 Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50032579
Found 50 hits Enz. Inhib. hit(s) with all data for entry = 50032579 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331382
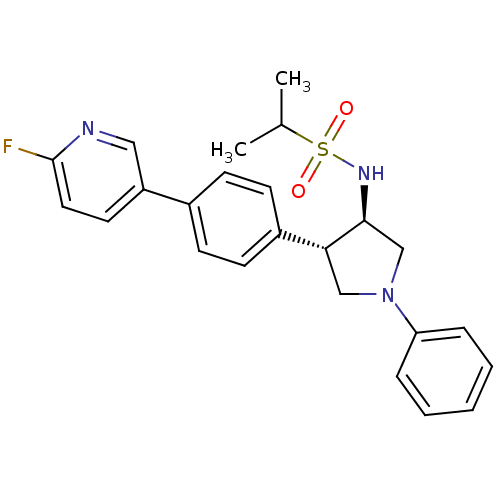
(CHEMBL1289063 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1)c1ccccc1 |r| Show InChI InChI=1S/C24H26FN3O2S/c1-17(2)31(29,30)27-23-16-28(21-6-4-3-5-7-21)15-22(23)19-10-8-18(9-11-19)20-12-13-24(25)26-14-20/h3-14,17,22-23,27H,15-16H2,1-2H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331371
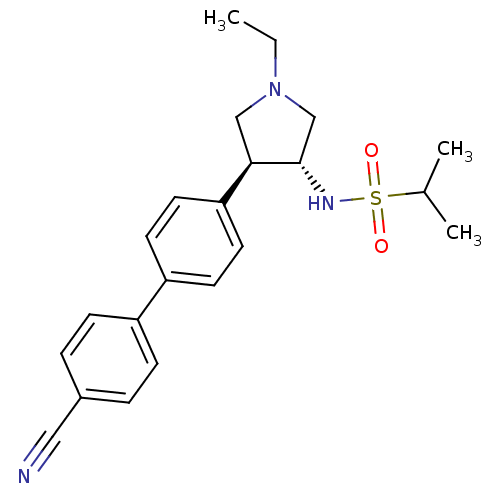
(CHEMBL1290275 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H27N3O2S/c1-4-25-14-21(22(15-25)24-28(26,27)16(2)3)20-11-9-19(10-12-20)18-7-5-17(13-23)6-8-18/h5-12,16,21-22,24H,4,14-15H2,1-3H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331373
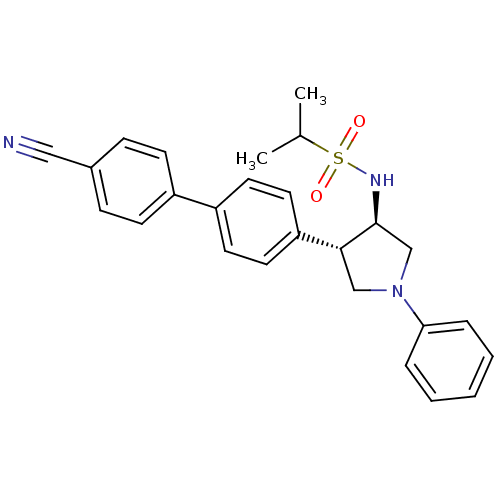
(CHEMBL1290391 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2S/c1-19(2)32(30,31)28-26-18-29(24-6-4-3-5-7-24)17-25(26)23-14-12-22(13-15-23)21-10-8-20(16-27)9-11-21/h3-15,19,25-26,28H,17-18H2,1-2H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331381
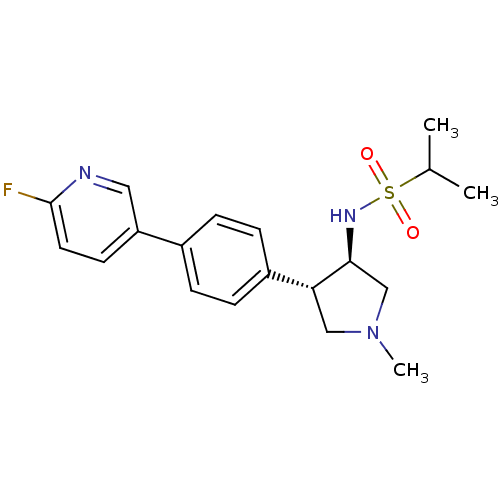
(CHEMBL1289062 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1 |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-6-4-14(5-7-15)16-8-9-19(20)21-10-16/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331369
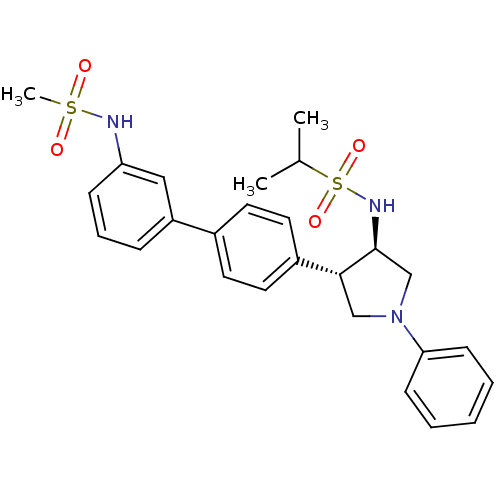
(CHEMBL1290165 | N-((3R,4R)-4-(3'-(methylsulfonamid...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1)c1ccccc1 |r| Show InChI InChI=1S/C26H31N3O4S2/c1-19(2)35(32,33)28-26-18-29(24-10-5-4-6-11-24)17-25(26)21-14-12-20(13-15-21)22-8-7-9-23(16-22)27-34(3,30)31/h4-16,19,25-28H,17-18H2,1-3H3/t25-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331370
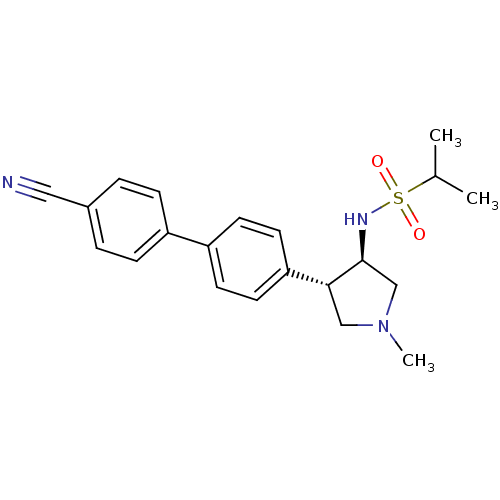
(CHEMBL1290166 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H25N3O2S/c1-15(2)27(25,26)23-21-14-24(3)13-20(21)19-10-8-18(9-11-19)17-6-4-16(12-22)5-7-17/h4-11,15,20-21,23H,13-14H2,1-3H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331367
