 Found 382 hits with Last Name = 'tatlock' and Initial = 'jh'
Found 382 hits with Last Name = 'tatlock' and Initial = 'jh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061306

((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 40: 3979-85 (1998)
Article DOI: 10.1021/jm9704098
BindingDB Entry DOI: 10.7270/Q2V69K71 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403490
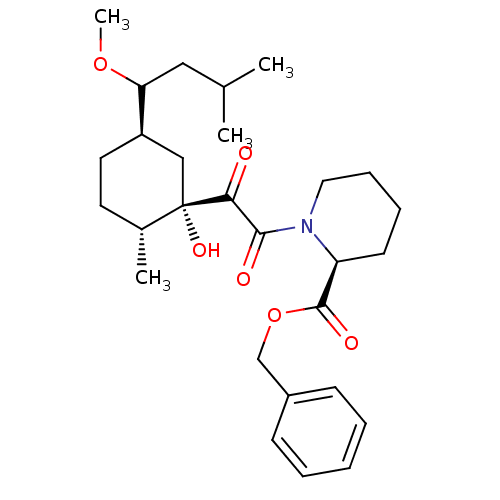
(CHEMBL312157)Show SMILES COC(CC(C)C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C28H41NO6/c1-19(2)16-24(34-4)22-14-13-20(3)28(33,17-22)25(30)26(31)29-15-9-8-12-23(29)27(32)35-18-21-10-6-5-7-11-21/h5-7,10-11,19-20,22-24,33H,8-9,12-18H2,1-4H3/t20-,22-,23+,24?,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061307

(AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H36N2O4S/c1-21-26(14-9-15-29(21)36)31(38)34-28(20-40-25-17-16-22-10-5-6-11-23(22)18-25)30(37)19-24-12-7-8-13-27(24)32(39)35-33(2,3)4/h5-18,28,30,36-37H,19-20H2,1-4H3,(H,34,38)(H,35,39)/t28-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 40: 3979-85 (1998)
Article DOI: 10.1021/jm9704098
BindingDB Entry DOI: 10.7270/Q2V69K71 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061307

(AG-1254 | CHEMBL128696 | N-[(1R,2R)-3-(2-tert-Buty...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H36N2O4S/c1-21-26(14-9-15-29(21)36)31(38)34-28(20-40-25-17-16-22-10-5-6-11-23(22)18-25)30(37)19-24-12-7-8-13-27(24)32(39)35-33(2,3)4/h5-18,28,30,36-37H,19-20H2,1-4H3,(H,34,38)(H,35,39)/t28-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286713
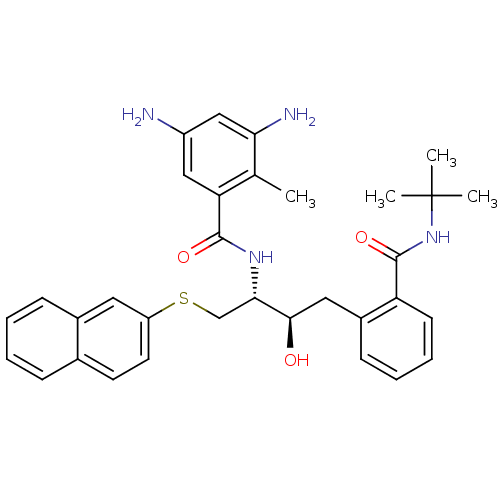
(3,5-Diamino-N-[(1R,2R)-3-(2-tert-butylcarbamoyl-ph...)Show SMILES Cc1c(N)cc(N)cc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H38N4O3S/c1-20-27(17-24(34)18-28(20)35)31(39)36-29(19-41-25-14-13-21-9-5-6-10-22(21)15-25)30(38)16-23-11-7-8-12-26(23)32(40)37-33(2,3)4/h5-15,17-18,29-30,38H,16,19,34-35H2,1-4H3,(H,36,39)(H,37,40)/t29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286699
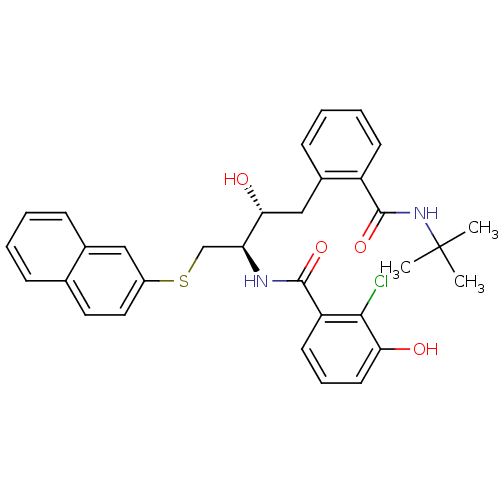
(AG-1309 | CHEMBL162065 | N-[(1R,2R)-3-(2-tert-Buty...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc(O)c1Cl Show InChI InChI=1S/C32H33ClN2O4S/c1-32(2,3)35-31(39)24-12-7-6-11-22(24)18-28(37)26(34-30(38)25-13-8-14-27(36)29(25)33)19-40-23-16-15-20-9-4-5-10-21(20)17-23/h4-17,26,28,36-37H,18-19H2,1-3H3,(H,34,38)(H,35,39)/t26-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286705
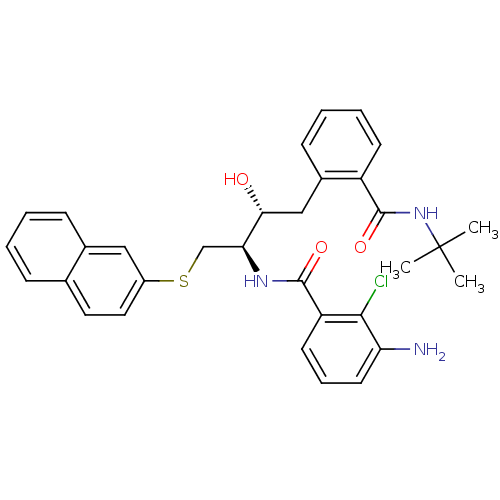
(3-Amino-N-[(1R,2R)-3-(2-tert-butylcarbamoyl-phenyl...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc(N)c1Cl Show InChI InChI=1S/C32H34ClN3O3S/c1-32(2,3)36-31(39)24-12-7-6-11-22(24)18-28(37)27(35-30(38)25-13-8-14-26(34)29(25)33)19-40-23-16-15-20-9-4-5-10-21(20)17-23/h4-17,27-28,37H,18-19,34H2,1-3H3,(H,35,38)(H,36,39)/t27-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286701

