

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50132083 (CHEMBL125622 | [3-Nitro-2-(2-nitro-phenyl)-4-oxo-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory concentration of compound towards cell surface aminopeptidase N (APN/CD13) | J Med Chem 46: 3900-13 (2003) Article DOI: 10.1021/jm021109f BindingDB Entry DOI: 10.7270/Q2ZP45JZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calnexin (Homo sapiens (Human)) | BDBM50421603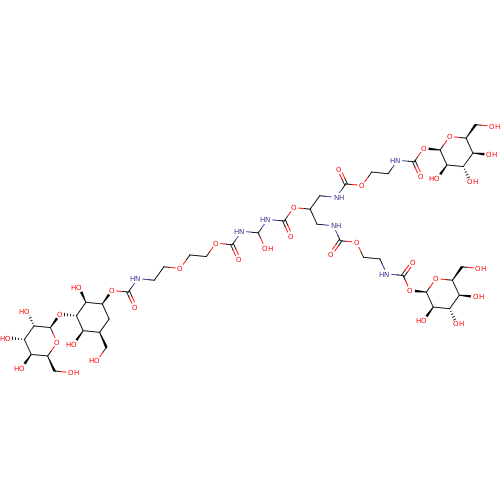 (CHEMBL2303776) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
UMR 176 CNRS-Institut Curie Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit binding of [3H]- Glc1Man9 GlcNAc2 to GST-fused calnexin immobilized on glutathione-agarose | Bioorg Med Chem Lett 12: 1237-40 (2002) BindingDB Entry DOI: 10.7270/Q26D5SBH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057812 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057803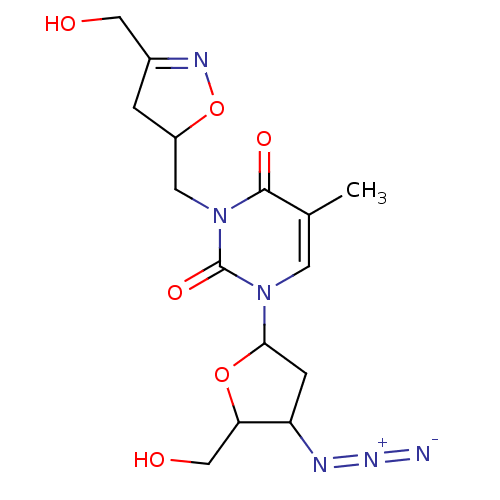 