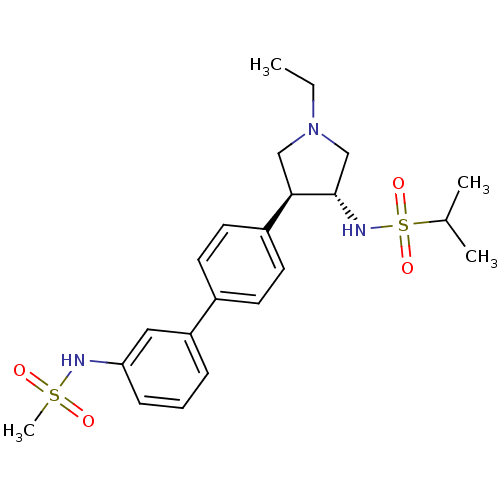
(CHEMBL1290058 | N-((3R,4R)-1-ethyl-4-(3'-(methylsu...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C22H31N3O4S2/c1-5-25-14-21(22(15-25)24-31(28,29)16(2)3)18-11-9-17(10-12-18)19-7-6-8-20(13-19)23-30(4,26)27/h6-13,16,21-24H,5,14-15H2,1-4H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331377
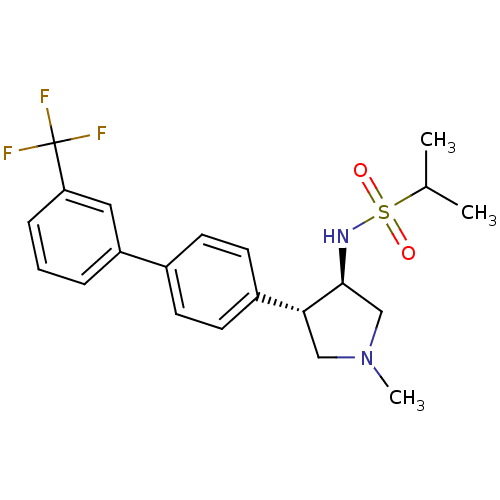
(CHEMBL1290612 | N-((3R,4R)-1-methyl-4-(3'-(trifluo...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H25F3N2O2S/c1-14(2)29(27,28)25-20-13-26(3)12-19(20)16-9-7-15(8-10-16)17-5-4-6-18(11-17)21(22,23)24/h4-11,14,19-20,25H,12-13H2,1-3H3/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331384
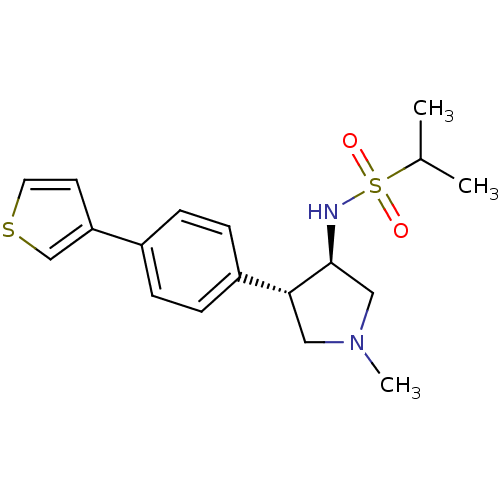
(CHEMBL1289180 | N-((3R,4R)-1-methyl-4-(4-(thiophen...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccsc1 |r| Show InChI InChI=1S/C18H24N2O2S2/c1-13(2)24(21,22)19-18-11-20(3)10-17(18)15-6-4-14(5-7-15)16-8-9-23-12-16/h4-9,12-13,17-19H,10-11H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331368
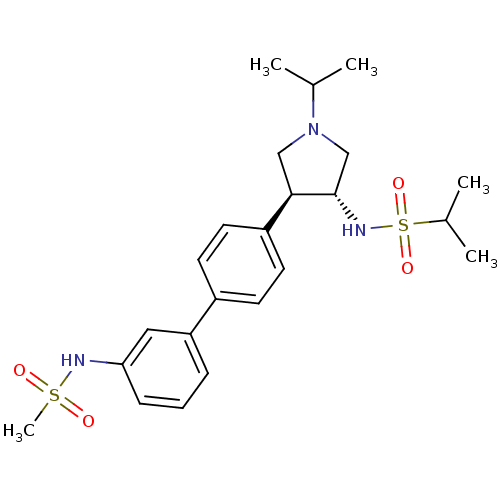
(CHEMBL1290059 | N-((3R,4R)-1-isopropyl-4-(3'-(meth...)Show SMILES CC(C)N1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C23H33N3O4S2/c1-16(2)26-14-22(23(15-26)25-32(29,30)17(3)4)19-11-9-18(10-12-19)20-7-6-8-21(13-20)24-31(5,27)28/h6-13,16-17,22-25H,14-15H2,1-5H3/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331380
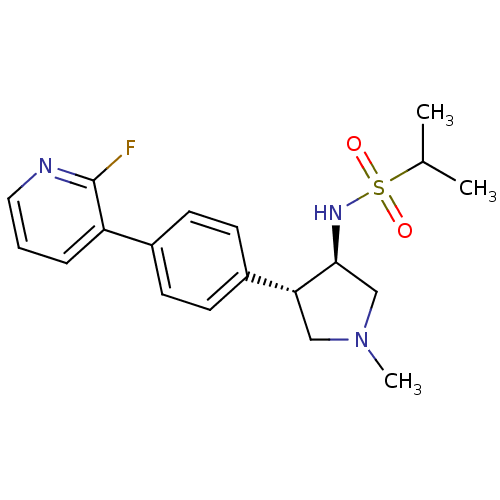
(CHEMBL1290732 | N-((3R,4R)-4-(4-(2-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccnc1F |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-8-6-14(7-9-15)16-5-4-10-21-19(16)20/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331376
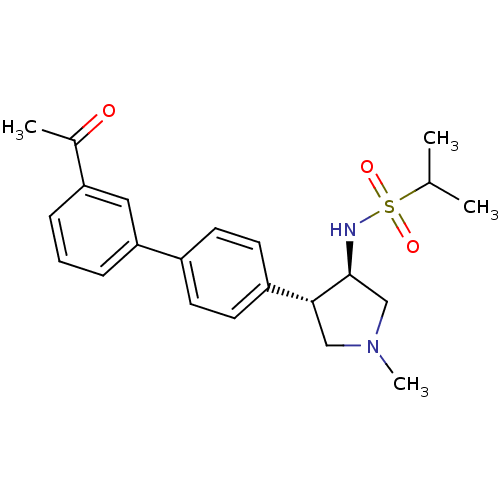
(CHEMBL1290504 | N-((3R,4R)-4-(3'-acetylbiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(C)=O |r| Show InChI InChI=1S/C22H28N2O3S/c1-15(2)28(26,27)23-22-14-24(4)13-21(22)18-10-8-17(9-11-18)20-7-5-6-19(12-20)16(3)25/h5-12,15,21-23H,13-14H2,1-4H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331387
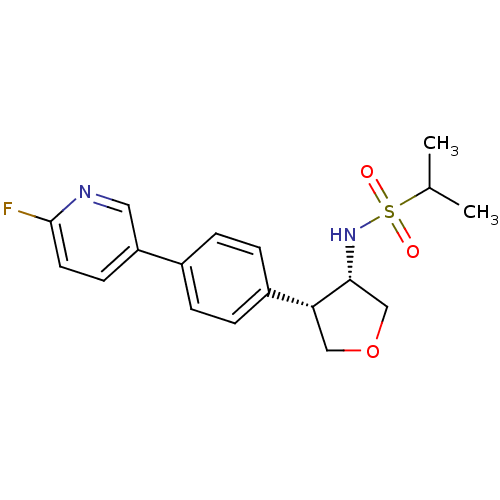