(5-Amino-N-[(1R,2R)-3-(2-tert-butylcarbamoyl-phenyl...)Show SMILES Cc1ccc(N)cc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H37N3O3S/c1-21-13-15-25(34)19-28(21)31(38)35-29(20-40-26-16-14-22-9-5-6-10-23(22)17-26)30(37)18-24-11-7-8-12-27(24)32(39)36-33(2,3)4/h5-17,19,29-30,37H,18,20,34H2,1-4H3,(H,35,38)(H,36,39)/t29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061308

((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C32H45N3O4/c1-21-25(15-10-16-28(21)36)30(38)33-26(17-22-11-6-5-7-12-22)29(37)20-35-19-24-14-9-8-13-23(24)18-27(35)31(39)34-32(2,3)4/h5-7,10-12,15-16,23-24,26-27,29,36-37H,8-9,13-14,17-20H2,1-4H3,(H,33,38)(H,34,39)/t23-,24+,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061308

((3S,4aS,8aS)-2-[(2R,3S)-2-Hydroxy-3-(3-hydroxy-2-m...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C32H45N3O4/c1-21-25(15-10-16-28(21)36)30(38)33-26(17-22-11-6-5-7-12-22)29(37)20-35-19-24-14-9-8-13-23(24)18-27(35)31(39)34-32(2,3)4/h5-7,10-12,15-16,23-24,26-27,29,36-37H,8-9,13-14,17-20H2,1-4H3,(H,33,38)(H,34,39)/t23-,24+,26-,27-,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 40: 3979-85 (1998)
Article DOI: 10.1021/jm9704098
BindingDB Entry DOI: 10.7270/Q2V69K71 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286703

(1,2,3,4-Tetrahydro-quinoline-5-carboxylic acid [(1...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2NCCCc12 Show InChI InChI=1S/C35H39N3O3S/c1-35(2,3)38-34(41)27-13-7-6-12-25(27)21-32(39)31(22-42-26-18-17-23-10-4-5-11-24(23)20-26)37-33(40)29-14-8-16-30-28(29)15-9-19-36-30/h4-8,10-14,16-18,20,31-32,36,39H,9,15,19,21-22H2,1-3H3,(H,37,40)(H,38,41)/t31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286709
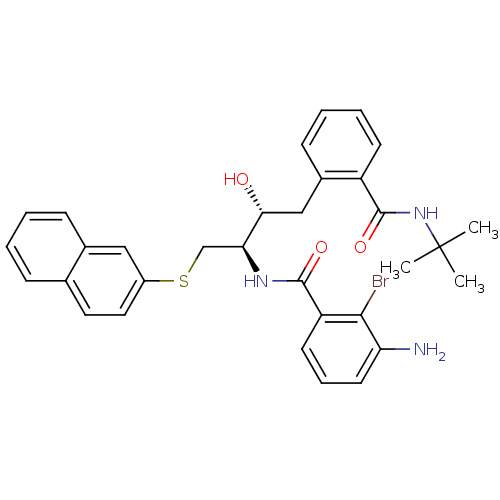
(3-Amino-2-bromo-N-[(1R,2R)-3-(2-tert-butylcarbamoy...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc(N)c1Br Show InChI InChI=1S/C32H34BrN3O3S/c1-32(2,3)36-31(39)24-12-7-6-11-22(24)18-28(37)27(35-30(38)25-13-8-14-26(34)29(25)33)19-40-23-16-15-20-9-4-5-10-21(20)17-23/h4-17,27-28,37H,18-19,34H2,1-3H3,(H,35,38)(H,36,39)/t27-,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286702