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057809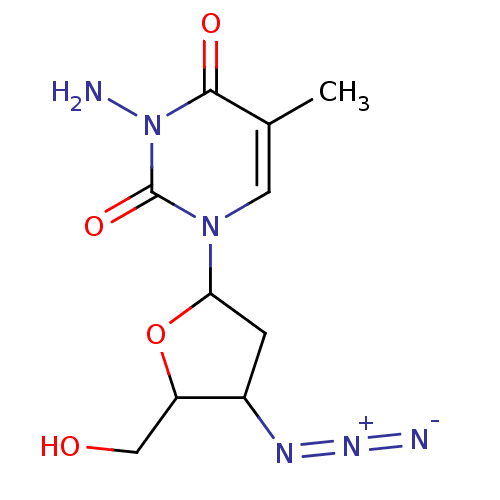 (3-Amino-1-(4-azido-5-hydroxymethyl-tetrahydro-fura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057819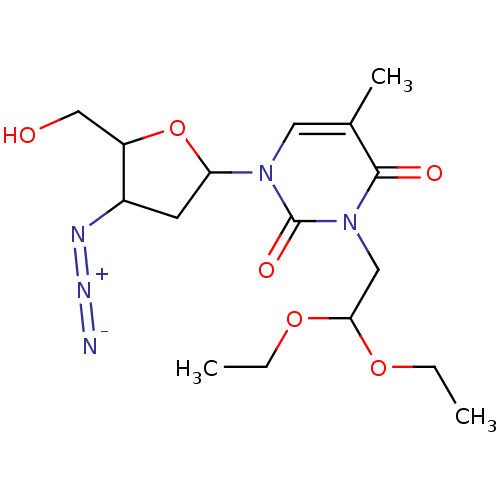 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057821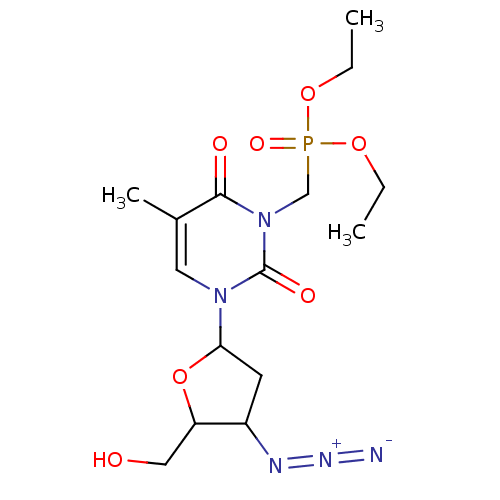 (CHEMBL39945 | [3-(4-Azido-5-hydroxymethyl-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057804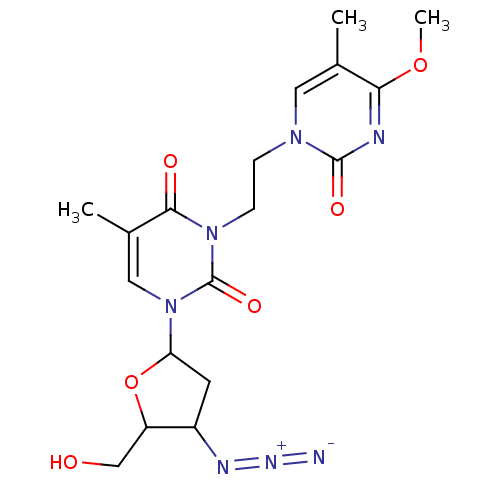 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.90E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057814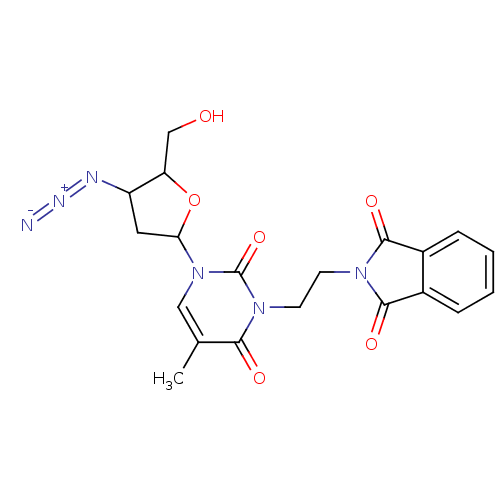 (2-{2-[3-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.14E+5 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057818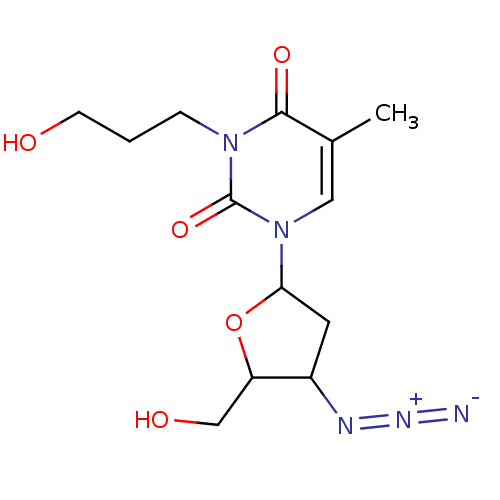 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 120 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057808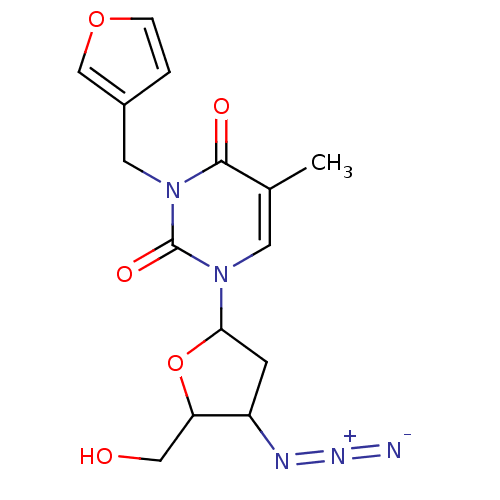 