-16(9-14-5-7-17(25-4)8-6-14)10-15-12-22-18(11-21)23-19(15)24/h5-8,10,12H,9,13H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223914
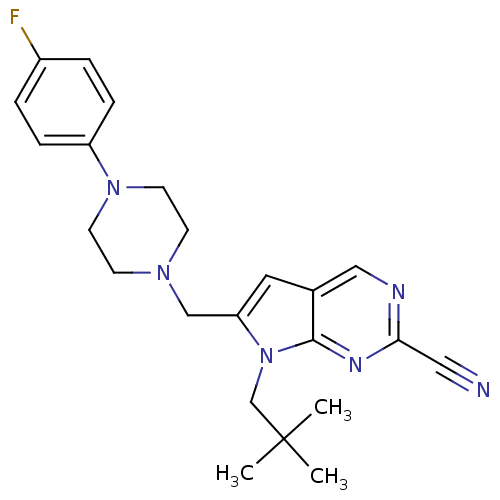
(6-((4-(4-fluorophenyl)piperazin-1-yl)methyl)-7-neo...)Show SMILES CC(C)(C)Cn1c(CN2CCN(CC2)c2ccc(F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C23H27FN6/c1-23(2,3)16-30-20(12-17-14-26-21(13-25)27-22(17)30)15-28-8-10-29(11-9-28)19-6-4-18(24)5-7-19/h4-7,12,14H,8-11,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223935
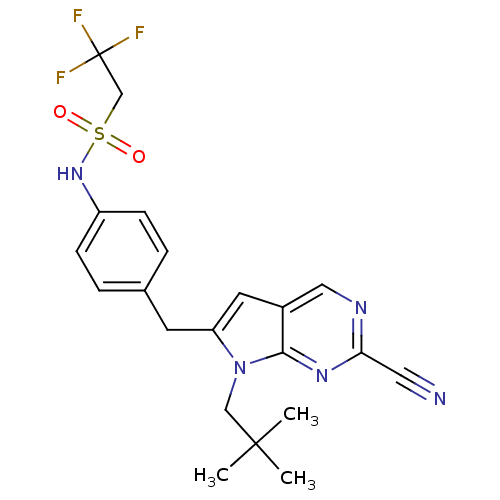
(CHEMBL399842 | N-(4-((2-cyano-7-neopentyl-7H-pyrro...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(NS(=O)(=O)CC(F)(F)F)cc2)cc2cnc(nc12)C#N Show InChI InChI=1S/C21H22F3N5O2S/c1-20(2,3)12-29-17(9-15-11-26-18(10-25)27-19(15)29)8-14-4-6-16(7-5-14)28-32(30,31)13-21(22,23)24/h4-7,9,11,28H,8,12-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223921
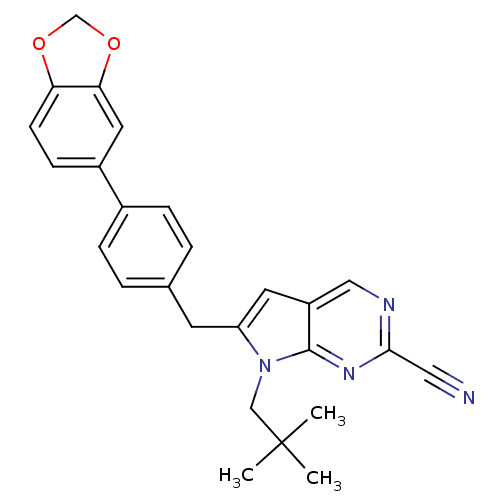
(6-(4-(benzo[d][1,3]dioxol-5-yl)benzyl)-7-neopentyl...)Show SMILES CC(C)(C)Cn1c(Cc2ccc(cc2)-c2ccc3OCOc3c2)cc2cnc(nc12)C#N Show InChI InChI=1S/C26H24N4O2/c1-26(2,3)15-30-21(11-20-14-28-24(13-27)29-25(20)30)10-17-4-6-18(7-5-17)19-8-9-22-23(12-19)32-16-31-22/h4-9,11-12,14H,10,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223915

(6-benzyl-7-neopentyl-7H-pyrrolo[2,3-d]pyrimidine-2...)Show InChI InChI=1S/C19H20N4/c1-19(2,3)13-23-16(9-14-7-5-4-6-8-14)10-15-12-21-17(11-20)22-18(15)23/h4-8,10,12H,9,13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223925
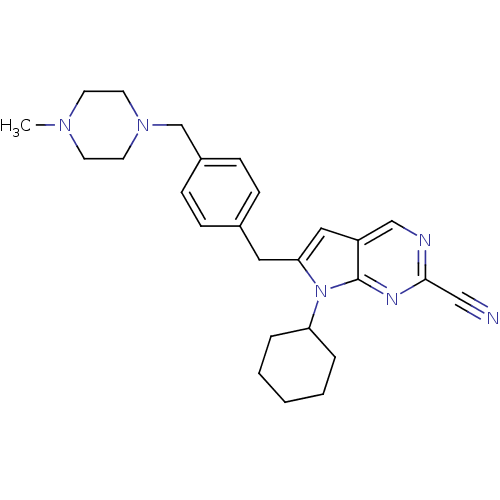
(6-(4-((4-methylpiperazin-1-yl)methyl)benzyl)-7-cyc...)Show SMILES CN1CCN(Cc2ccc(Cc3cc4cnc(nc4n3C3CCCCC3)C#N)cc2)CC1 Show InChI InChI=1S/C26H32N6/c1-30-11-13-31(14-12-30)19-21-9-7-20(8-10-21)15-24-16-22-18-28-25(17-27)29-26(22)32(24)23-5-3-2-4-6-23/h7-10,16,18,23H,2-6,11-15,19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223919
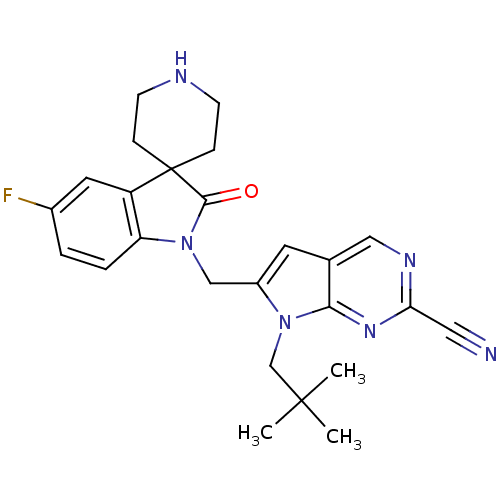
(7-(2,2-dimethylpropyl)-6-[(5-fluoro-2-oxospiro[ind...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)C3(CCNCC3)c3cc(F)ccc23)cc2cnc(nc12)C#N Show InChI InChI=1S/C25H27FN6O/c1-24(2,3)15-32-18(10-16-13-29-21(12-27)30-22(16)32)14-31-20-5-4-17(26)11-19(20)25(23(31)33)6-8-28-9-7-25/h4-5,10-11,13,28H,6-9,14-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223910

(6-(4-chlorobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3-d]p...)Show InChI InChI=1S/C20H19ClN4/c21-16-8-6-14(7-9-16)10-18-11-15-13-23-19(12-22)24-20(15)25(18)17-4-2-1-3-5-17/h6-9,11,13,17H,1-5,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223911