(CHEMBL1289294 | N-((3S,4S)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(F)nc1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-17-11-24-10-16(17)14-5-3-13(4-6-14)15-7-8-18(19)20-9-15/h3-9,12,16-17,21H,10-11H2,1-2H3/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331375
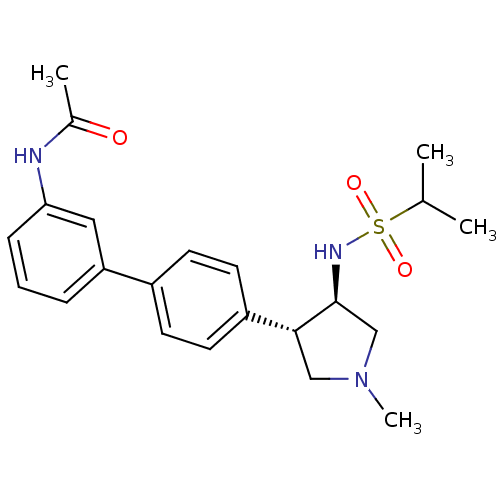
(CHEMBL1290503 | N-(4'-((3R,4R)-1-methyl-4-(1-methy...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(NC(C)=O)c1 |r| Show InChI InChI=1S/C22H29N3O3S/c1-15(2)29(27,28)24-22-14-25(4)13-21(22)18-10-8-17(9-11-18)19-6-5-7-20(12-19)23-16(3)26/h5-12,15,21-22,24H,13-14H2,1-4H3,(H,23,26)/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331385
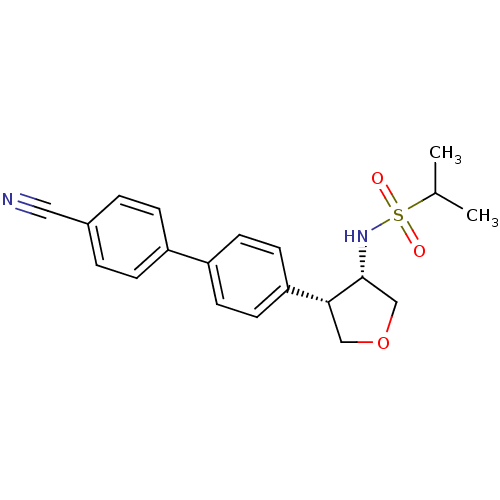
(CHEMBL1289181 | N-((3S,4S)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N2O3S/c1-14(2)26(23,24)22-20-13-25-12-19(20)18-9-7-17(8-10-18)16-5-3-15(11-21)4-6-16/h3-10,14,19-20,22H,12-13H2,1-2H3/t19-,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331379
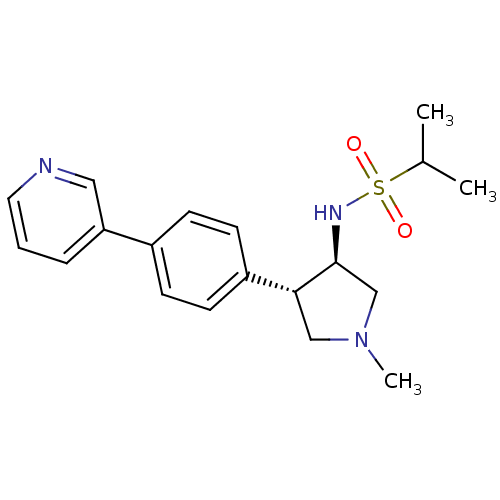
(CHEMBL1290731 | N-((3R,4R)-1-methyl-4-(4-(pyridin-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccnc1 |r| Show InChI InChI=1S/C19H25N3O2S/c1-14(2)25(23,24)21-19-13-22(3)12-18(19)16-8-6-15(7-9-16)17-5-4-10-20-11-17/h4-11,14,18-19,21H,12-13H2,1-3H3/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331366
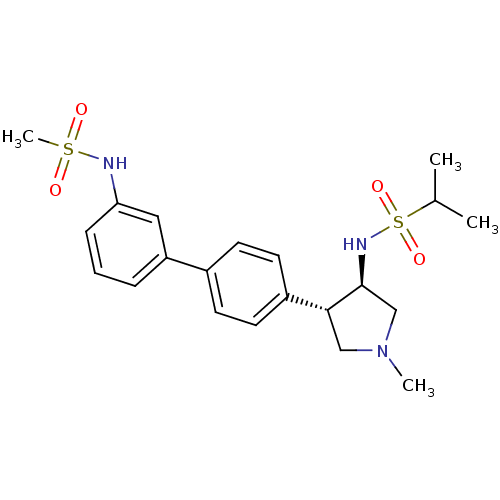
(CHEMBL1289960 | N-((3R,4R)-1-methyl-4-(3'-(methyls...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C21H29N3O4S2/c1-15(2)30(27,28)23-21-14-24(3)13-20(21)17-10-8-16(9-11-17)18-6-5-7-19(12-18)22-29(4,25)26/h5-12,15,20-23H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331386
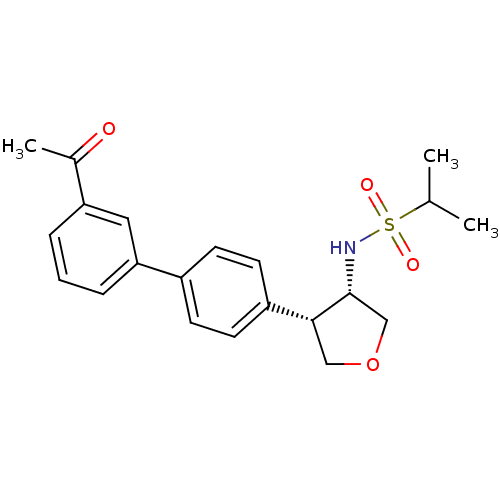
(CHEMBL1289293 | N-((3S,4S)-4-(3'-acetylbiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cccc(c1)C(C)=O |r| Show InChI InChI=1S/C21H25NO4S/c1-14(2)27(24,25)22-21-13-26-12-20(21)17-9-7-16(8-10-17)19-6-4-5-18(11-19)15(3)23/h4-11,14,20-22H,12-13H2,1-3H3/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331390
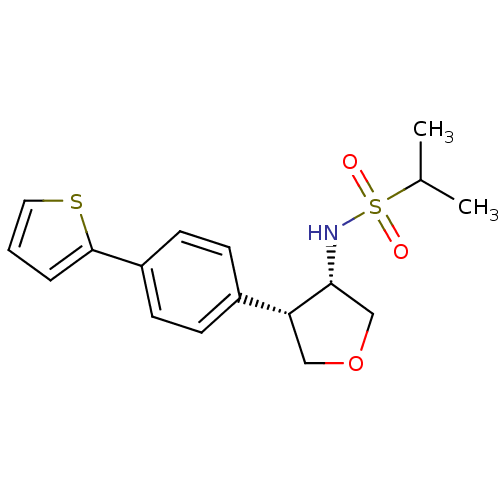