(3-Amino-N-[(1R,2R)-3-(2-tert-butylcarbamoyl-phenyl...)Show SMILES Cc1c(N)cccc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H37N3O3S/c1-21-26(14-9-15-28(21)34)31(38)35-29(20-40-25-17-16-22-10-5-6-11-23(22)18-25)30(37)19-24-12-7-8-13-27(24)32(39)36-33(2,3)4/h5-18,29-30,37H,19-20,34H2,1-4H3,(H,35,38)(H,36,39)/t29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403488
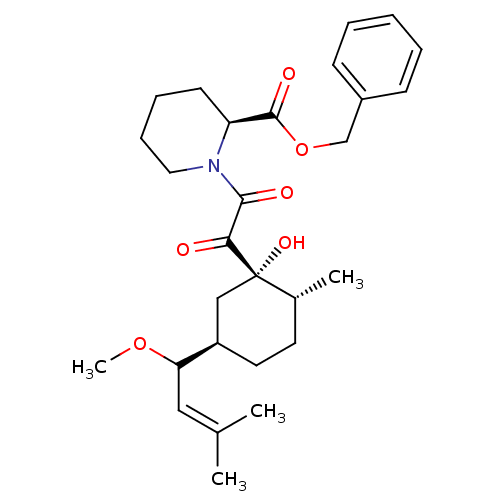
(CHEMBL81439)Show SMILES [#6]-[#8]-[#6](\[#6]=[#6](/[#6])-[#6])-[#6@@H]-1-[#6]-[#6]-[#6@@H](-[#6])[C@@]([#8])([#6]-1)[#6](=O)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#8]-[#6]-c1ccccc1 Show InChI InChI=1S/C28H39NO6/c1-19(2)16-24(34-4)22-14-13-20(3)28(33,17-22)25(30)26(31)29-15-9-8-12-23(29)27(32)35-18-21-10-6-5-7-11-21/h5-7,10-11,16,20,22-24,33H,8-9,12-15,17-18H2,1-4H3/t20-,22-,23+,24?,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403487
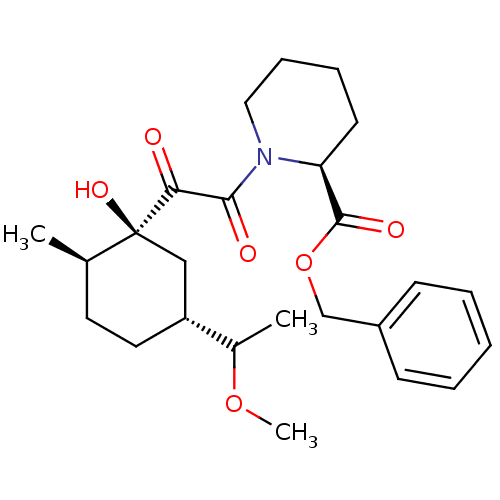
(CHEMBL82511)Show SMILES COC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C25H35NO6/c1-17-12-13-20(18(2)31-3)15-25(17,30)22(27)23(28)26-14-8-7-11-21(26)24(29)32-16-19-9-5-4-6-10-19/h4-6,9-10,17-18,20-21,30H,7-8,11-16H2,1-3H3/t17-,18?,20-,21+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50061305

((3S,4aS,8aS)-2-[(2R,3R)-2-Hydroxy-3-(3-hydroxy-2-m...)Show SMILES Cc1c(O)cccc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C36H47N3O4S/c1-23-29(14-9-15-32(23)40)34(42)37-30(22-44-28-17-16-24-10-5-6-11-25(24)18-28)33(41)21-39-20-27-13-8-7-12-26(27)19-31(39)35(43)38-36(2,3)4/h5-6,9-11,14-18,26-27,30-31,33,40-41H,7-8,12-13,19-22H2,1-4H3,(H,37,42)(H,38,43)/t26-,27+,30-,31-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
J Med Chem 40: 3979-85 (1998)
Article DOI: 10.1021/jm9704098
BindingDB Entry DOI: 10.7270/Q2V69K71 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403489
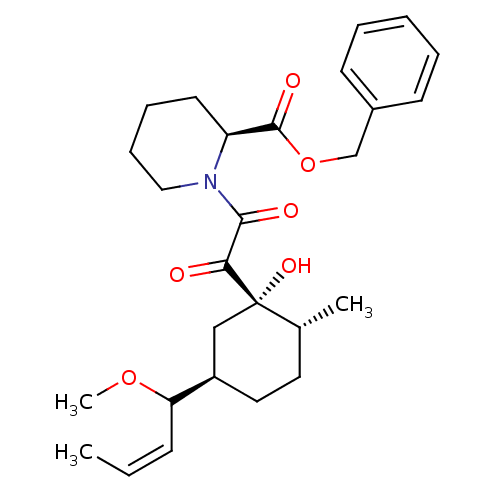
(CHEMBL79747)Show SMILES COC(\C=C/C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C27H37NO6/c1-4-10-23(33-3)21-15-14-19(2)27(32,17-21)24(29)25(30)28-16-9-8-13-22(28)26(31)34-18-20-11-6-5-7-12-20/h4-7,10-12,19,21-23,32H,8-9,13-18H2,1-3H3/b10-4-/t19-,21-,22+,23?,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286711
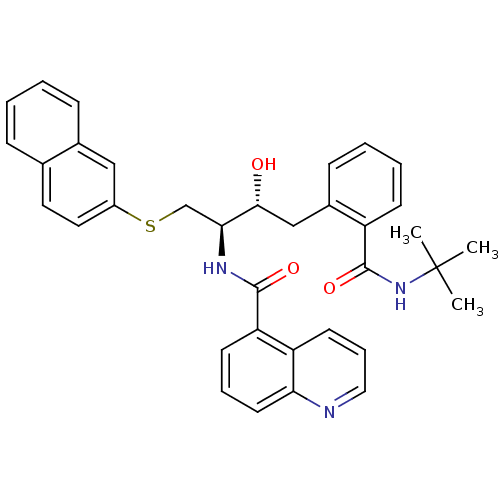
(CHEMBL354231 | Quinoline-5-carboxylic acid [(1R,2R...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2ncccc12 Show InChI InChI=1S/C35H35N3O3S/c1-35(2,3)38-34(41)27-13-7-6-12-25(27)21-32(39)31(22-42-26-18-17-23-10-4-5-11-24(23)20-26)37-33(40)29-14-8-16-30-28(29)15-9-19-36-30/h4-20,31-32,39H,21-22H2,1-3H3,(H,37,40)(H,38,41)/t31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408099

