

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Translocator protein (Rattus norvegicus (rat)) | BDBM21363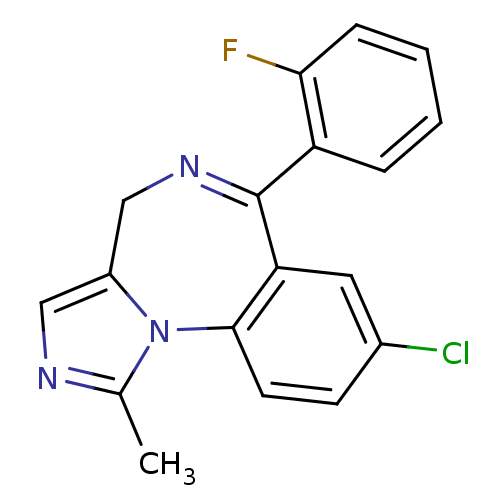 (12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triaza...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120343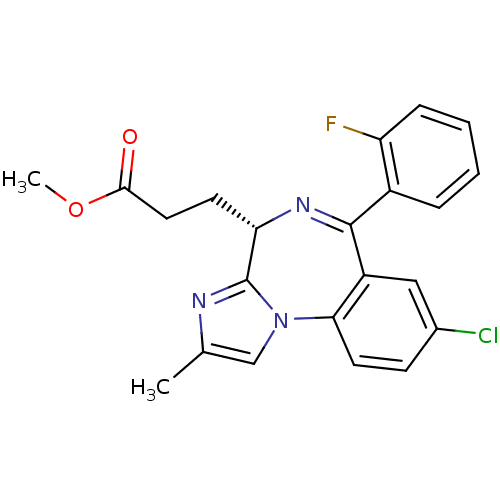 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-2-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120340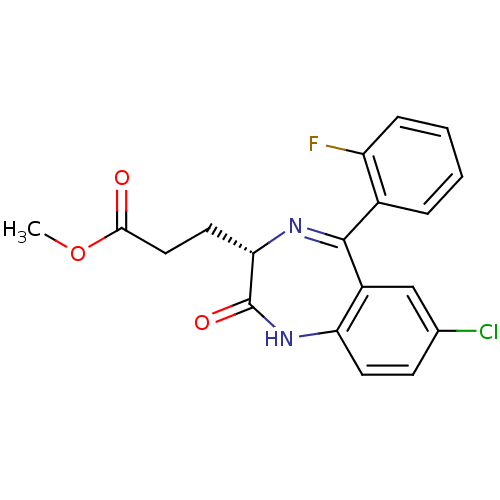 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-oxo-2,3-dihy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120353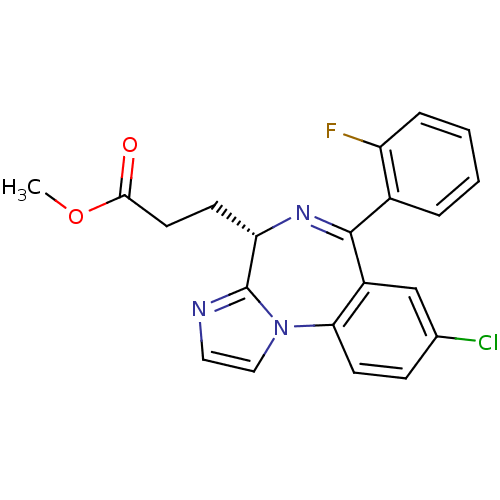 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-4H-3,5,10b-tri...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120360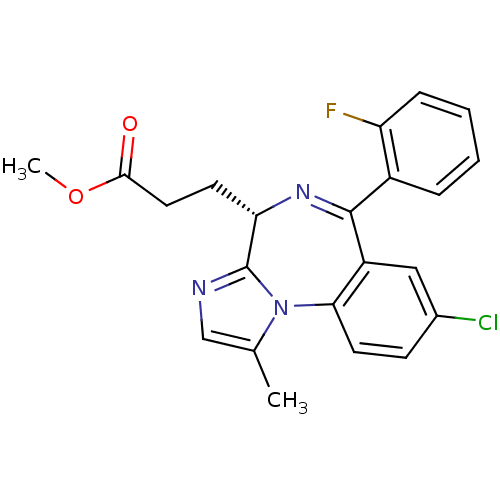 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-3,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120344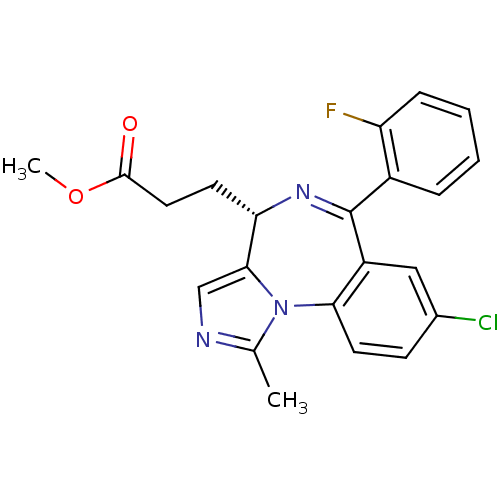 (3-[(S)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120359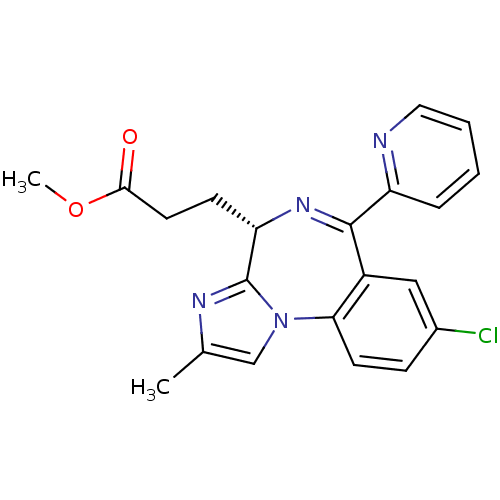 (3-((S)-8-Chloro-2-methyl-6-pyridin-2-yl-4H-3,5,10b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120357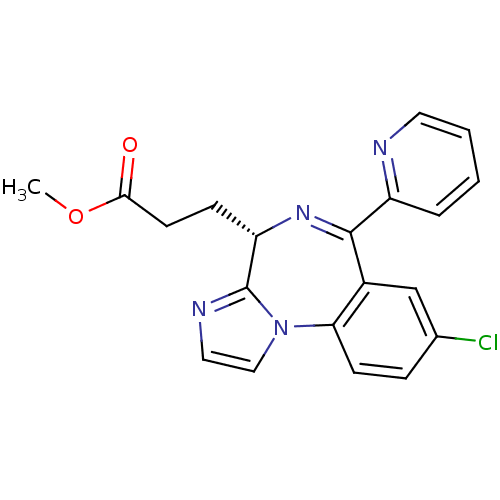 (3-((S)-8-Chloro-6-pyridin-2-yl-4H-3,5,10b-triaza-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120345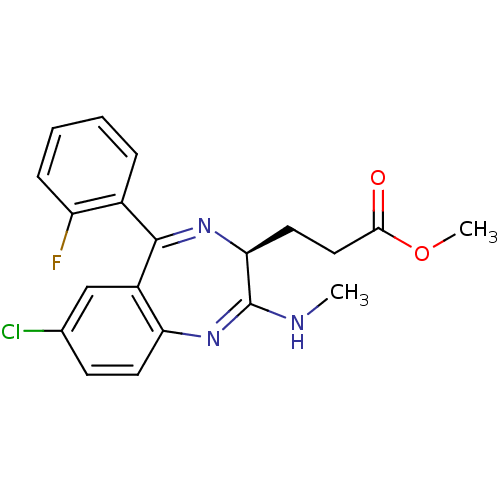 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-methylamino-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120358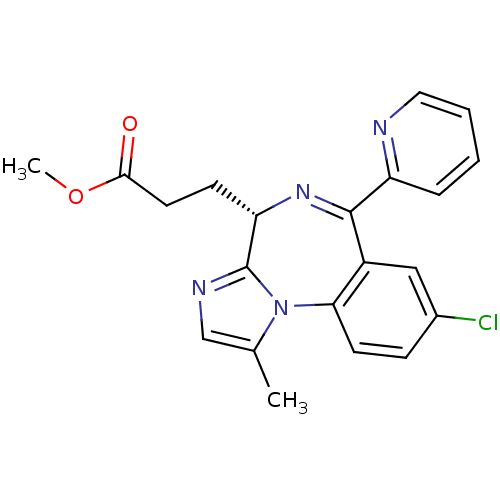 (3-((S)-8-Chloro-1-methyl-6-pyridin-2-yl-4H-3,5,10b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120354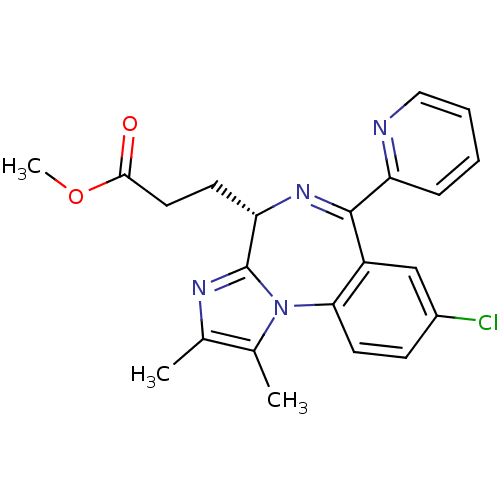 (3-((S)-8-Chloro-1,2-dimethyl-6-pyridin-2-yl-4H-3,5...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120347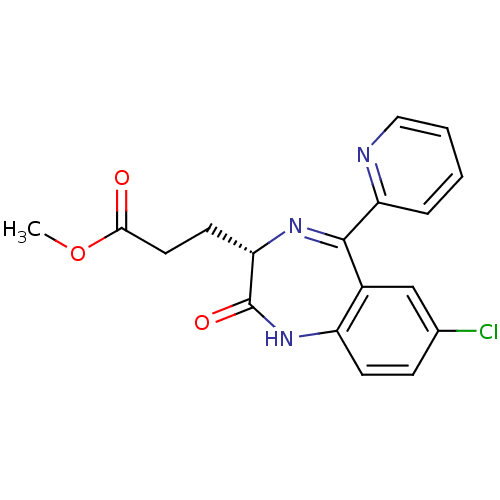 (3-((S)-7-Chloro-2-oxo-5-pyridin-2-yl-2,3-dihydro-1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120349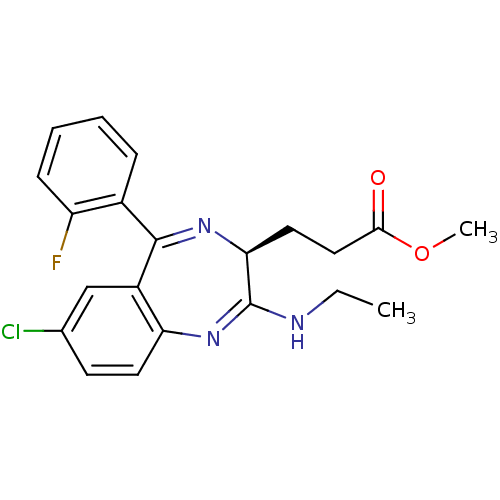 (3-[(S)-7-Chloro-2-ethylamino-5-(2-fluoro-phenyl)-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120352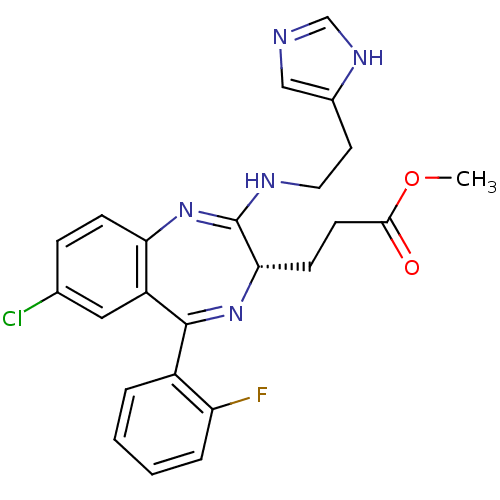 (3-{(S)-7-Chloro-5-(2-fluoro-phenyl)-2-[2-(1H-imida...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120351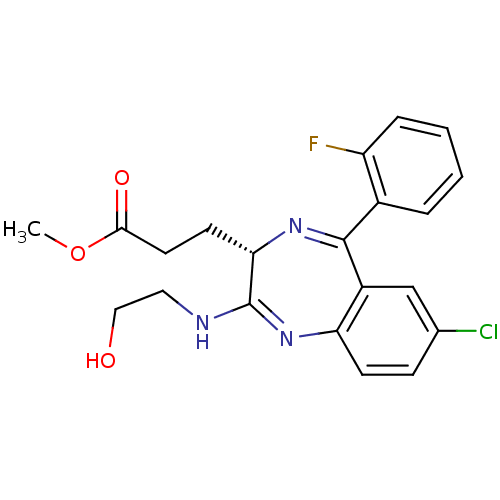 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-(2-hydroxy-e...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 303 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50007664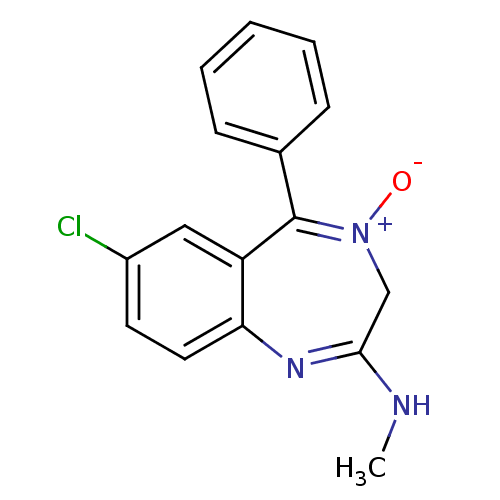 (7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazep...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 438 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120355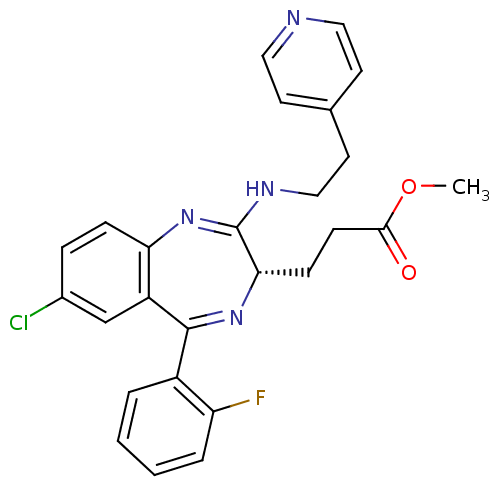 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-(2-pyridin-4...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 792 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120350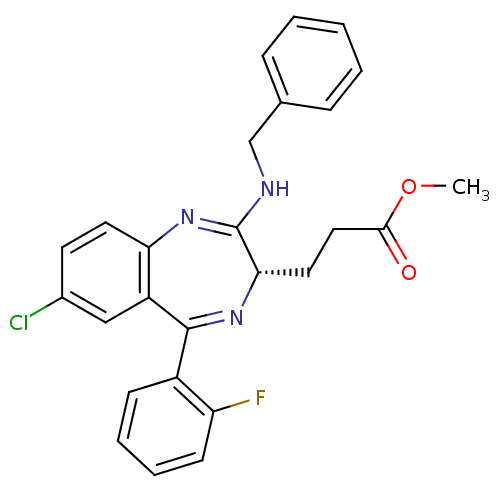 (3-[(S)-2-Benzylamino-7-chloro-5-(2-fluoro-phenyl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 