(CHEMBL1289410 | N-((3S,4S)-4-(4-(thiophen-2-yl)phe...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cccs1 |r| Show InChI InChI=1S/C17H21NO3S2/c1-12(2)23(19,20)18-16-11-21-10-15(16)13-5-7-14(8-6-13)17-4-3-9-22-17/h3-9,12,15-16,18H,10-11H2,1-2H3/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331391
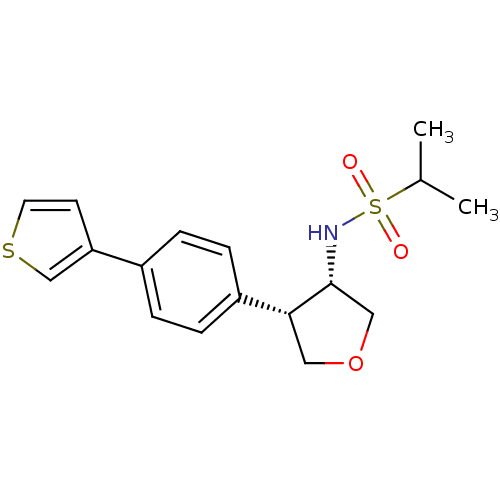
(CHEMBL1289523 | N-((3S,4S)-4-(4-(thiophen-3-yl)phe...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccsc1 |r| Show InChI InChI=1S/C17H21NO3S2/c1-12(2)23(19,20)18-17-10-21-9-16(17)14-5-3-13(4-6-14)15-7-8-22-11-15/h3-8,11-12,16-18H,9-10H2,1-2H3/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331383
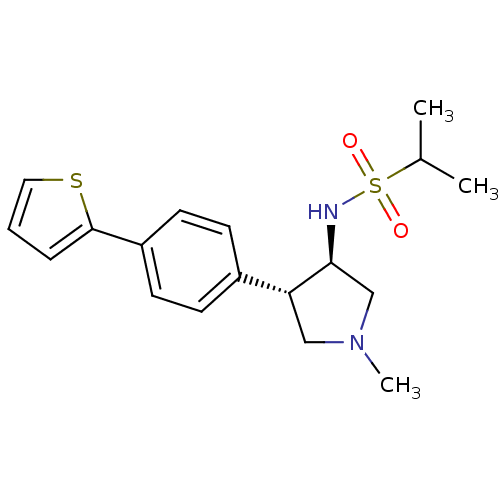
(CHEMBL1289179 | N-((3R,4R)-1-methyl-4-(4-(thiophen...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccs1 |r| Show InChI InChI=1S/C18H24N2O2S2/c1-13(2)24(21,22)19-17-12-20(3)11-16(17)14-6-8-15(9-7-14)18-5-4-10-23-18/h4-10,13,16-17,19H,11-12H2,1-3H3/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331389
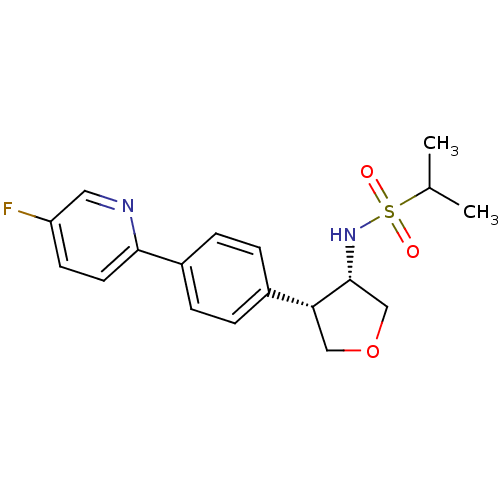
(CHEMBL1289409 | N-((3S,4S)-4-(4-(5-fluoropyridin-2...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(F)cn1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-18-11-24-10-16(18)13-3-5-14(6-4-13)17-8-7-15(19)9-20-17/h3-9,12,16,18,21H,10-11H2,1-2H3/t16-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331374
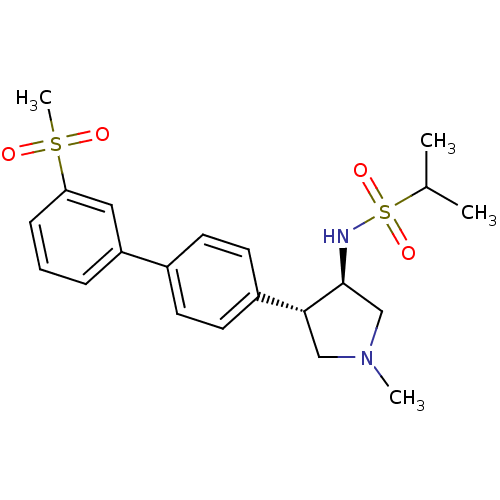
(CHEMBL1290392 | N-((3R,4R)-1-methyl-4-(3'-(methyls...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S2/c1-15(2)29(26,27)22-21-14-23(3)13-20(21)17-10-8-16(9-11-17)18-6-5-7-19(12-18)28(4,24)25/h5-12,15,20-22H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50331388
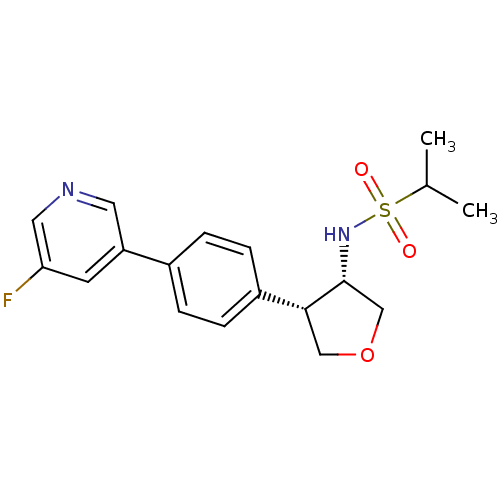
(CHEMBL1289408 | N-((3S,4S)-4-(4-(5-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cncc(F)c1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-18-11-24-10-17(18)14-5-3-13(4-6-14)15-7-16(19)9-20-8-15/h3-9,12,17-18,21H,10-11H2,1-2H3/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]-dofetilide from human hERG |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331377