(CHEMBL57087)Show SMILES CC1(CNC(=O)N2CCCC[C@H]2C(=O)OCCCCc2ccccc2)CCCCC1 Show InChI InChI=1S/C25H38N2O3/c1-25(16-8-3-9-17-25)20-26-24(29)27-18-10-6-15-22(27)23(28)30-19-11-7-14-21-12-4-2-5-13-21/h2,4-5,12-13,22H,3,6-11,14-20H2,1H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408102

(CHEMBL55646)Show SMILES CC(C)(C)CNC(=O)N1CCCC[C@H]1C(=O)OCCCCc1ccccc1 Show InChI InChI=1S/C22H34N2O3/c1-22(2,3)17-23-21(26)24-15-9-7-14-19(24)20(25)27-16-10-8-13-18-11-5-4-6-12-18/h4-6,11-12,19H,7-10,13-17H2,1-3H3,(H,23,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286710
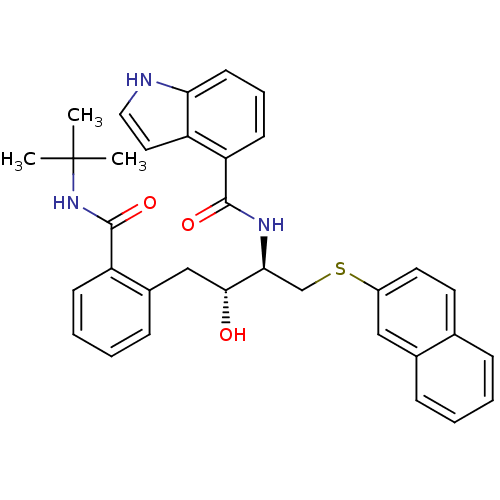
(1H-Indole-4-carboxylic acid [(1R,2R)-3-(2-tert-but...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2[nH]ccc12 Show InChI InChI=1S/C34H35N3O3S/c1-34(2,3)37-33(40)26-12-7-6-11-24(26)20-31(38)30(21-41-25-16-15-22-9-4-5-10-23(22)19-25)36-32(39)28-13-8-14-29-27(28)17-18-35-29/h4-19,30-31,35,38H,20-21H2,1-3H3,(H,36,39)(H,37,40)/t30-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286712
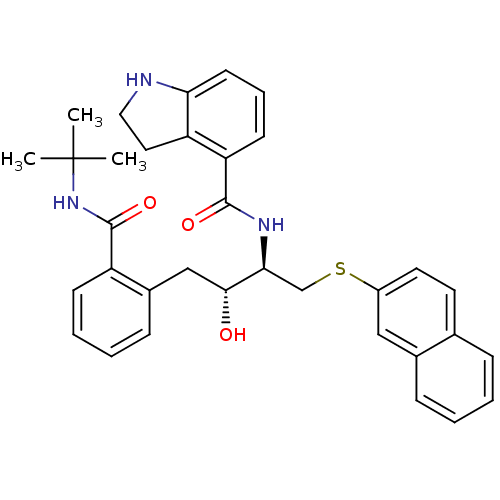
(2,3-Dihydro-1H-indole-4-carboxylic acid [(1R,2R)-3...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2NCCc12 Show InChI InChI=1S/C34H37N3O3S/c1-34(2,3)37-33(40)26-12-7-6-11-24(26)20-31(38)30(21-41-25-16-15-22-9-4-5-10-23(22)19-25)36-32(39)28-13-8-14-29-27(28)17-18-35-29/h4-16,19,30-31,35,38H,17-18,20-21H2,1-3H3,(H,36,39)(H,37,40)/t30-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286704
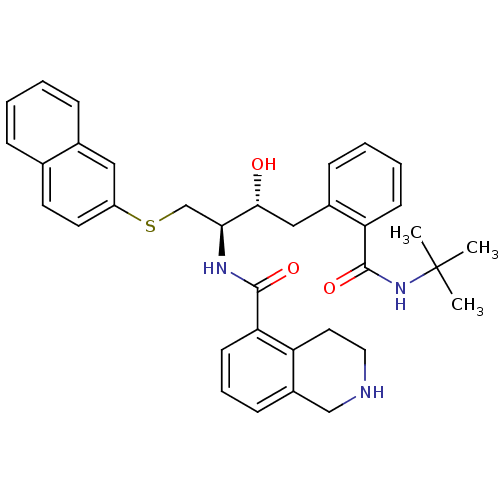
(1,2,3,4-Tetrahydro-isoquinoline-5-carboxylic acid ...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2CNCCc12 Show InChI InChI=1S/C35H39N3O3S/c1-35(2,3)38-34(41)29-13-7-6-11-25(29)20-32(39)31(22-42-27-16-15-23-9-4-5-10-24(23)19-27)37-33(40)30-14-8-12-26-21-36-18-17-28(26)30/h4-16,19,31-32,36,39H,17-18,20-22H2,1-3H3,(H,37,40)(H,38,41)/t31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408104
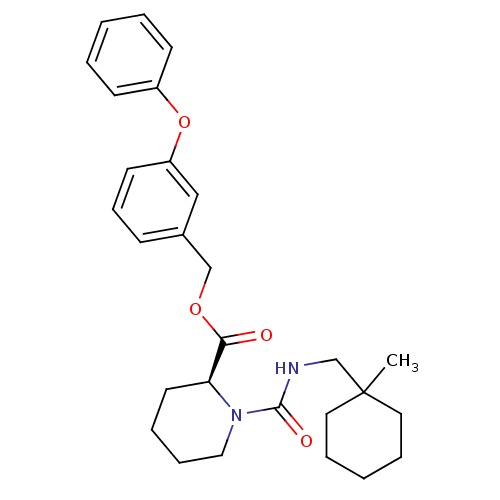
(CHEMBL292238)Show SMILES CC1(CNC(=O)N2CCCC[C@H]2C(=O)OCc2cccc(Oc3ccccc3)c2)CCCCC1 Show InChI InChI=1S/C28H36N2O4/c1-28(16-7-3-8-17-28)21-29-27(32)30-18-9-6-15-25(30)26(31)33-20-22-11-10-14-24(19-22)34-23-12-4-2-5-13-23/h2,4-5,10-14,19,25H,3,6-9,15-18,20-21H2,1H3,(H,29,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288769