(7-neopentyl-6-((pyridin-4-yloxy)methyl)-7H-pyrrolo...)Show InChI InChI=1S/C18H19N5O/c1-18(2,3)12-23-14(11-24-15-4-6-20-7-5-15)8-13-10-21-16(9-19)22-17(13)23/h4-8,10H,11-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223920
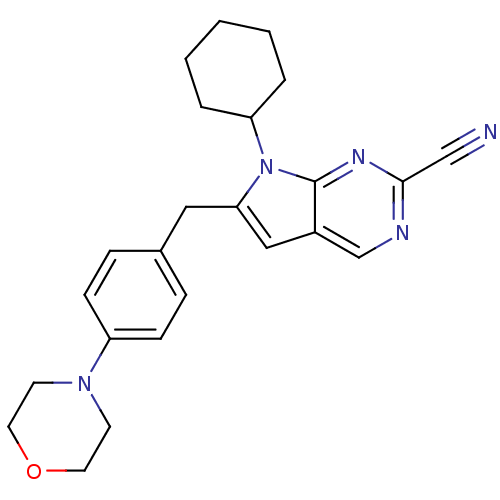
(6-(4-morpholinobenzyl)-7-cyclohexyl-7H-pyrrolo[2,3...)Show SMILES N#Cc1ncc2cc(Cc3ccc(cc3)N3CCOCC3)n(C3CCCCC3)c2n1 Show InChI InChI=1S/C24H27N5O/c25-16-23-26-17-19-15-22(29(24(19)27-23)21-4-2-1-3-5-21)14-18-6-8-20(9-7-18)28-10-12-30-13-11-28/h6-9,15,17,21H,1-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184049
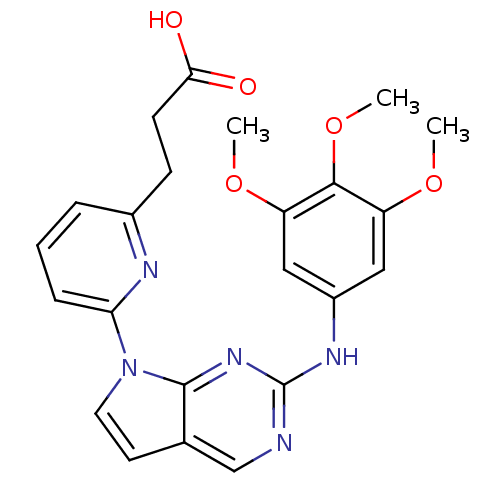
(3-(6-(2-(3,4,5-trimethoxyphenylamino)-7H-pyrrolo[2...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(CCC(O)=O)n4)c3n2)cc(OC)c1OC Show InChI InChI=1S/C23H23N5O5/c1-31-17-11-16(12-18(32-2)21(17)33-3)26-23-24-13-14-9-10-28(22(14)27-23)19-6-4-5-15(25-19)7-8-20(29)30/h4-6,9-13H,7-8H2,1-3H3,(H,29,30)(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223936

(6-((5,5-dimethyl-2,4-dioxooxazolidin-3-yl)methyl)-...)Show SMILES CC(C)(C)Cn1c(CN2C(=O)OC(C)(C)C2=O)cc2cnc(nc12)C#N Show InChI InChI=1S/C18H21N5O3/c1-17(2,3)10-23-12(6-11-8-20-13(7-19)21-14(11)23)9-22-15(24)18(4,5)26-16(22)25/h6,8H,9-10H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223940
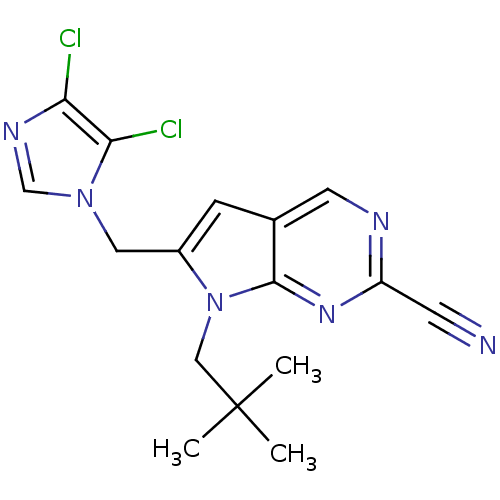
(6-((4,5-dichloro-1H-imidazol-1-yl)methyl)-7-neopen...)Show SMILES CC(C)(C)Cn1c(Cn2cnc(Cl)c2Cl)cc2cnc(nc12)C#N Show InChI InChI=1S/C16H16Cl2N6/c1-16(2,3)8-24-11(7-23-9-21-13(17)14(23)18)4-10-6-20-12(5-19)22-15(10)24/h4,6,9H,7-8H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223941
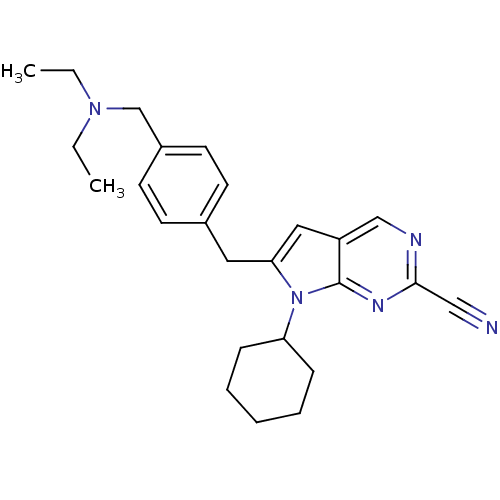
(6-(4-((diethylamino)methyl)benzyl)-7-cyclohexyl-7H...)Show SMILES CCN(CC)Cc1ccc(Cc2cc3cnc(nc3n2C2CCCCC2)C#N)cc1 Show InChI InChI=1S/C25H31N5/c1-3-29(4-2)18-20-12-10-19(11-13-20)14-23-15-21-17-27-24(16-26)28-25(21)30(23)22-8-6-5-7-9-22/h10-13,15,17,22H,3-9,14,18H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223937

(6-((1H-1,2,3-triazol-1-yl)methyl)-7-neopentyl-7H-p...)Show InChI InChI=1S/C15H17N7/c1-15(2,3)10-22-12(9-21-5-4-18-20-21)6-11-8-17-13(7-16)19-14(11)22/h4-6,8H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223918
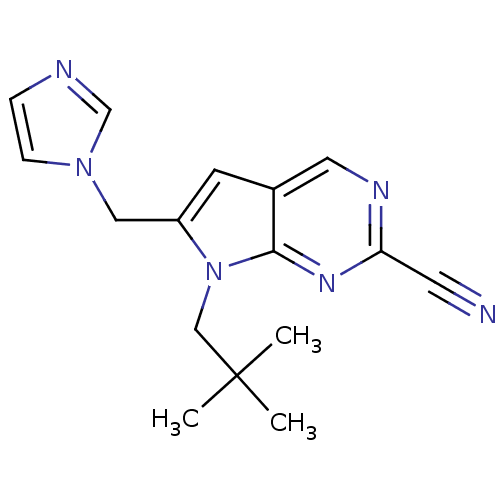
(6-((1H-imidazol-1-yl)methyl)-7-neopentyl-7H-pyrrol...)Show InChI InChI=1S/C16H18N6/c1-16(2,3)10-22-13(9-21-5-4-18-11-21)6-12-8-19-14(7-17)20-15(12)22/h4-6,8,11H,9-10H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223923
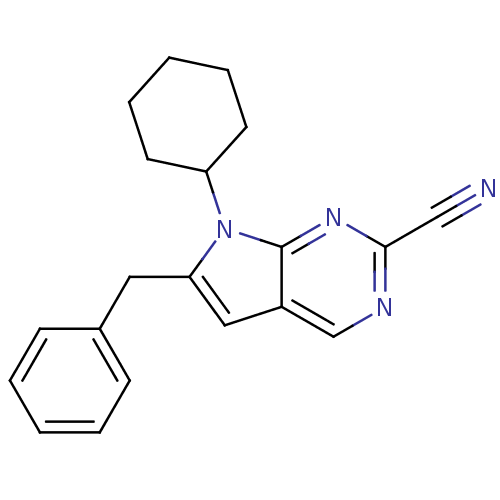