875 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120348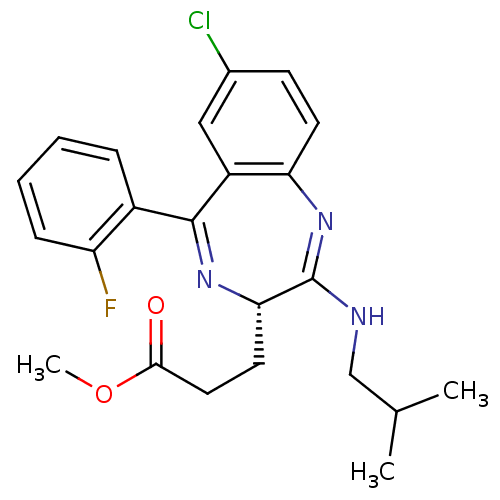 (3-[(S)-7-Chloro-5-(2-fluoro-phenyl)-2-isobutylamin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120361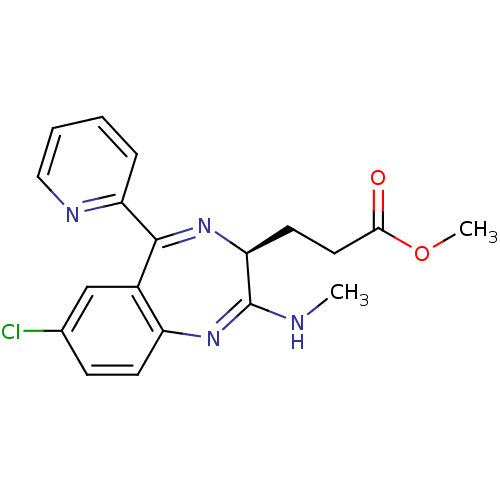 (3-((S)-7-Chloro-2-methylamino-5-pyridin-2-yl-3H-be...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120356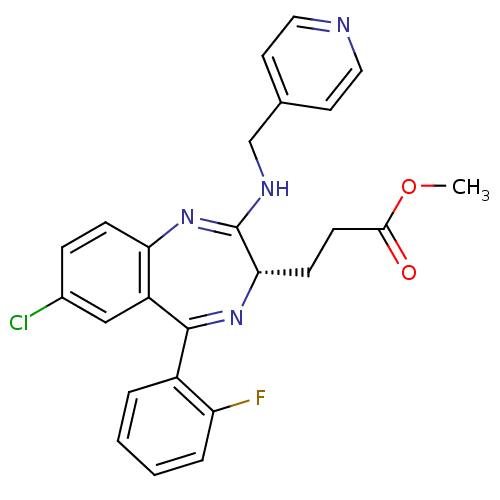 (3-{(S)-7-Chloro-5-(2-fluoro-phenyl)-2-[(pyridin-4-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Rattus norvegicus (rat)) | BDBM50120346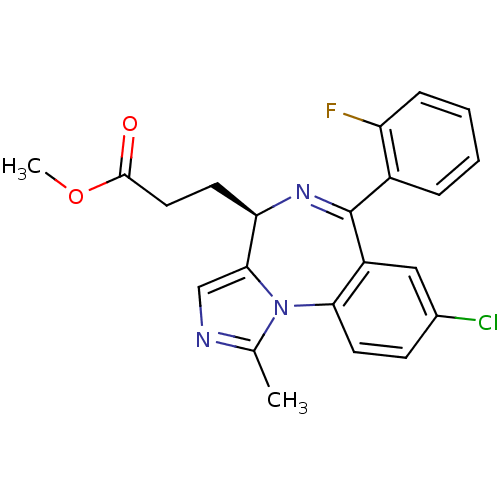 (3-[(R)-8-Chloro-6-(2-fluoro-phenyl)-1-methyl-4H-2,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against rat benzodiazepine (BZD) receptor | Bioorg Med Chem Lett 12: 3219-22 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6X4F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Nav 1.8 channel expressed in HEK293 cells | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231774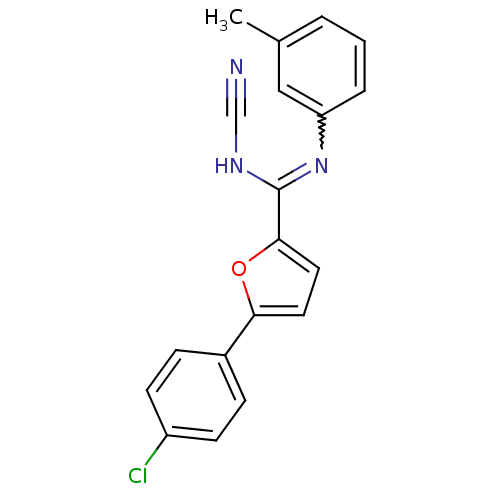 (5-(4-chlorophenyl)-N-cyano-N'-(3-methylphenyl)fura...