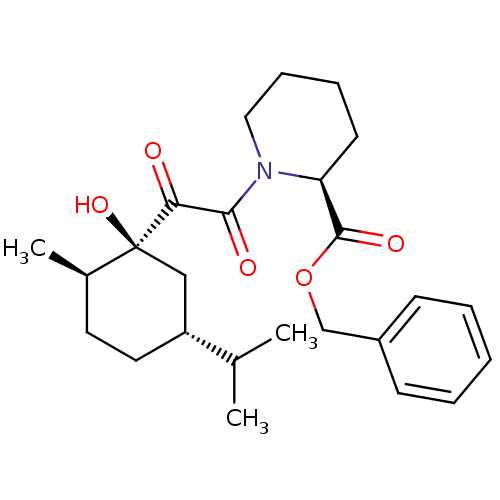
((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C25H35NO5/c1-17(2)20-13-12-18(3)25(30,15-20)22(27)23(28)26-14-8-7-11-21(26)24(29)31-16-19-9-5-4-6-10-19/h4-6,9-10,17-18,20-21,30H,7-8,11-16H2,1-3H3/t18-,20-,21+,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408110
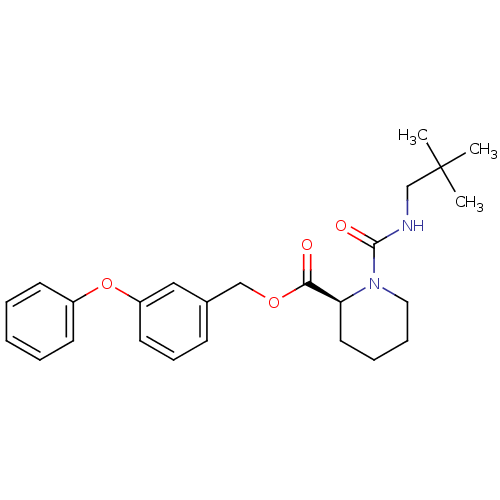
(CHEMBL293284)Show SMILES CC(C)(C)CNC(=O)N1CCCC[C@H]1C(=O)OCc1cccc(Oc2ccccc2)c1 Show InChI InChI=1S/C25H32N2O4/c1-25(2,3)18-26-24(29)27-15-8-7-14-22(27)23(28)30-17-19-10-9-13-21(16-19)31-20-11-5-4-6-12-20/h4-6,9-13,16,22H,7-8,14-15,17-18H2,1-3H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50403486
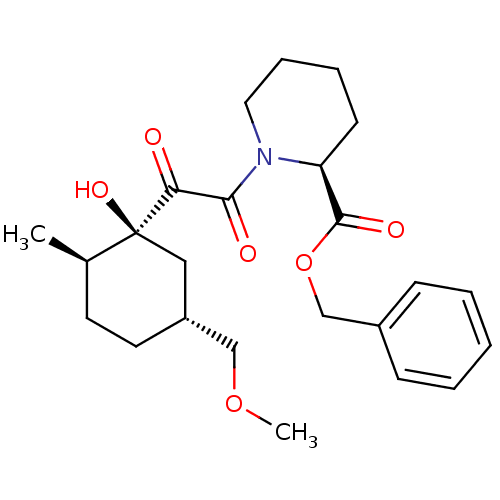
(CHEMBL82427)Show SMILES COC[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H33NO6/c1-17-11-12-19(15-30-2)14-24(17,29)21(26)22(27)25-13-7-6-10-20(25)23(28)31-16-18-8-4-3-5-9-18/h3-5,8-9,17,19-20,29H,6-7,10-16H2,1-2H3/t17-,19-,20+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity of compound against FK506 binding protein 12 was determined |
Bioorg Med Chem Lett 5: 2489-2494 (1995)
Article DOI: 10.1016/0960-894X(95)00429-W
BindingDB Entry DOI: 10.7270/Q24Q7W66 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286700
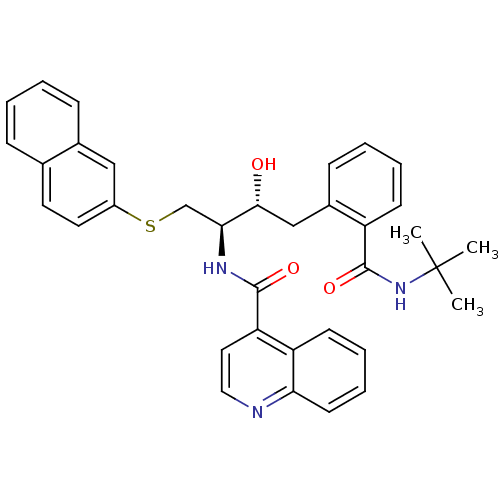
(CHEMBL162866 | Quinoline-4-carboxylic acid [(1R,2R...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1ccnc2ccccc12 Show InChI InChI=1S/C35H35N3O3S/c1-35(2,3)38-34(41)27-13-7-6-12-25(27)21-32(39)31(22-42-26-17-16-23-10-4-5-11-24(23)20-26)37-33(40)29-18-19-36-30-15-9-8-14-28(29)30/h4-20,31-32,39H,21-22H2,1-3H3,(H,37,40)(H,38,41)/t31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 265 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288762
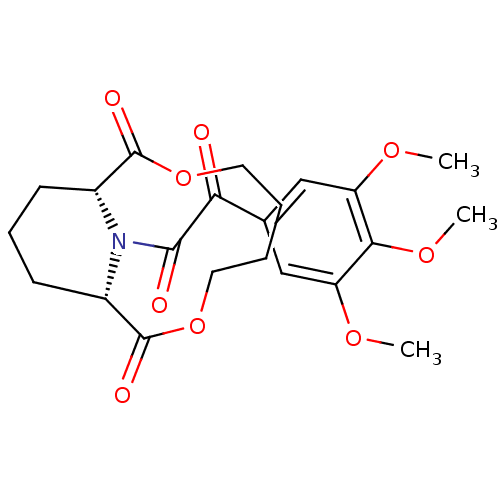
((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCCOC2=O Show InChI InChI=1S/C22H27NO9/c1-28-16-11-13(12-17(29-2)19(16)30-3)18(24)20(25)23-14-7-6-8-15(23)22(27)32-10-5-4-9-31-21(14)26/h11-12,14-15H,4-10H2,1-3H3/t14-,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408096