(6-benzyl-7-cyclohexyl-7H-pyrrolo[2,3-d]pyrimidine-...)Show InChI InChI=1S/C20H20N4/c21-13-19-22-14-16-12-18(11-15-7-3-1-4-8-15)24(20(16)23-19)17-9-5-2-6-10-17/h1,3-4,7-8,12,14,17H,2,5-6,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50312718
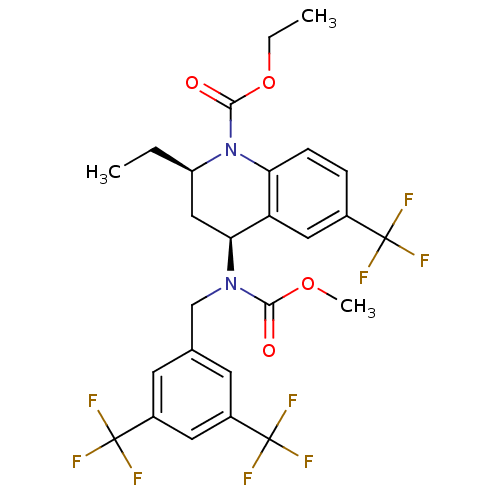
(CHEMBL479527 | torcetrapib)Show SMILES CCOC(=O)N1[C@H](CC)C[C@H](N(Cc2cc(cc(c2)C(F)(F)F)C(F)(F)F)C(=O)OC)c2cc(ccc12)C(F)(F)F |r| Show InChI InChI=1S/C26H25F9N2O4/c1-4-18-12-21(19-11-15(24(27,28)29)6-7-20(19)37(18)23(39)41-5-2)36(22(38)40-3)13-14-8-16(25(30,31)32)10-17(9-14)26(33,34)35/h6-11,18,21H,4-5,12-13H2,1-3H3/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50348228
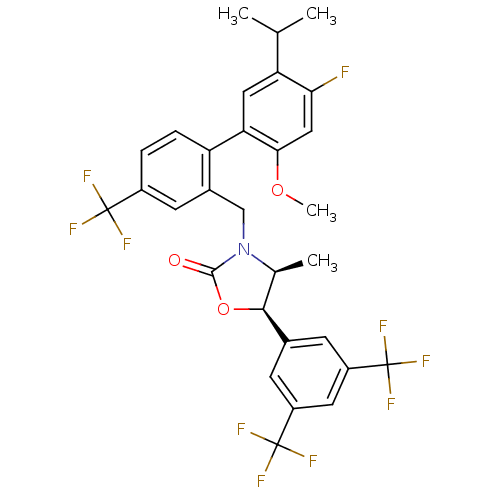
(CHEMBL1800807)Show SMILES COc1cc(F)c(cc1-c1ccc(cc1CN1[C@@H](C)[C@H](OC1=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)C(F)(F)F)C(C)C |r| Show InChI InChI=1S/C30H25F10NO3/c1-14(2)22-11-23(25(43-4)12-24(22)31)21-6-5-18(28(32,33)34)9-17(21)13-41-15(3)26(44-27(41)42)16-7-19(29(35,36)37)10-20(8-16)30(38,39)40/h5-12,14-15,26H,13H2,1-4H3/t15-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124871

(US8759365, 9-4 | US8759365, 9-5)Show SMILES CCC(C)OC(=O)N1C[C@H](C[C@H]1CC)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C28H32F6N6O2/c1-5-17(3)42-26(41)40-16-24(10-23(40)6-2)39(25-35-11-19(12-36-25)20-13-37-38(4)15-20)14-18-7-21(27(29,30)31)9-22(8-18)28(32,33)34/h7-9,11-13,15,17,23-24H,5-6,10,14,16H2,1-4H3/t17?,23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124882
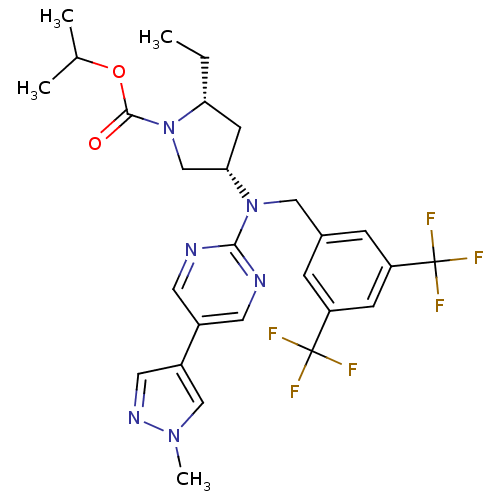
(US8759365, 6)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C27H30F6N6O2/c1-5-22-9-23(15-39(22)25(40)41-16(2)3)38(24-34-10-18(11-35-24)19-12-36-37(4)14-19)13-17-6-20(26(28,29)30)8-21(7-17)27(31,32)33/h6-8,10-12,14,16,22-23H,5,9,13,15H2,1-4H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124884
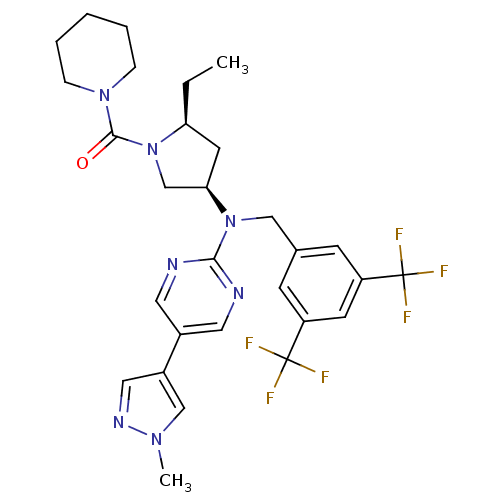
(US8759365, 16-2)Show SMILES CC[C@H]1C[C@H](CN1C(=O)N1CCCCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 Show InChI InChI=1S/C29H33F6N7O/c1-3-24-12-25(18-42(24)27(43)40-7-5-4-6-8-40)41(26-36-13-20(14-37-26)21-15-38-39(2)17-21)16-19-9-22(28(30,31)32)11-23(10-19)29(33,34)35/h9-11,13-15,17,24-25H,3-8,12,16,18H2,1-2H3/t24-,25+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50183986
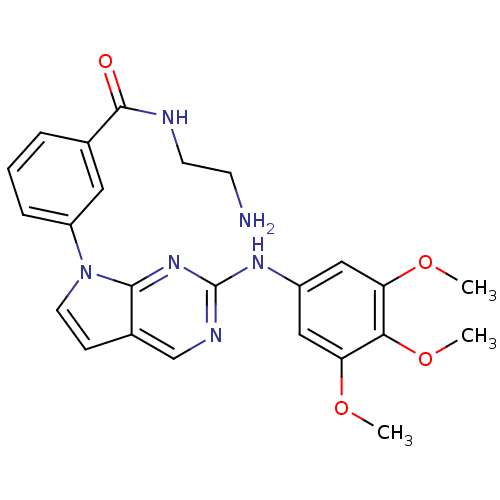
(CHEMBL207084 | N-(2-aminoethyl)-3-(2-(3,4,5-trimet...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(c4)C(=O)NCCN)c3n2)cc(OC)c1OC Show InChI InChI=1S/C24H26N6O4/c1-32-19-12-17(13-20(33-2)21(19)34-3)28-24-27-14-16-7-10-30(22(16)29-24)18-6-4-5-15(11-18)23(31)26-9-8-25/h4-7,10-14H,8-9,25H2,1-3H3,(H,26,31)(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124878