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Homo sapiens (Human)) | BDBM50231767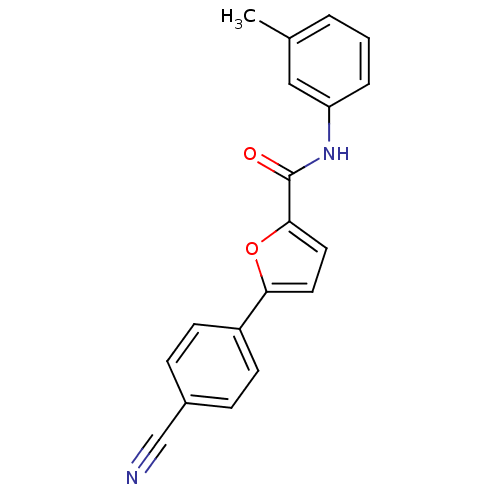 (5-(4-cyanophenyl)-N-(3-methylphenyl)furan-2-carbox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant Nav 1.8 channel expressed in HEK293 cells | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231777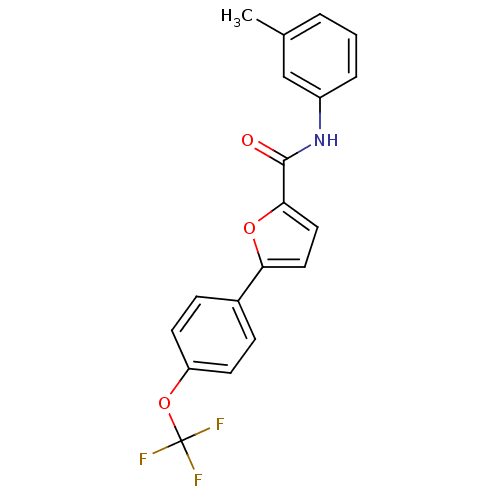 (5-[(4-trifluoromethoxy)phenyl]-N-(3-methylphenyl)f...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371254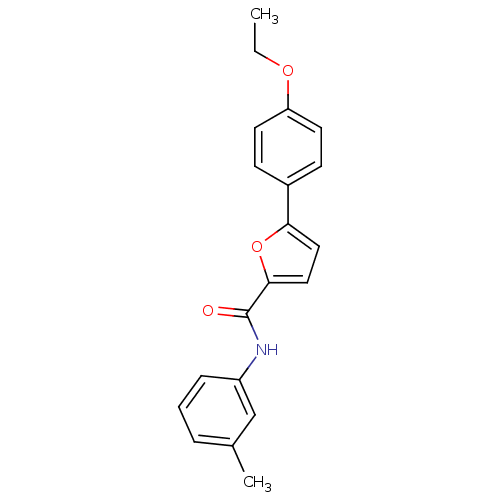 (CHEMBL429830) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231779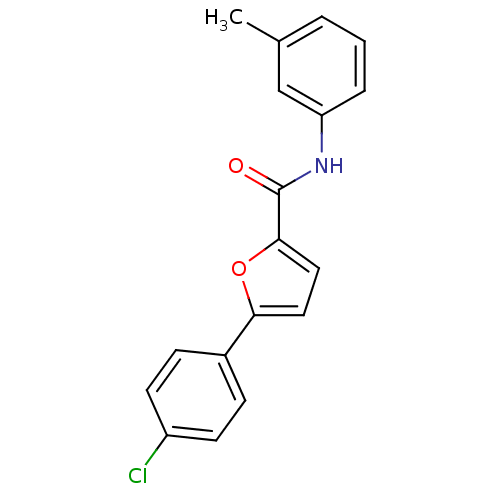 (5-(4-chlorophenyl)-N-(3-methylphenyl)furan-2-carbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231767 (5-(4-cyanophenyl)-N-(3-methylphenyl)furan-2-carbox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50231775 ((R)-5-(4-chlorophenyl)-N-(1-(4-(3-(4-(cyclopropane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of