(CHEMBL301025)Show SMILES CC(C)(C)CNC(=O)N1CCCC[C@H]1C(=O)O[C@H](CCc1ccccc1)c1ccccc1 Show InChI InChI=1S/C27H36N2O3/c1-27(2,3)20-28-26(31)29-19-11-10-16-23(29)25(30)32-24(22-14-8-5-9-15-22)18-17-21-12-6-4-7-13-21/h4-9,12-15,23-24H,10-11,16-20H2,1-3H3,(H,28,31)/t23-,24+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408101

(CHEMBL293519)Show SMILES COc1cccc(CCCOC(=O)[C@@H]2CCCCN2C(=O)NCC(C)(C)C)c1 Show InChI InChI=1S/C22H34N2O4/c1-22(2,3)16-23-21(26)24-13-6-5-12-19(24)20(25)28-14-8-10-17-9-7-11-18(15-17)27-4/h7,9,11,15,19H,5-6,8,10,12-14,16H2,1-4H3,(H,23,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408094

(CHEMBL59256)Show SMILES COc1cc(CCCOC(=O)[C@@H]2CCCCN2C(=O)NCC(C)(C)C)cc(OC)c1OC Show InChI InChI=1S/C24H38N2O6/c1-24(2,3)16-25-23(28)26-12-8-7-11-18(26)22(27)32-13-9-10-17-14-19(29-4)21(31-6)20(15-17)30-5/h14-15,18H,7-13,16H2,1-6H3,(H,25,28)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286706
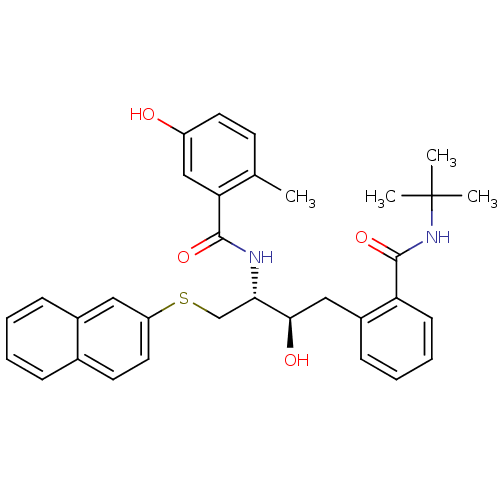
(AG-1273 | CHEMBL351021 | N-[(1R,2R)-3-(2-tert-Buty...)Show SMILES Cc1ccc(O)cc1C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H36N2O4S/c1-21-13-15-25(36)19-28(21)31(38)34-29(20-40-26-16-14-22-9-5-6-10-23(22)17-26)30(37)18-24-11-7-8-12-27(24)32(39)35-33(2,3)4/h5-17,19,29-30,36-37H,18,20H2,1-4H3,(H,34,38)(H,35,39)/t29-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286708

(AG-1224 | CHEMBL351391 | N-[(1R,2R)-3-(2-tert-Buty...)Show SMILES Cc1c(cccc1[N+]([O-])=O)C(=O)N[C@@H](CSc1ccc2ccccc2c1)[C@H](O)Cc1ccccc1C(=O)NC(C)(C)C Show InChI InChI=1S/C33H35N3O5S/c1-21-26(14-9-15-29(21)36(40)41)31(38)34-28(20-42-25-17-16-22-10-5-6-11-23(22)18-25)30(37)19-24-12-7-8-13-27(24)32(39)35-33(2,3)4/h5-18,28,30,37H,19-20H2,1-4H3,(H,34,38)(H,35,39)/t28-,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408105