(US8759365, 23-10)Show SMILES CC[C@@H]1C[C@@H](CN1c1nc(ncc1Cl)N1CCC(O)CC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C32H34ClF6N9O/c1-3-24-11-25(18-47(24)28-27(33)15-42-30(44-28)46-6-4-26(49)5-7-46)48(29-40-12-20(13-41-29)21-14-43-45(2)17-21)16-19-8-22(31(34,35)36)10-23(9-19)32(37,38)39/h8-10,12-15,17,24-26,49H,3-7,11,16,18H2,1-2H3/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124876

(US8759365, 17)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)N1CCOCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 Show InChI InChI=1S/C28H31F6N7O2/c1-3-23-11-24(17-41(23)26(42)39-4-6-43-7-5-39)40(25-35-12-19(13-36-25)20-14-37-38(2)16-20)15-18-8-21(27(29,30)31)10-22(9-18)28(32,33)34/h8-10,12-14,16,23-24H,3-7,11,15,17H2,1-2H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124881

(US8759365, 14)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cn[nH]c1 Show InChI InChI=1S/C26H28F6N6O2/c1-4-21-8-22(14-38(21)24(39)40-15(2)3)37(23-33-9-17(10-34-23)18-11-35-36-12-18)13-16-5-19(25(27,28)29)7-20(6-16)26(30,31)32/h5-7,9-12,15,21-22H,4,8,13-14H2,1-3H3,(H,35,36)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50183989
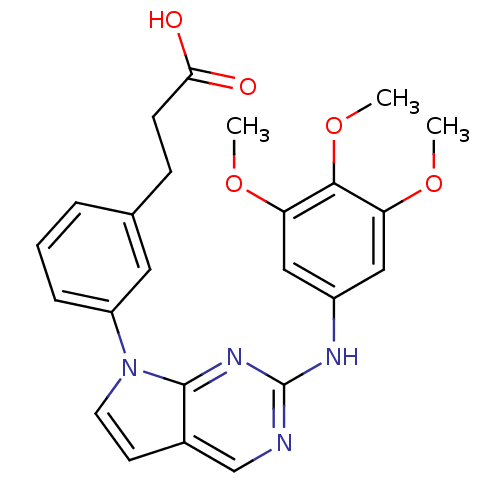
(3-(3-(2-(3,4,5-trimethoxyphenylamino)-7H-pyrrolo[2...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(CCC(O)=O)c4)c3n2)cc(OC)c1OC Show InChI InChI=1S/C24H24N4O5/c1-31-19-12-17(13-20(32-2)22(19)33-3)26-24-25-14-16-9-10-28(23(16)27-24)18-6-4-5-15(11-18)7-8-21(29)30/h4-6,9-14H,7-8H2,1-3H3,(H,29,30)(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184028
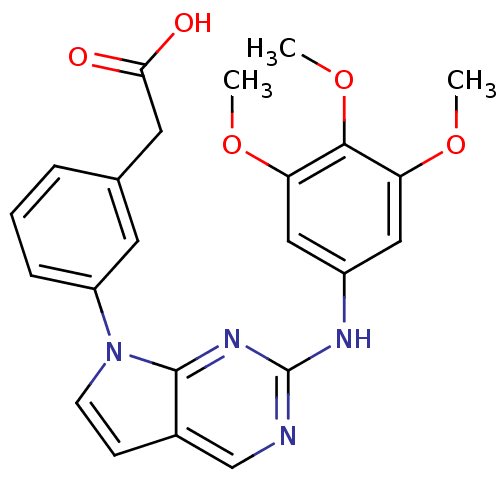
(2-(3-(2-(3,4,5-trimethoxyphenylamino)-7H-pyrrolo[2...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(CC(O)=O)c4)c3n2)cc(OC)c1OC Show InChI InChI=1S/C23H22N4O5/c1-30-18-11-16(12-19(31-2)21(18)32-3)25-23-24-13-15-7-8-27(22(15)26-23)17-6-4-5-14(9-17)10-20(28)29/h4-9,11-13H,10H2,1-3H3,(H,28,29)(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184034
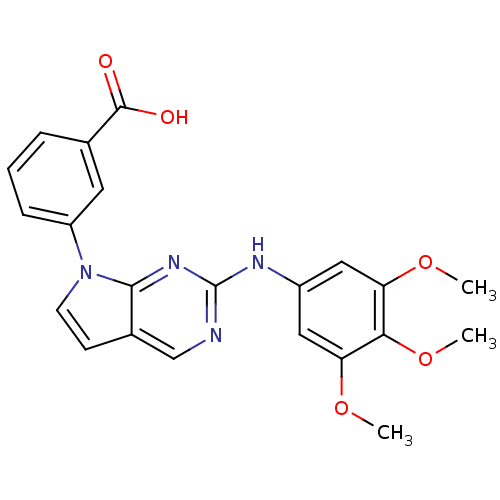
(3-(2-(3,4,5-trimethoxyphenylamino)-7H-pyrrolo[2,3-...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(c4)C(O)=O)c3n2)cc(OC)c1OC Show InChI InChI=1S/C22H20N4O5/c1-29-17-10-15(11-18(30-2)19(17)31-3)24-22-23-12-14-7-8-26(20(14)25-22)16-6-4-5-13(9-16)21(27)28/h4-12H,1-3H3,(H,27,28)(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184019
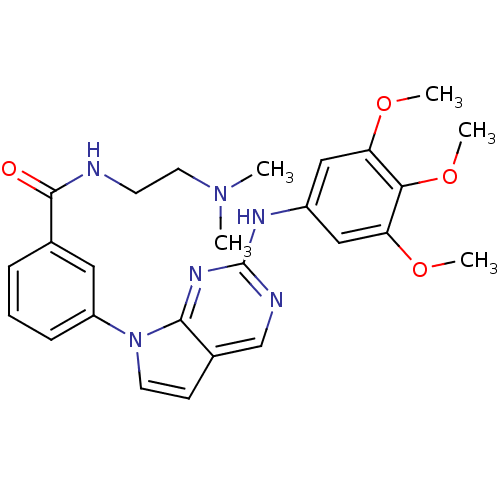