hERG potassium channel expressed in CHO cells by isotope efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231770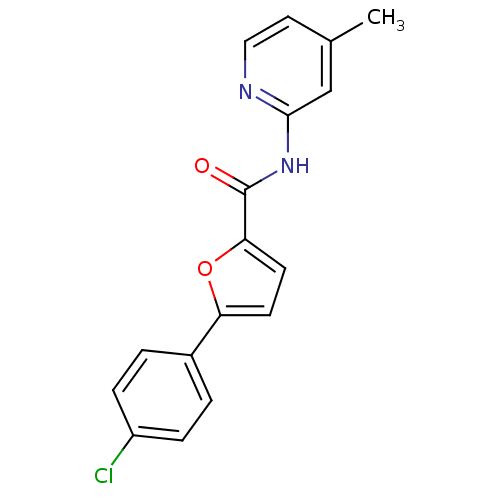 (5-(4-chlorophenyl)-N-[(5-methyl)pyridin-3-yl]furan...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371245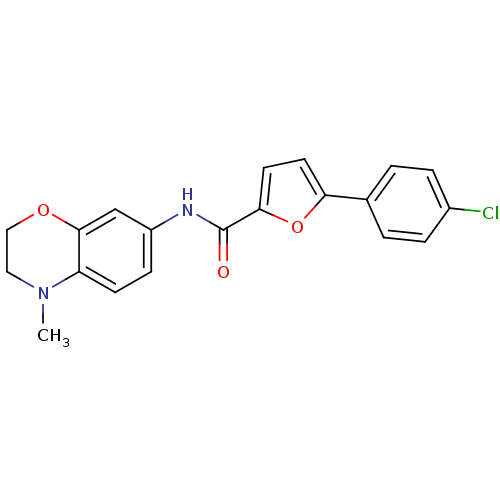 (CHEMBL399792) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371253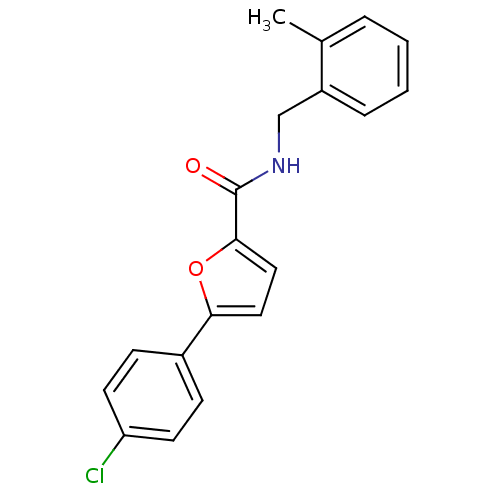 (CHEMBL427714) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371252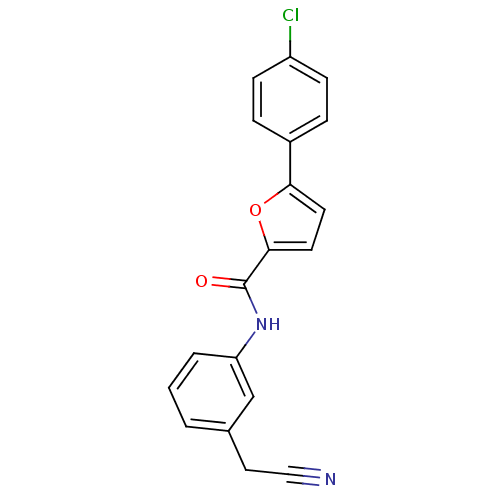 (CHEMBL400769) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231782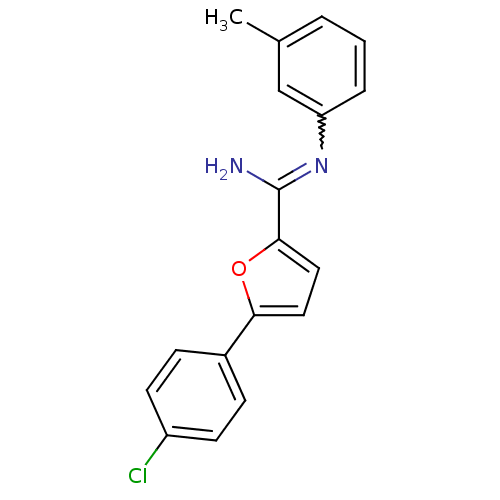 (5-(4-chlorophenyl)-N-(3-methylphenyl)furan-2-carbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371241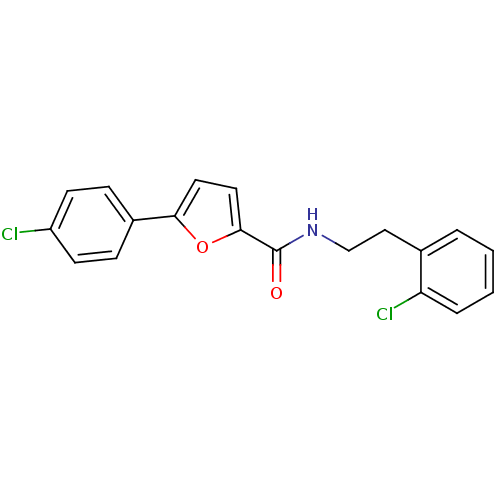 (CHEMBL253560) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav1.5 channel at 1 uM | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to peripheral benzodiazepine receptor | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Translocator protein (Homo sapiens (Human)) | BDBM50231767 (5-(4-cyanophenyl)-N-(3-methylphenyl)furan-2-carbox...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to peripheral benzodiazepine receptor | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to 5HT2A receptor | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50231767 (5-(4-cyanophenyl)-N-(3-methylphenyl)furan-2-carbox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human Nav1.5 channel at 1 uM | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholecystokinin receptor type A (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to CCKAR | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50212239 (5-(4-chlorophenyl)-N-(3,5-dimethoxyphenyl)furan-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity to dopamine D1 receptor | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50231782 (5-(4-chlorophenyl)-N-(3-methylphenyl)furan-2-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of hERG potassium channel expressed in CHO cells by isotope efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371243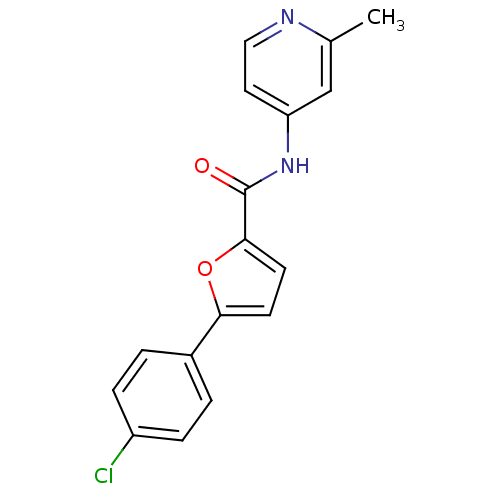 (CHEMBL251066) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371249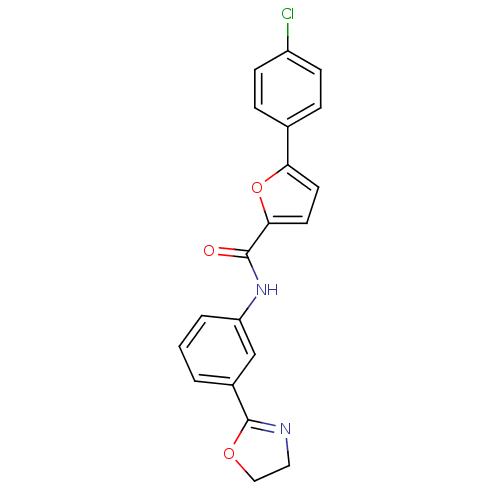 (CHEMBL250698) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50231772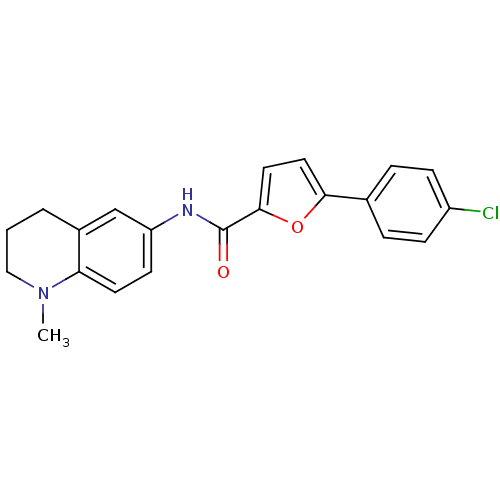 (5-(4-chlorophenyl)-N-[(1-methyl-1,2,3,4-tetrahydro...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371240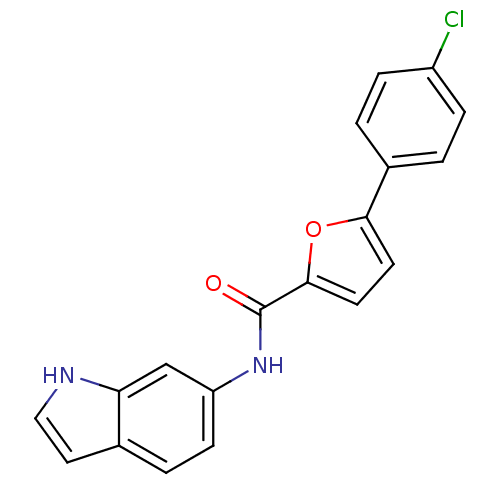 (CHEMBL400355) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 10 subunit alpha (Mus musculus) | BDBM50371242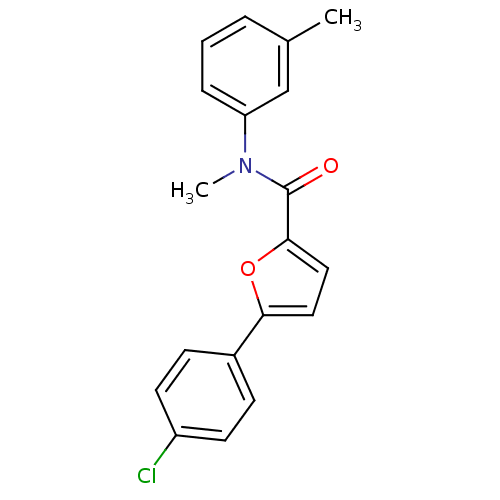 (CHEMBL400958) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Nav 1.8 channel expressed in HEK293 cells by isotopic efflux assay | J Med Chem 51: 407-16 (2008) Article DOI: 10.1021/jm070637u BindingDB Entry DOI: 10.7270/Q2KK9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 67 total ) | Next | Last >> |