

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383031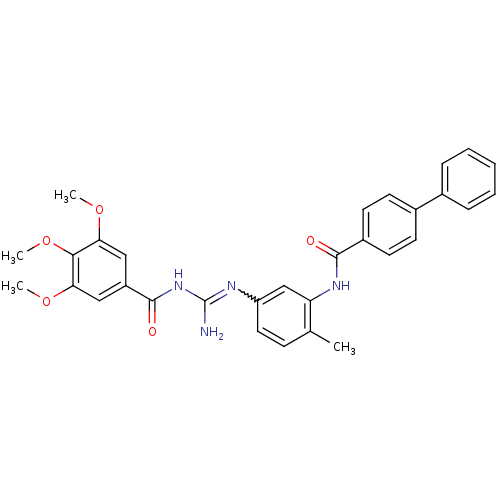 (CHEMBL2031290) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50249522 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50249522 (2-chloro-N-(4-chloro-3-(pyridin-2-yl)phenyl)-4-(me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383020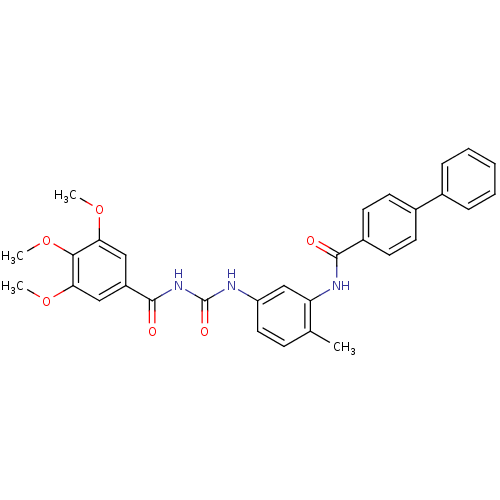 (CHEMBL2031277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383029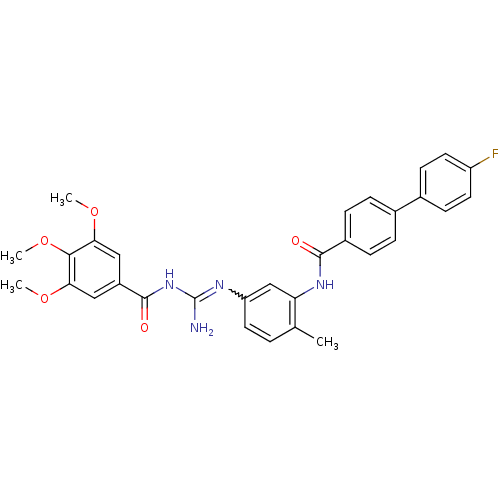 (CHEMBL2031288) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383031 (CHEMBL2031290) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383020 (CHEMBL2031277) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383015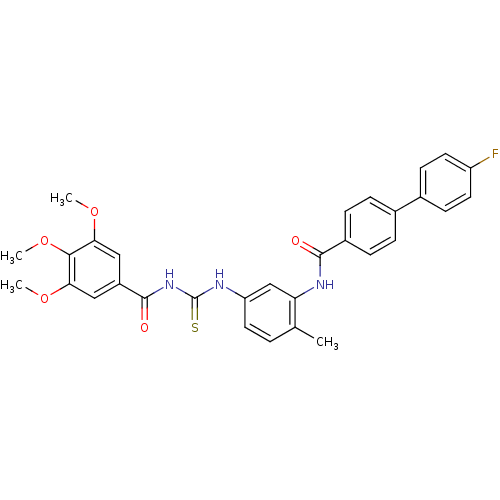 (CHEMBL2031263) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383028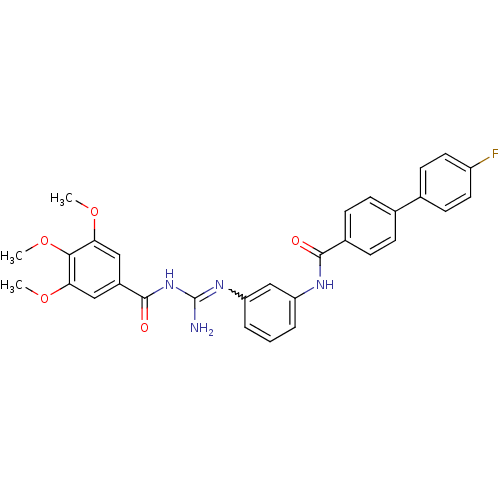 (CHEMBL2031287) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383016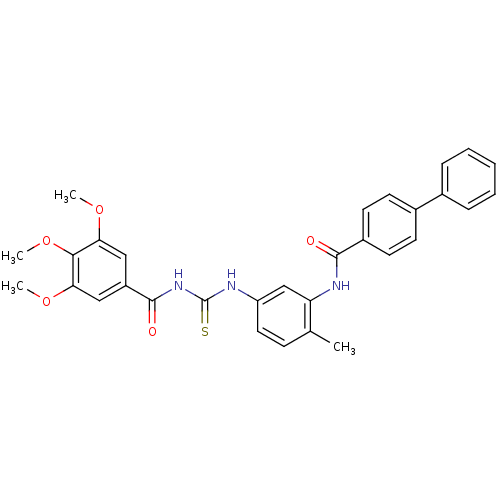 (CHEMBL2031268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383016 (CHEMBL2031268) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50232973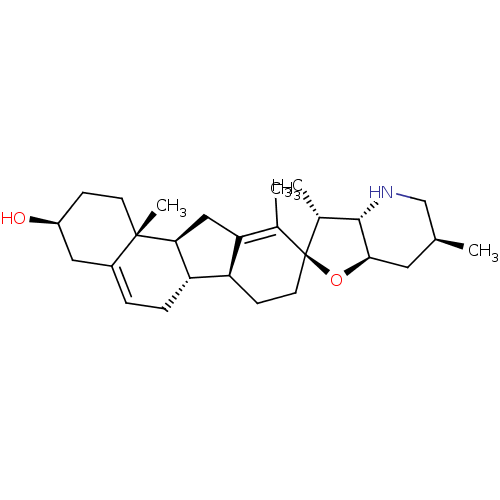 (CHEMBL254129 | CYCLOPAMINE) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Displacement of BODIPY-labelled cyclopamine from human Smo receptor expressed in HEK293 cells after 2 hrs by fluorescence microscopy | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383024 (CHEMBL2031283) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383030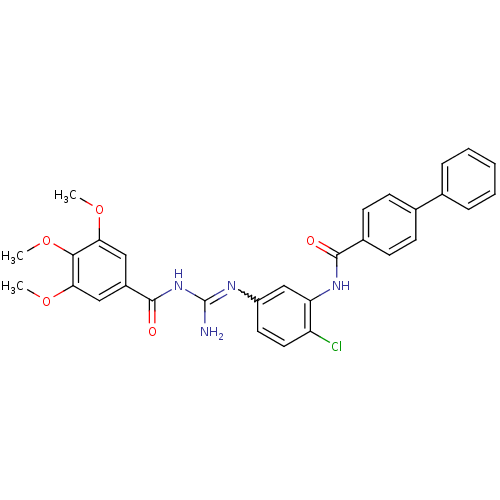 (CHEMBL2031289) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383022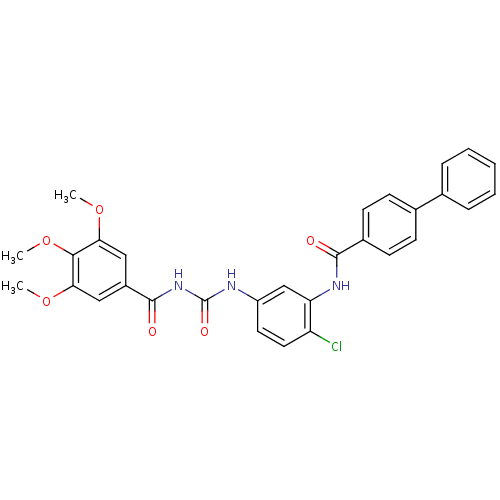 (CHEMBL2031077) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383036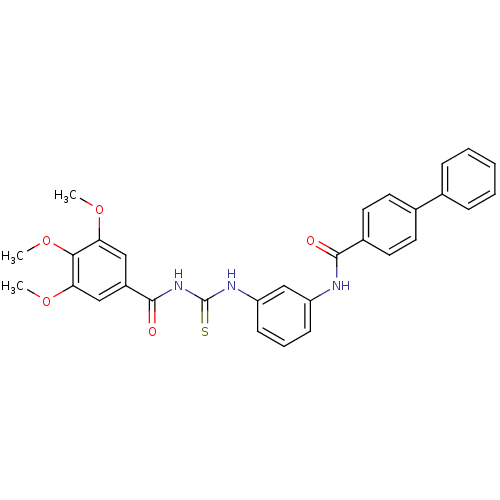 (CHEMBL2031098) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316285 (2-Dimethylamino-5-[6-(4-fluoro-2-methoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316288 (US10294218, Example 116 | US9617225, Example 116 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316293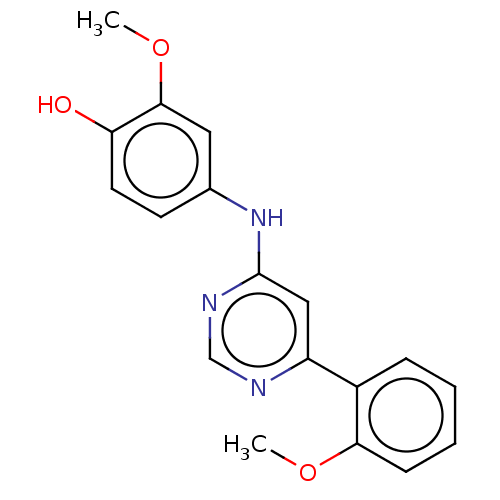 (2-Methoxy-4-[6-(2-methoxy-phenyl)-pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316301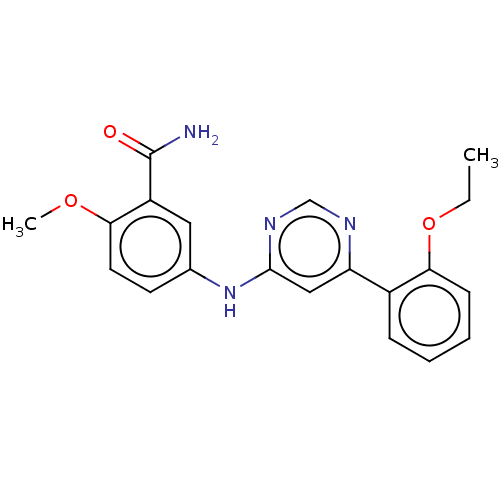 (5-[6-(2-Ethoxy-phenyl)-pyrimidin-4- ylamino]-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316302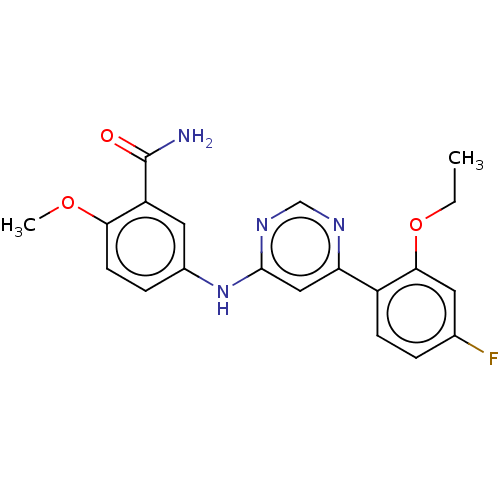 (5-[6-(2-Ethoxy-4-fluoro-phenyl)- pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM7585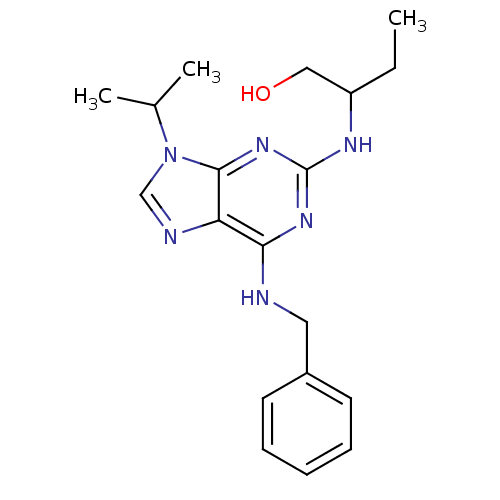 ((R,S)-Roscovitine | 2,6,9-Trisubstituted purine de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316203 (N-(3-((1H-benzo[d]imidazol-1- yl)methyl)-4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316217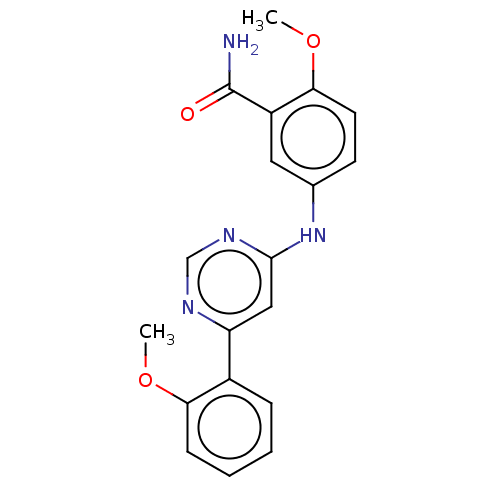 (2-methoxy-5-(6-(2- methoxyphenyl)pyrimidin-4- ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM5655 (2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50383037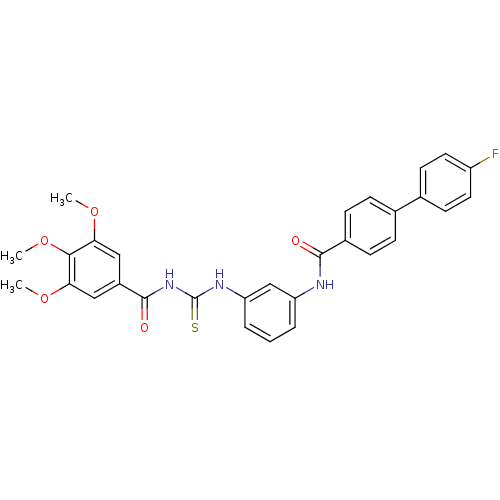 (CHEMBL2031245) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of Smo-mediated Hh signaling in human Shh-light2 cells by luciferase reporter gene assay | J Med Chem 55: 1559-71 (2012) Article DOI: 10.1021/jm2013369 BindingDB Entry DOI: 10.7270/Q24B32BR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316174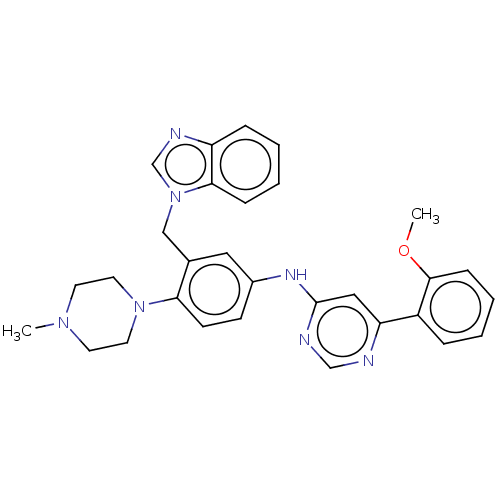 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-(4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316176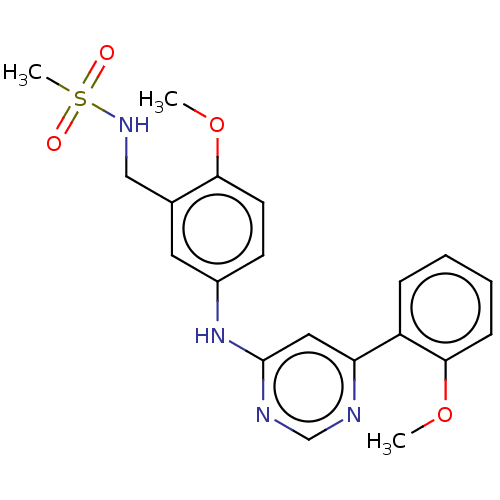 (N-(2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316179 (2-chloro-5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316181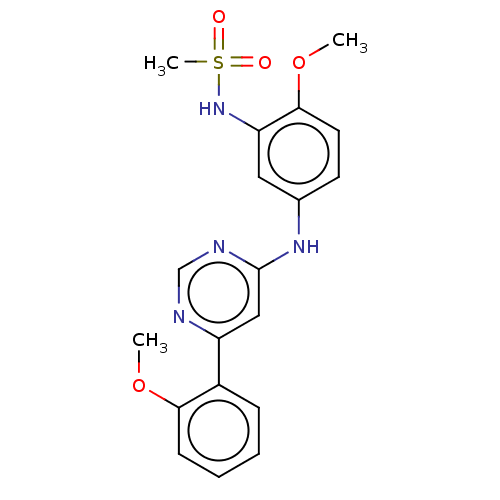 (N-(2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-yla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316182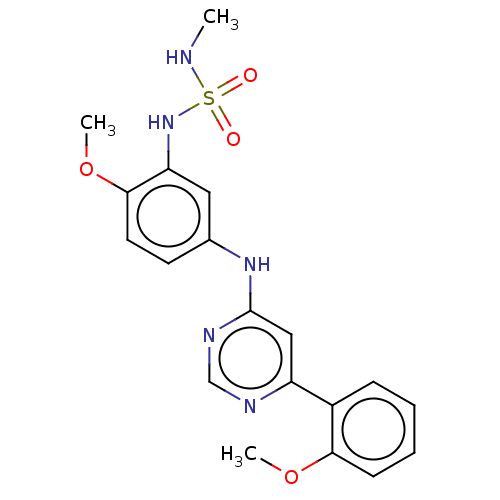 ((2-methoxy-5-(6-(2-methoxyphenyl) pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316188 (N-(3-((1H-benzo[d]imidazol-1- yl)methyl)-4-chlorop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316189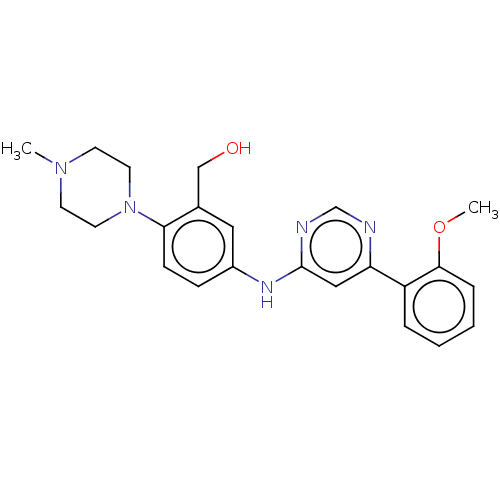 ((5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino)-2-(4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316202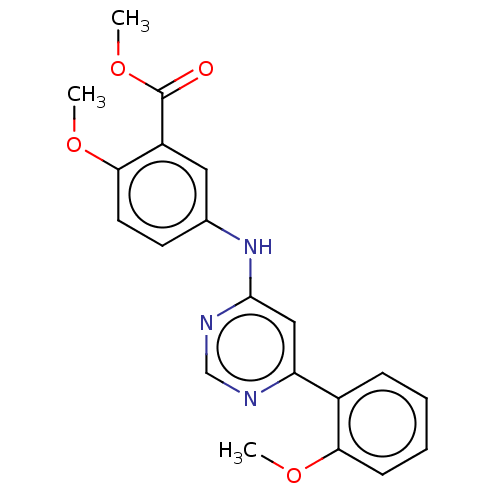 (US10294218, Example 30 | US9617225, Example 30 | m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316204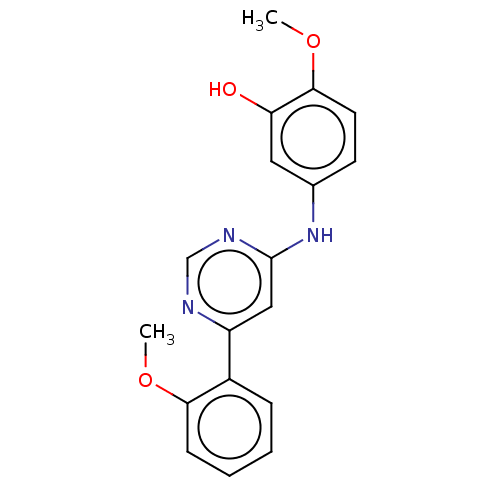 (2-methoxy-5-(6-(2- methoxyphenyl)pyrimidin-4- ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316205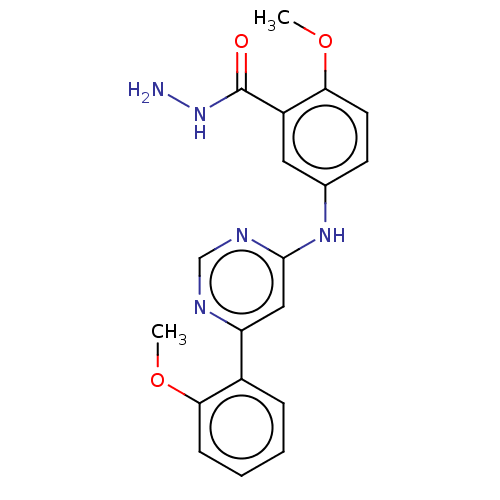 (2-methoxy-5-(6-(2-methoxyphenyl)pyrimidin-4-ylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316207 (N-(3-((dimethylamino)methyl)-4-methoxyphenyl)-6-(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316209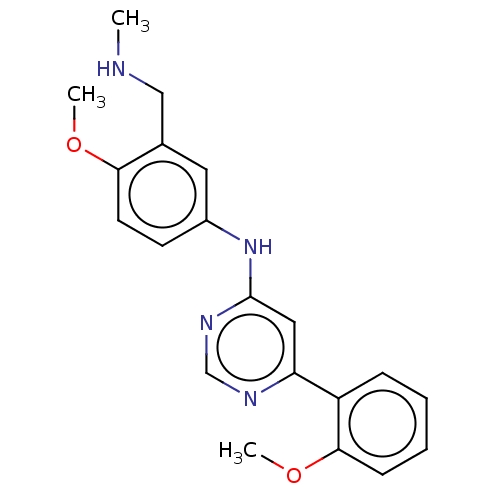 (N-(4-methoxy-3-((methylamino)methyl)phenyl)-6-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316210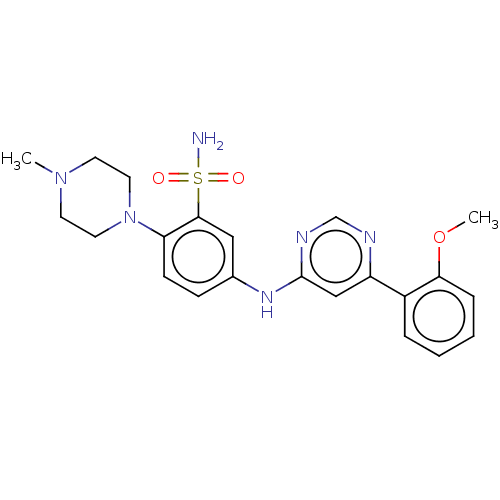 (5-(6-(2-methoxyphenyl)pyrimidin-4-ylamino)-2-(4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316211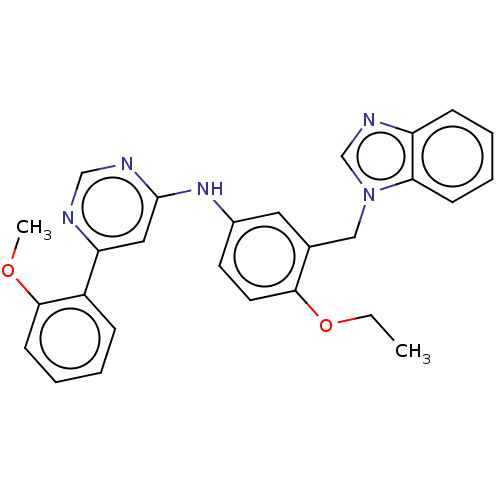 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-ethoxyph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316212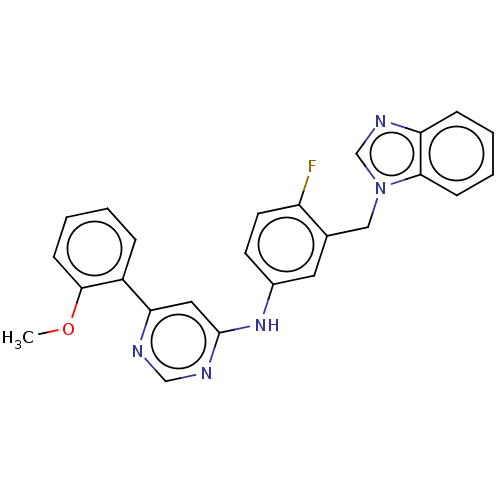 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-fluoroph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316227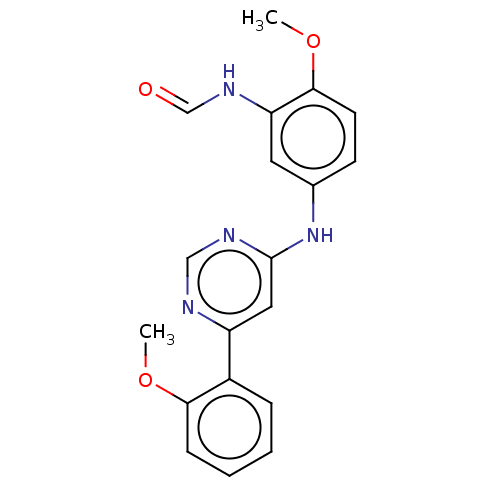 (N-{2-Methoxy-5-[6-(2-methoxy-phenyl)-pyrimidin-4-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316285 (2-Dimethylamino-5-[6-(4-fluoro-2-methoxy-phenyl)-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316288 (US10294218, Example 116 | US9617225, Example 116 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316293 (2-Methoxy-4-[6-(2-methoxy-phenyl)-pyrimidin-4-ylam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316301 (5-[6-(2-Ethoxy-phenyl)-pyrimidin-4- ylamino]-2-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316302 (5-[6-(2-Ethoxy-4-fluoro-phenyl)- pyrimidin-4-ylami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM7585 ((R,S)-Roscovitine | 2,6,9-Trisubstituted purine de...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
VIROSTATICS SRL US Patent | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | US Patent US9617225 (2017) BindingDB Entry DOI: 10.7270/Q29W0HK6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-T1/Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM316174 (N-(3-((1H-benzo[d]imidazol-1-yl)methyl)-4-(4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
GSK | Assay Description The activity of the compounds described in the present invention can be determined by measuring the phosphorylation of a fluorescently-labeled peptid... | Bioorg Med Chem Lett 18: 1022-6 (2008) BindingDB Entry DOI: 10.7270/Q2RB76X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 372 total ) | Next | Last >> |