(CHEMBL379837 | N-(2-(dimethylamino)ethyl)-3-(2-(3,...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(c4)C(=O)NCCN(C)C)c3n2)cc(OC)c1OC Show InChI InChI=1S/C26H30N6O4/c1-31(2)12-10-27-25(33)17-7-6-8-20(13-17)32-11-9-18-16-28-26(30-24(18)32)29-19-14-21(34-3)23(36-5)22(15-19)35-4/h6-9,11,13-16H,10,12H2,1-5H3,(H,27,33)(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124875
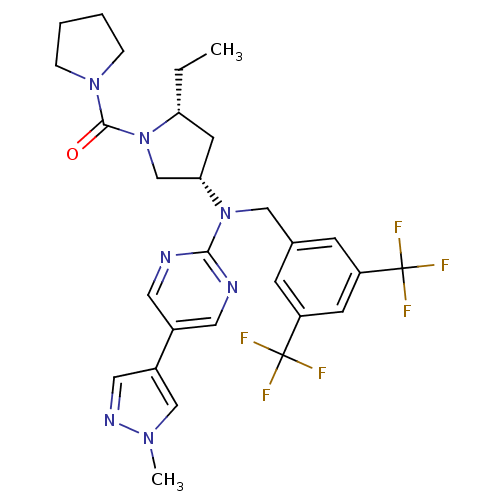
(US8759365, 16)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)N1CCCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 Show InChI InChI=1S/C28H31F6N7O/c1-3-23-11-24(17-41(23)26(42)39-6-4-5-7-39)40(25-35-12-19(13-36-25)20-14-37-38(2)16-20)15-18-8-21(27(29,30)31)10-22(9-18)28(32,33)34/h8-10,12-14,16,23-24H,3-7,11,15,17H2,1-2H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124868
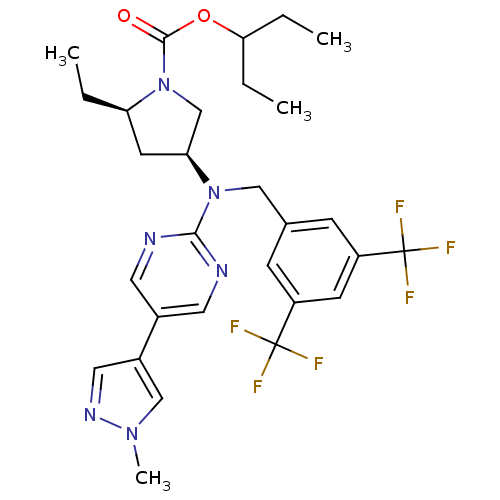
(US8759365, 9-1)Show SMILES CCC(CC)OC(=O)N1C[C@H](C[C@H]1CC)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C29H34F6N6O2/c1-5-23-11-24(17-41(23)27(42)43-25(6-2)7-3)40(26-36-12-19(13-37-26)20-14-38-39(4)16-20)15-18-8-21(28(30,31)32)10-22(9-18)29(33,34)35/h8-10,12-14,16,23-25H,5-7,11,15,17H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124867
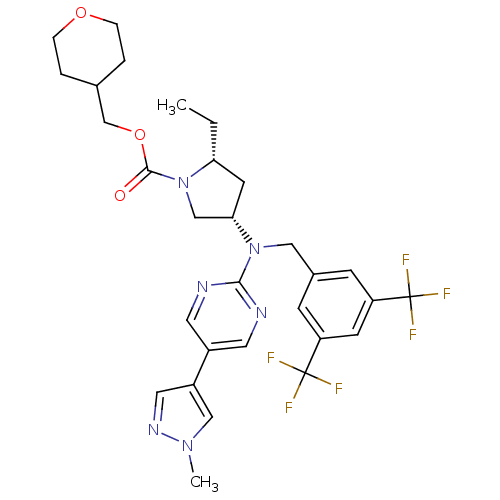
(US8759365, 7-6)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC1CCOCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C30H34F6N6O3/c1-3-25-11-26(17-42(25)28(43)45-18-19-4-6-44-7-5-19)41(27-37-12-21(13-38-27)22-14-39-40(2)16-22)15-20-8-23(29(31,32)33)10-24(9-20)30(34,35)36/h8-10,12-14,16,19,25-26H,3-7,11,15,17-18H2,1-2H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124865
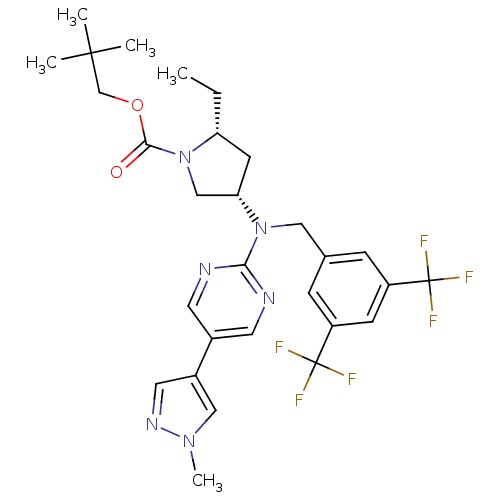
(US8759365, 6-2)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C29H34F6N6O2/c1-6-23-10-24(16-41(23)26(42)43-17-27(2,3)4)40(25-36-11-19(12-37-25)20-13-38-39(5)15-20)14-18-7-21(28(30,31)32)9-22(8-18)29(33,34)35/h7-9,11-13,15,23-24H,6,10,14,16-17H2,1-5H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50223928
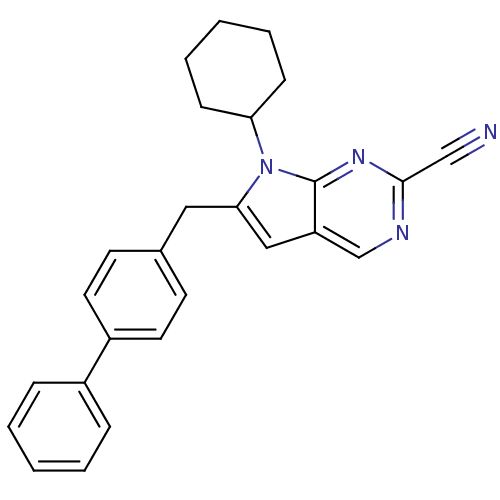
(6-biphenyl-4-ylmethyl-7-cyclohexyl-7H-pyrrolo[2,3-...)Show SMILES N#Cc1ncc2cc(Cc3ccc(cc3)-c3ccccc3)n(C3CCCCC3)c2n1 Show InChI InChI=1S/C26H24N4/c27-17-25-28-18-22-16-24(30(26(22)29-25)23-9-5-2-6-10-23)15-19-11-13-21(14-12-19)20-7-3-1-4-8-20/h1,3-4,7-8,11-14,16,18,23H,2,5-6,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin K by fluorescence assay |
Bioorg Med Chem Lett 17: 6096-100 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.047
BindingDB Entry DOI: 10.7270/Q2G160K6 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124883
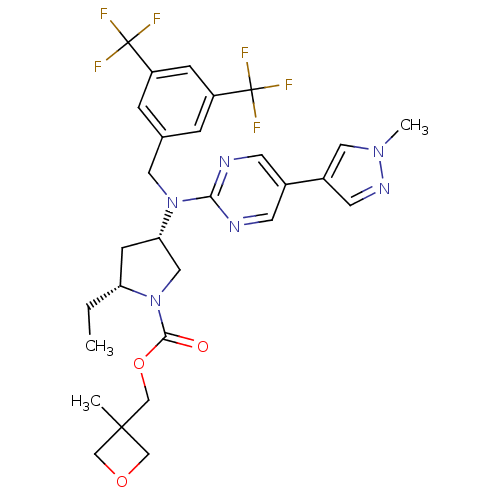