(CHEMBL1290612 | N-((3R,4R)-1-methyl-4-(3'-(trifluo...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H25F3N2O2S/c1-14(2)29(27,28)25-20-13-26(3)12-19(20)16-9-7-15(8-10-16)17-5-4-6-18(11-17)21(22,23)24/h4-11,14,19-20,25H,12-13H2,1-3H3/t19-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.94E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331376

(CHEMBL1290504 | N-((3R,4R)-4-(3'-acetylbiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)C(C)=O |r| Show InChI InChI=1S/C22H28N2O3S/c1-15(2)28(26,27)23-22-14-24(4)13-21(22)18-10-8-17(9-11-18)20-7-5-6-19(12-20)16(3)25/h5-12,15,21-23H,13-14H2,1-4H3/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331375

(CHEMBL1290503 | N-(4'-((3R,4R)-1-methyl-4-(1-methy...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(NC(C)=O)c1 |r| Show InChI InChI=1S/C22H29N3O3S/c1-15(2)29(27,28)24-22-14-25(4)13-21(22)18-10-8-17(9-11-18)19-6-5-7-20(12-19)23-16(3)26/h5-12,15,21-22,24H,13-14H2,1-4H3,(H,23,26)/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331374

(CHEMBL1290392 | N-((3R,4R)-1-methyl-4-(3'-(methyls...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(c1)S(C)(=O)=O |r| Show InChI InChI=1S/C21H28N2O4S2/c1-15(2)29(26,27)22-21-14-23(3)13-20(21)17-10-8-16(9-11-17)18-6-5-7-19(12-18)28(4,24)25/h5-12,15,20-22H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331373