(CHEMBL294146)Show SMILES CC1(CNC(=O)N2CCCC[C@H]2C(=O)OCc2ccccc2)CCCCC1 Show InChI InChI=1S/C22H32N2O3/c1-22(13-7-3-8-14-22)17-23-21(26)24-15-9-6-12-19(24)20(25)27-16-18-10-4-2-5-11-18/h2,4-5,10-11,19H,3,6-9,12-17H2,1H3,(H,23,26)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408100
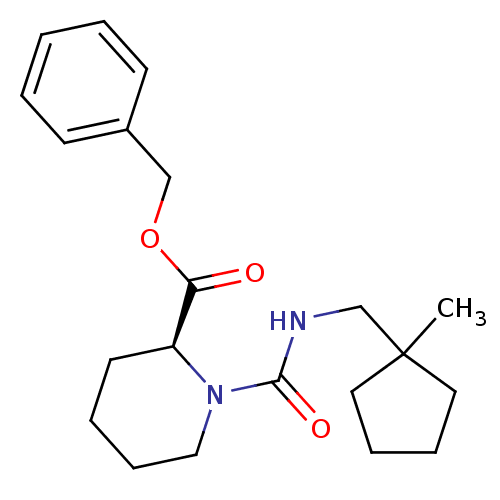
(CHEMBL57674)Show SMILES CC1(CNC(=O)N2CCCC[C@H]2C(=O)OCc2ccccc2)CCCC1 Show InChI InChI=1S/C21H30N2O3/c1-21(12-6-7-13-21)16-22-20(25)23-14-8-5-11-18(23)19(24)26-15-17-9-3-2-4-10-17/h2-4,9-10,18H,5-8,11-16H2,1H3,(H,22,25)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408103

(CHEMBL293294)Show InChI InChI=1S/C19H28N2O3/c1-19(2,3)14-20-18(23)21-12-8-7-11-16(21)17(22)24-13-15-9-5-4-6-10-15/h4-6,9-10,16H,7-8,11-14H2,1-3H3,(H,20,23)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50286707
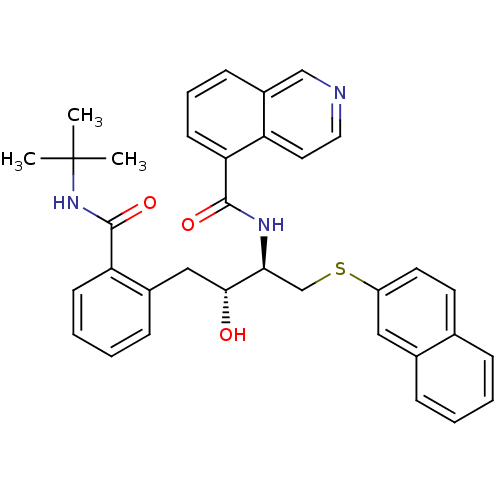
(CHEMBL445642 | Isoquinoline-5-carboxylic acid [(1R...)Show SMILES CC(C)(C)NC(=O)c1ccccc1C[C@@H](O)[C@H](CSc1ccc2ccccc2c1)NC(=O)c1cccc2cnccc12 Show InChI InChI=1S/C35H35N3O3S/c1-35(2,3)38-34(41)29-13-7-6-11-25(29)20-32(39)31(22-42-27-16-15-23-9-4-5-10-24(23)19-27)37-33(40)30-14-8-12-26-21-36-18-17-28(26)30/h4-19,21,31-32,39H,20,22H2,1-3H3,(H,37,40)(H,38,41)/t31-,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity HIV-1 protease enzyme |
Bioorg Med Chem Lett 5: 727-732 (1995)
Article DOI: 10.1016/0960-894X(95)00103-Z
BindingDB Entry DOI: 10.7270/Q29S1R0T |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288763

((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(COCc1ccccc1)COC2=O Show InChI InChI=1S/C29H33NO10/c1-35-23-12-20(13-24(36-2)26(23)37-3)25(31)27(32)30-21-10-7-11-22(30)29(34)40-17-19(16-39-28(21)33)15-38-14-18-8-5-4-6-9-18/h4-6,8-9,12-13,19,21-22H,7,10-11,14-17H2,1-3H3/t19?,21-,22+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288765

((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO[Si](C)(C)C(C)(C)C)COC2=O Show InChI InChI=1S/C28H41NO10Si/c1-28(2,3)40(7,8)39-16-17-14-37-26(32)19-10-9-11-20(27(33)38-15-17)29(19)25(31)23(30)18-12-21(34-4)24(36-6)22(13-18)35-5/h12-13,17,19-20H,9-11,14-16H2,1-8H3/t17?,19-,20+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408107
(CHEMBL56789)Show InChI InChI=1S/C21H24N2O3/c24-20(26-16-18-11-5-2-6-12-18)19-13-7-8-14-23(19)21(25)22-15-17-9-3-1-4-10-17/h1-6,9-12,19H,7-8,13-16H2,(H,22,25)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408097