(US8759365, 9)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC1(C)COC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C29H32F6N6O3/c1-4-23-8-24(14-41(23)26(42)44-17-27(2)15-43-16-27)40(25-36-9-19(10-37-25)20-11-38-39(3)13-20)12-18-5-21(28(30,31)32)7-22(6-18)29(33,34)35/h5-7,9-11,13,23-24H,4,8,12,14-17H2,1-3H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124871

(US8759365, 9-4 | US8759365, 9-5)Show SMILES CCC(C)OC(=O)N1C[C@H](C[C@H]1CC)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C28H32F6N6O2/c1-5-17(3)42-26(41)40-16-24(10-23(40)6-2)39(25-35-11-19(12-36-25)20-13-37-38(4)15-20)14-18-7-21(27(29,30)31)9-22(8-18)28(32,33)34/h7-9,11-13,15,17,23-24H,5-6,10,14,16H2,1-4H3/t17?,23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184002
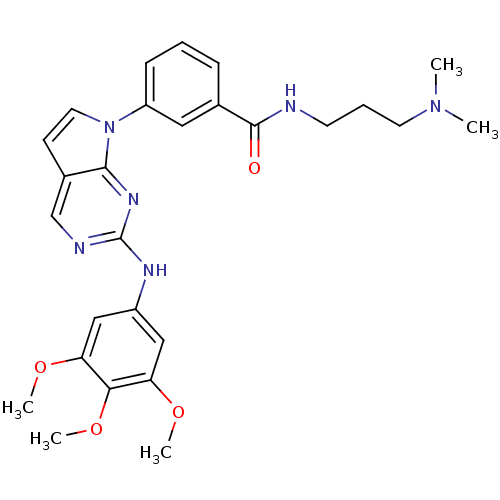
(CHEMBL207961 | N-(3-(dimethylamino)propyl)-3-(2-(3...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(c4)C(=O)NCCCN(C)C)c3n2)cc(OC)c1OC Show InChI InChI=1S/C27H32N6O4/c1-32(2)12-7-11-28-26(34)18-8-6-9-21(14-18)33-13-10-19-17-29-27(31-25(19)33)30-20-15-22(35-3)24(37-5)23(16-20)36-4/h6,8-10,13-17H,7,11-12H2,1-5H3,(H,28,34)(H,29,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124869
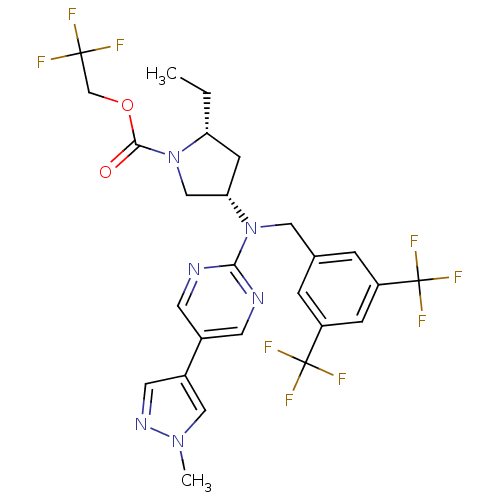
(US8759365, 9-2)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC(F)(F)F)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C26H25F9N6O2/c1-3-20-7-21(13-41(20)23(42)43-14-24(27,28)29)40(22-36-8-16(9-37-22)17-10-38-39(2)12-17)11-15-4-18(25(30,31)32)6-19(5-15)26(33,34)35/h4-6,8-10,12,20-21H,3,7,11,13-14H2,1-2H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
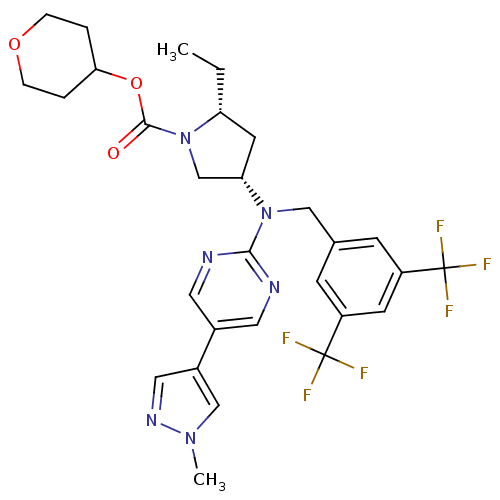
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124866
(US8759365, 7-4)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OC1CCOCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C29H32F6N6O3/c1-3-23-11-24(17-41(23)27(42)44-25-4-6-43-7-5-25)40(26-36-12-19(13-37-26)20-14-38-39(2)16-20)15-18-8-21(28(30,31)32)10-22(9-18)29(33,34)35/h8-10,12-14,16,23-25H,3-7,11,15,17H2,1-2H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250371
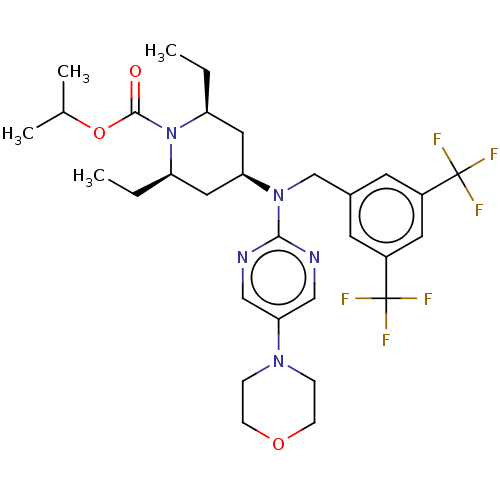
(CHEMBL4064793)Show SMILES CC[C@H]1C[C@H](C[C@@H](CC)N1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CCOCC1 |r| Show InChI InChI=1S/C30H39F6N5O3/c1-5-23-14-25(15-24(6-2)41(23)28(42)44-19(3)4)40(27-37-16-26(17-38-27)39-7-9-43-10-8-39)18-20-11-21(29(31,32)33)13-22(12-20)30(34,35)36/h11-13,16-17,19,23-25H,5-10,14-15,18H2,1-4H3/t23-,24+,25+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250366
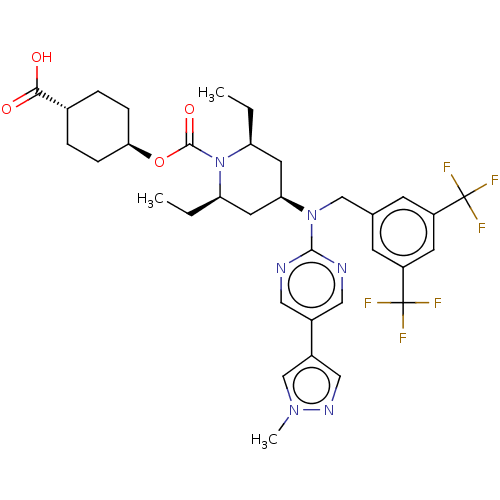