(CHEMBL1290391 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N)c1ccccc1 |r| Show InChI InChI=1S/C26H27N3O2S/c1-19(2)32(30,31)28-26-18-29(24-6-4-3-5-7-24)17-25(26)23-14-12-22(13-15-23)21-10-8-20(16-27)9-11-21/h3-15,19,25-26,28H,17-18H2,1-2H3/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331372
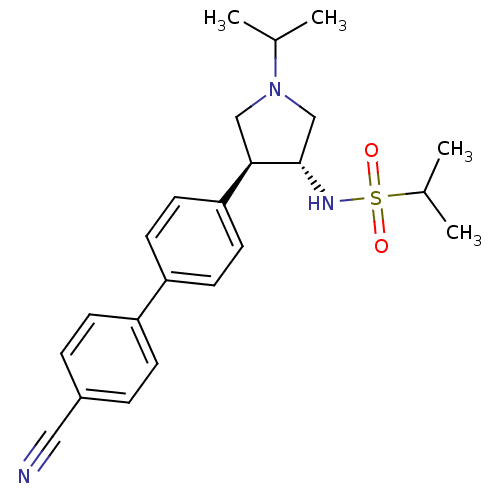
(CHEMBL1290276 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)N1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C23H29N3O2S/c1-16(2)26-14-22(23(15-26)25-29(27,28)17(3)4)21-11-9-20(10-12-21)19-7-5-18(13-24)6-8-19/h5-12,16-17,22-23,25H,14-15H2,1-4H3/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331371