(CHEMBL56270)Show InChI InChI=1S/C18H24N2O3/c1-14(2)12-19-18(22)20-11-7-6-10-16(20)17(21)23-13-15-8-4-3-5-9-15/h3-5,8-9,16H,1,6-7,10-13H2,2H3,(H,19,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
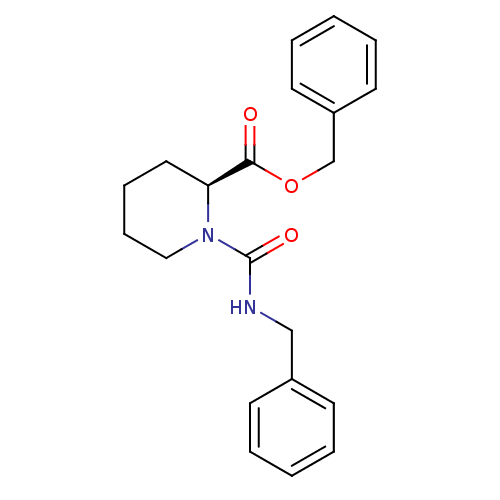
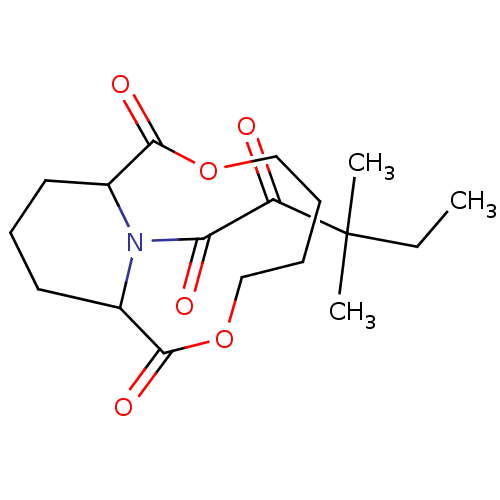
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288764
((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...)Show SMILES CC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C22H33NO7/c1-13(2)15-9-8-14(3)22(28,12-15)18(24)19(25)23-16-6-4-7-17(23)21(27)30-11-5-10-29-20(16)26/h13-17,28H,4-12H2,1-3H3/t14-,15-,16-,17+,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
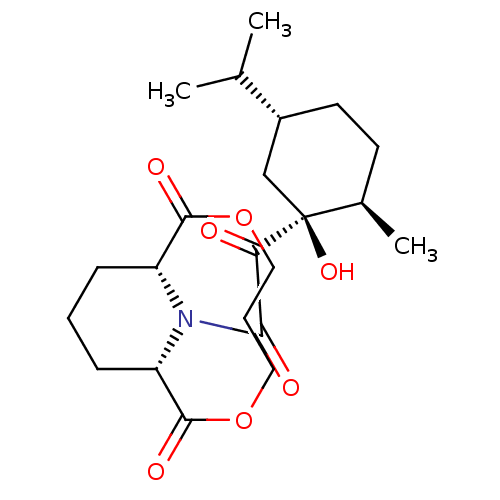
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288768
((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...)Show SMILES CCC(C)(C)C(=O)C(=O)N1C2CCCC1C(=O)OCCCCOC2=O Show InChI InChI=1S/C18H27NO6/c1-4-18(2,3)14(20)15(21)19-12-8-7-9-13(19)17(23)25-11-6-5-10-24-16(12)22/h12-13H,4-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408109

(CHEMBL54055)Show SMILES CC(C)(CO)CNC(=O)N1CCCC[C@H]1C(=O)OCc1ccccc1 Show InChI InChI=1S/C19H28N2O4/c1-19(2,14-22)13-20-18(24)21-11-7-6-10-16(21)17(23)25-12-15-8-4-3-5-9-15/h3-5,8-9,16,22H,6-7,10-14H2,1-2H3,(H,20,24)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288767
((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...)Show SMILES CCC(C)(C)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCCCOC2=O Show InChI InChI=1S/C17H25NO6/c1-4-17(2,3)13(19)14(20)18-11-7-5-8-12(18)16(22)24-10-6-9-23-15(11)21/h11-12H,4-10H2,1-3H3/t11-,12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408106

(CHEMBL56927)Show SMILES O=C(OCc1ccccc1)[C@@H]1CCC[C@H]2CCN(Cc3ccccc3)C(=O)N12 Show InChI InChI=1S/C23H26N2O3/c26-22(28-17-19-10-5-2-6-11-19)21-13-7-12-20-14-15-24(23(27)25(20)21)16-18-8-3-1-4-9-18/h1-6,8-11,20-21H,7,12-17H2/t20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408098

(CHEMBL298828)Show SMILES CCCN1CC[C@@H]2CCC[C@H](N2C1=O)C(=O)OCc1ccccc1 Show InChI InChI=1S/C19H26N2O3/c1-2-12-20-13-11-16-9-6-10-17(21(16)19(20)23)18(22)24-14-15-7-4-3-5-8-15/h3-5,7-8,16-17H,2,6,9-14H2,1H3/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50288766
((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...)Show SMILES COCC(C)[C@@H]1CC[C@@H](C)[C@@](O)(C1)C(=O)C(=O)N1[C@@H]2CCC[C@H]1C(=O)OCC(CO)COC2=O Show InChI InChI=1S/C24H37NO9/c1-14(11-32-3)17-8-7-15(2)24(31,9-17)20(27)21(28)25-18-5-4-6-19(25)23(30)34-13-16(10-26)12-33-22(18)29/h14-19,26,31H,4-13H2,1-3H3/t14?,15-,16?,17-,18-,19+,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibitory activity against human FKBP-12 rotamase |
Bioorg Med Chem Lett 6: 385-390 (1996)
Article DOI: 10.1016/0960-894X(96)00032-7
BindingDB Entry DOI: 10.7270/Q2862GFJ |
More data for this
Ligand-Target Pair | |
Peptidyl-prolyl cis-trans isomerase FKBP1A
(Homo sapiens (Human)) | BDBM50408095

(CHEMBL292166)Show SMILES COC(=O)[C@@H]1CCC[C@H]2CCN(Cc3ccccc3)C(=O)N12 Show InChI InChI=1S/C17H22N2O3/c1-22-16(20)15-9-5-8-14-10-11-18(17(21)19(14)15)12-13-6-3-2-4-7-13/h2-4,6-7,14-15H,5,8-12H2,1H3/t14-,15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Apparent inhibition constant of Wild-type human recombinant FK506 binding protein 12 |
J Med Chem 39: 1872-84 (1996)
Article DOI: 10.1021/jm950798a
BindingDB Entry DOI: 10.7270/Q2CZ38C2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data