(CHEMBL4075773)Show SMILES CC[C@H]1C[C@H](C[C@@H](CC)N1C(=O)O[C@H]1CC[C@@H](CC1)C(O)=O)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r,wU:4.23,6.6,2.1,13.13,wD:16.20,(37.57,-12.9,;38.91,-13.68,;40.24,-12.9,;40.24,-11.36,;41.57,-10.59,;42.9,-11.36,;42.9,-12.9,;44.24,-13.68,;45.57,-12.9,;41.57,-13.67,;41.57,-15.21,;40.24,-15.97,;42.91,-15.97,;42.91,-17.51,;44.24,-18.28,;44.23,-19.82,;42.89,-20.59,;41.56,-19.82,;41.57,-18.28,;42.89,-22.13,;41.55,-22.9,;44.22,-22.91,;41.57,-9.05,;40.24,-8.27,;40.24,-6.73,;41.58,-5.97,;41.58,-4.43,;40.24,-3.66,;38.9,-4.44,;38.91,-5.98,;37.57,-3.67,;37.56,-2.13,;36.23,-4.45,;36.23,-2.9,;42.91,-3.66,;44.25,-4.44,;42.91,-2.12,;44.24,-2.89,;42.9,-8.28,;44.24,-9.05,;45.57,-8.28,;45.57,-6.74,;44.23,-5.97,;42.9,-6.74,;46.9,-5.97,;48.3,-6.59,;49.34,-5.45,;48.56,-4.11,;49.19,-2.71,;47.05,-4.44,)| Show InChI InChI=1S/C34H40F6N6O4/c1-4-26-13-28(14-27(5-2)46(26)32(49)50-29-8-6-21(7-9-29)30(47)48)45(31-41-15-22(16-42-31)23-17-43-44(3)19-23)18-20-10-24(33(35,36)37)12-25(11-20)34(38,39)40/h10-12,15-17,19,21,26-29H,4-9,13-14,18H2,1-3H3,(H,47,48)/t21-,26-,27+,28+,29- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184035
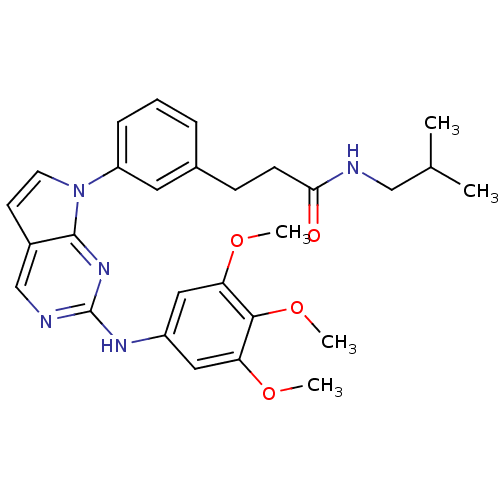
(CHEMBL207499 | N-isobutyl-3-(3-(2-(3,4,5-trimethox...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(CCC(=O)NCC(C)C)c4)c3n2)cc(OC)c1OC Show InChI InChI=1S/C28H33N5O4/c1-18(2)16-29-25(34)10-9-19-7-6-8-22(13-19)33-12-11-20-17-30-28(32-27(20)33)31-21-14-23(35-3)26(37-5)24(15-21)36-4/h6-8,11-15,17-18H,9-10,16H2,1-5H3,(H,29,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50184000
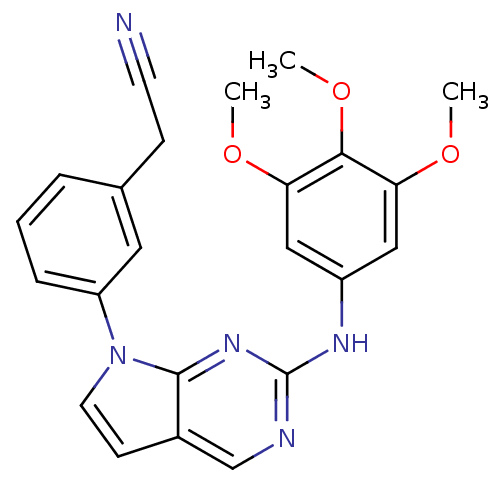
(2-(3-(2-(3,4,5-trimethoxyphenylamino)-7H-pyrrolo[2...)Show SMILES COc1cc(Nc2ncc3ccn(-c4cccc(CC#N)c4)c3n2)cc(OC)c1OC Show InChI InChI=1S/C23H21N5O3/c1-29-19-12-17(13-20(30-2)21(19)31-3)26-23-25-14-16-8-10-28(22(16)27-23)18-6-4-5-15(11-18)7-9-24/h4-6,8,10-14H,7H2,1-3H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation (GNF)
Curated by ChEMBL
| Assay Description
Inhibition of FAK |
Bioorg Med Chem Lett 16: 2689-92 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.032
BindingDB Entry DOI: 10.7270/Q2NK3DN0 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124877

(US8759365, 18)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)N(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 Show InChI InChI=1S/C26H29F6N7O/c1-5-21-9-22(15-39(21)24(40)36(2)3)38(23-33-10-17(11-34-23)18-12-35-37(4)14-18)13-16-6-19(25(27,28)29)8-20(7-16)26(30,31)32/h6-8,10-12,14,21-22H,5,9,13,15H2,1-4H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124864
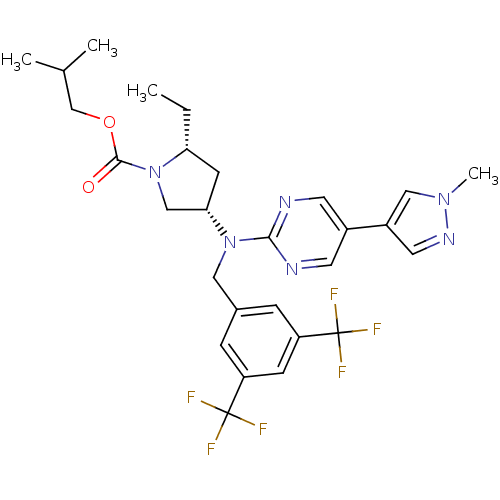