(CHEMBL1290275 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C22H27N3O2S/c1-4-25-14-21(22(15-25)24-28(26,27)16(2)3)20-11-9-19(10-12-20)18-7-5-17(13-23)6-8-18/h5-12,16,21-22,24H,4,14-15H2,1-3H3/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331370

(CHEMBL1290166 | N-((3R,4R)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H25N3O2S/c1-15(2)27(25,26)23-21-14-24(3)13-20(21)19-10-8-18(9-11-19)17-6-4-16(12-22)5-7-17/h4-11,15,20-21,23H,13-14H2,1-3H3/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331369

(CHEMBL1290165 | N-((3R,4R)-4-(3'-(methylsulfonamid...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1)c1ccccc1 |r| Show InChI InChI=1S/C26H31N3O4S2/c1-19(2)35(32,33)28-26-18-29(24-10-5-4-6-11-24)17-25(26)21-14-12-20(13-15-21)22-8-7-9-23(16-22)27-34(3,30)31/h4-16,19,25-28H,17-18H2,1-3H3/t25-,26+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331368

(CHEMBL1290059 | N-((3R,4R)-1-isopropyl-4-(3'-(meth...)Show SMILES CC(C)N1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C23H33N3O4S2/c1-16(2)26-14-22(23(15-26)25-32(29,30)17(3)4)19-11-9-18(10-12-19)20-7-6-8-21(13-20)24-31(5,27)28/h6-13,16-17,22-25H,14-15H2,1-5H3/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331367

(CHEMBL1290058 | N-((3R,4R)-1-ethyl-4-(3'-(methylsu...)Show SMILES CCN1C[C@H](NS(=O)(=O)C(C)C)[C@H](C1)c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C22H31N3O4S2/c1-5-25-14-21(22(15-25)24-31(28,29)16(2)3)18-11-9-17(10-12-18)19-7-6-8-20(13-19)23-30(4,26)27/h6-13,16,21-24H,5,14-15H2,1-4H3/t21-,22+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331366

(CHEMBL1289960 | N-((3R,4R)-1-methyl-4-(3'-(methyls...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccc(NS(C)(=O)=O)c1 |r| Show InChI InChI=1S/C21H29N3O4S2/c1-15(2)30(27,28)23-21-14-24(3)13-20(21)17-10-8-16(9-11-17)18-6-5-7-19(12-18)22-29(4,25)26/h5-12,15,20-23H,13-14H2,1-4H3/t20-,21+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331391

(CHEMBL1289523 | N-((3S,4S)-4-(4-(thiophen-3-yl)phe...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccsc1 |r| Show InChI InChI=1S/C17H21NO3S2/c1-12(2)23(19,20)18-17-10-21-9-16(17)14-5-3-13(4-6-14)15-7-8-22-11-15/h3-8,11-12,16-18H,9-10H2,1-2H3/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331390

(CHEMBL1289410 | N-((3S,4S)-4-(4-(thiophen-2-yl)phe...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cccs1 |r| Show InChI InChI=1S/C17H21NO3S2/c1-12(2)23(19,20)18-16-11-21-10-15(16)13-5-7-14(8-6-13)17-4-3-9-22-17/h3-9,12,15-16,18H,10-11H2,1-2H3/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331389

(CHEMBL1289409 | N-((3S,4S)-4-(4-(5-fluoropyridin-2...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(F)cn1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-18-11-24-10-16(18)13-3-5-14(6-4-13)17-8-7-15(19)9-20-17/h3-9,12,16,18,21H,10-11H2,1-2H3/t16-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331388

(CHEMBL1289408 | N-((3S,4S)-4-(4-(5-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cncc(F)c1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-18-11-24-10-17(18)14-5-3-13(4-6-14)15-7-16(19)9-20-8-15/h3-9,12,17-18,21H,10-11H2,1-2H3/t17-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331387