(US8759365, 6-1)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C28H32F6N6O2/c1-5-23-9-24(15-40(23)26(41)42-16-17(2)3)39(25-35-10-19(11-36-25)20-12-37-38(4)14-20)13-18-6-21(27(29,30)31)8-22(7-18)28(32,33)34/h6-8,10-12,14,17,23-24H,5,9,13,15-16H2,1-4H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124873

(US8759365, 9-6)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OCC1(CC)COC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C30H34F6N6O3/c1-4-24-9-25(15-42(24)27(43)45-18-28(5-2)16-44-17-28)41(26-37-10-20(11-38-26)21-12-39-40(3)14-21)13-19-6-22(29(31,32)33)8-23(7-19)30(34,35)36/h6-8,10-12,14,24-25H,4-5,9,13,15-18H2,1-3H3/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124870
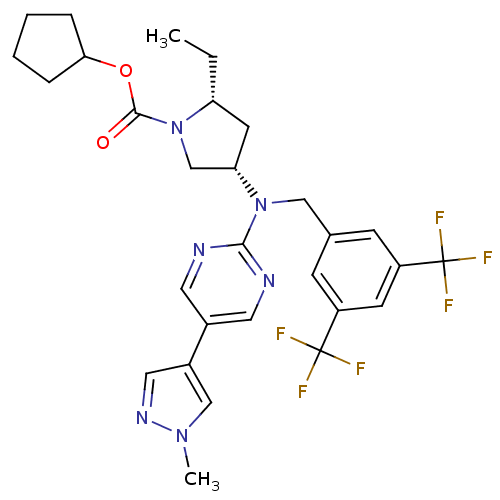
(US8759365, 9-3)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OC1CCCC1)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C29H32F6N6O2/c1-3-23-11-24(17-41(23)27(42)43-25-6-4-5-7-25)40(26-36-12-19(13-37-26)20-14-38-39(2)16-20)15-18-8-21(28(30,31)32)10-22(9-18)29(33,34)35/h8-10,12-14,16,23-25H,3-7,11,15,17H2,1-2H3/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM50250363
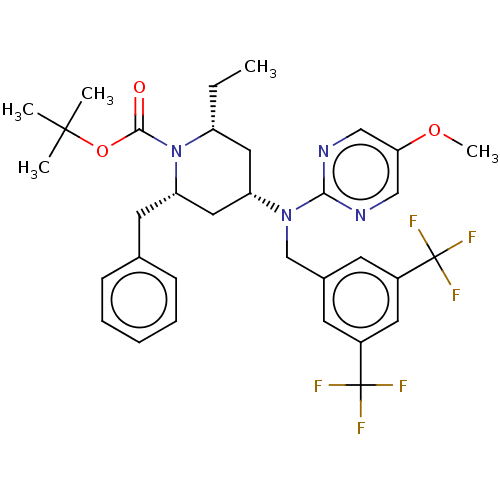
(CHEMBL4070653)Show SMILES CC[C@@H]1C[C@@H](C[C@H](Cc2ccccc2)N1C(=O)OC(C)(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(OC)cn1 |r| Show InChI InChI=1S/C33H38F6N4O3/c1-6-25-16-26(17-27(14-21-10-8-7-9-11-21)43(25)30(44)46-31(2,3)4)42(29-40-18-28(45-5)19-41-29)20-22-12-23(32(34,35)36)15-24(13-22)33(37,38)39/h7-13,15,18-19,25-27H,6,14,16-17,20H2,1-5H3/t25-,26+,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CETP in human plasma measured every 30 mins for 120 mins by fluorescence method |
J Med Chem 60: 8466-8481 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00900
BindingDB Entry DOI: 10.7270/Q27083WQ |
More data for this
Ligand-Target Pair | |
Cholesteryl ester transfer protein
(Homo sapiens (Human)) | BDBM124874

(US8759365, 11)Show SMILES CC[C@@H]1C[C@@H](CN1C(=O)OC(C)C)N(Cc1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1ncc(cn1)N1CC[C@H](O)C1 Show InChI InChI=1S/C27H33F6N5O3/c1-4-20-10-21(14-38(20)25(40)41-16(2)3)37(24-34-11-22(12-35-24)36-6-5-23(39)15-36)13-17-7-18(26(28,29)30)9-19(8-17)27(31,32)33/h7-9,11-12,16,20-21,23,39H,4-6,10,13-15H2,1-3H3/t20-,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Novartis AG
US Patent
| Assay Description
CETP Activity Kit (#RB-RPAK) was purchased from Roar Biochemical, Inc. (New York, N.Y., USA). To each well of a 96-well NBS half-area plate (costar #... |
US Patent US8759365 (2014)
BindingDB Entry DOI: 10.7270/Q2NG4P95 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data