(CHEMBL1289294 | N-((3S,4S)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(F)nc1 |r| Show InChI InChI=1S/C18H21FN2O3S/c1-12(2)25(22,23)21-17-11-24-10-16(17)14-5-3-13(4-6-14)15-7-8-18(19)20-9-15/h3-9,12,16-17,21H,10-11H2,1-2H3/t16-,17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331386

(CHEMBL1289293 | N-((3S,4S)-4-(3'-acetylbiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1cccc(c1)C(C)=O |r| Show InChI InChI=1S/C21H25NO4S/c1-14(2)27(24,25)22-21-13-26-12-20(21)17-9-7-16(8-10-17)19-6-4-5-18(11-19)15(3)23/h4-11,14,20-22H,12-13H2,1-3H3/t20-,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331385

(CHEMBL1289181 | N-((3S,4S)-4-(4'-cyanobiphenyl-4-y...)Show SMILES CC(C)S(=O)(=O)N[C@@H]1COC[C@@H]1c1ccc(cc1)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C20H22N2O3S/c1-14(2)26(23,24)22-20-13-25-12-19(20)18-9-7-17(8-10-18)16-5-3-15(11-21)4-6-16/h3-10,14,19-20,22H,12-13H2,1-2H3/t19-,20-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331384

(CHEMBL1289180 | N-((3R,4R)-1-methyl-4-(4-(thiophen...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccsc1 |r| Show InChI InChI=1S/C18H24N2O2S2/c1-13(2)24(21,22)19-18-11-20(3)10-17(18)15-6-4-14(5-7-15)16-8-9-23-12-16/h4-9,12-13,17-19H,10-11H2,1-3H3/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331383

(CHEMBL1289179 | N-((3R,4R)-1-methyl-4-(4-(thiophen...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccs1 |r| Show InChI InChI=1S/C18H24N2O2S2/c1-13(2)24(21,22)19-17-12-20(3)11-16(17)14-6-8-15(9-7-14)18-5-4-10-23-18/h4-10,13,16-17,19H,11-12H2,1-3H3/t16-,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331382

(CHEMBL1289063 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1)c1ccccc1 |r| Show InChI InChI=1S/C24H26FN3O2S/c1-17(2)31(29,30)27-23-16-28(21-6-4-3-5-7-21)15-22(23)19-10-8-18(9-11-19)20-12-13-24(25)26-14-20/h3-14,17,22-23,27H,15-16H2,1-2H3/t22-,23+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331381

(CHEMBL1289062 | N-((3R,4R)-4-(4-(6-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccc(F)nc1 |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-6-4-14(5-7-15)16-8-9-19(20)21-10-16/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331378
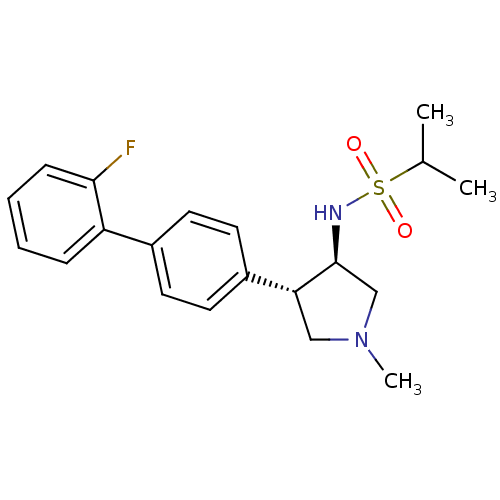
(CHEMBL1290613 | N-((3R,4R)-4-(2'-fluorobiphenyl-4-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1ccccc1F |r| Show InChI InChI=1S/C20H25FN2O2S/c1-14(2)26(24,25)22-20-13-23(3)12-18(20)16-10-8-15(9-11-16)17-6-4-5-7-19(17)21/h4-11,14,18,20,22H,12-13H2,1-3H3/t18-,20+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331379

(CHEMBL1290731 | N-((3R,4R)-1-methyl-4-(4-(pyridin-...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccnc1 |r| Show InChI InChI=1S/C19H25N3O2S/c1-14(2)25(23,24)21-19-13-22(3)12-18(19)16-8-6-15(7-9-16)17-5-4-10-20-11-17/h4-11,14,18-19,21H,12-13H2,1-3H3/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50331380

(CHEMBL1290732 | N-((3R,4R)-4-(4-(2-fluoropyridin-3...)Show SMILES CC(C)S(=O)(=O)N[C@H]1CN(C)C[C@@H]1c1ccc(cc1)-c1cccnc1F |r| Show InChI InChI=1S/C19H24FN3O2S/c1-13(2)26(24,25)22-18-12-23(3)11-17(18)15-8-6-14(7-9-15)16-5-4-10-21-19(16)20/h4-10,13,17-18,22H,11-12H2,1-3H3/t17-,18+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Positive modulation of human GluA2 flip receptor by FLIPR assay |
Bioorg Med Chem Lett 20: 7116-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.062
BindingDB Entry DOI: 10.7270/Q2WQ042G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data