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.43E+5 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057805 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057810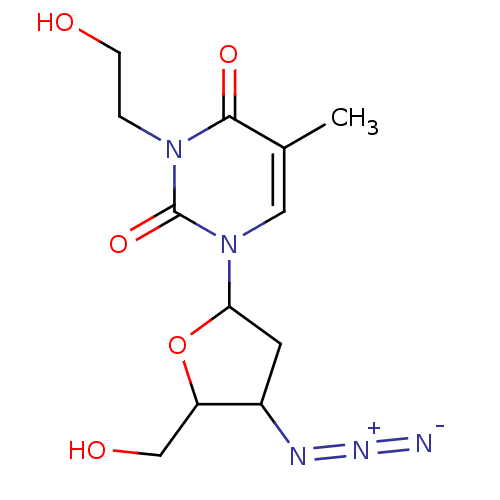 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057822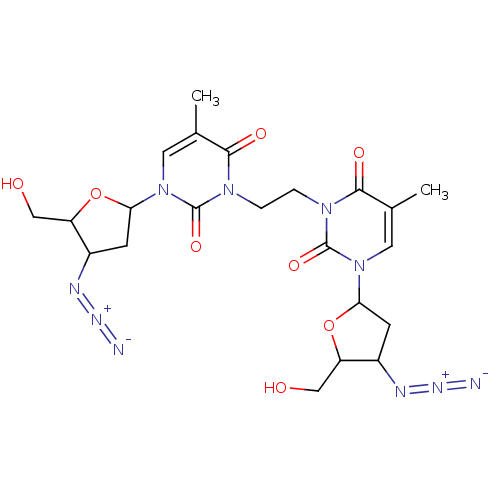 (1-(4-azido-5-hydroxymethyltetrahydro-2-furanyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057807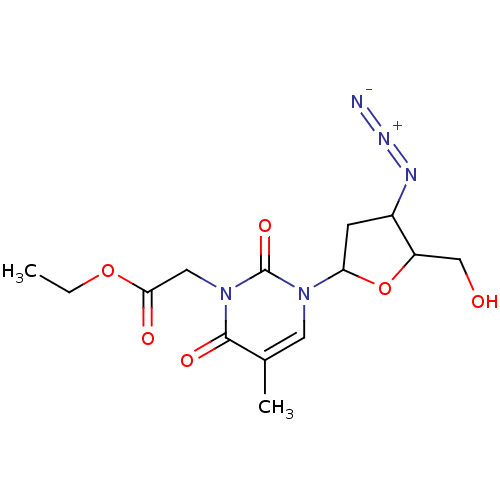 (CHEMBL38606 | [3-(4-Azido-5-hydroxymethyl-tetrahyd...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057811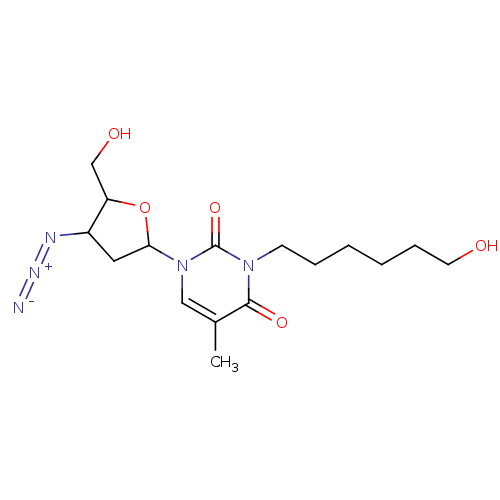 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057823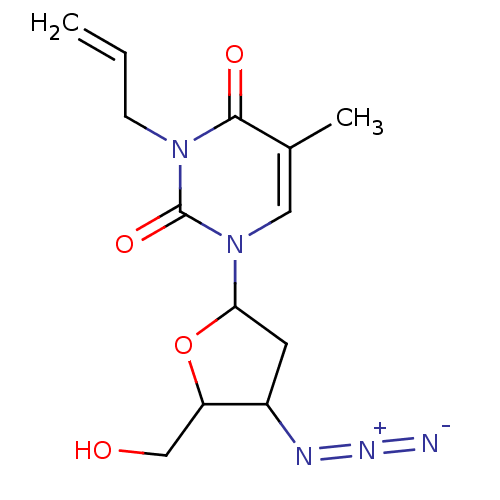 (3-Allyl-1-(4-azido-5-hydroxymethyl-tetrahydro-fura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057815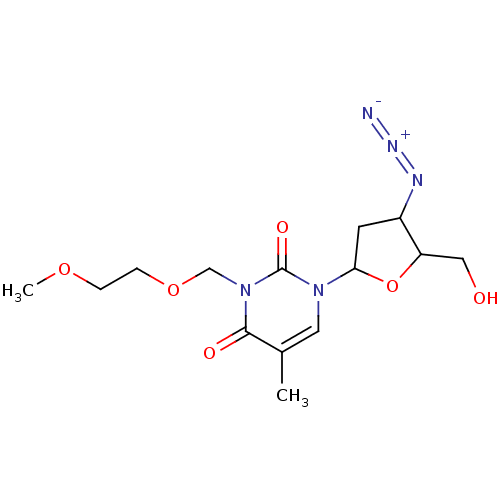 (1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-2-yl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50057816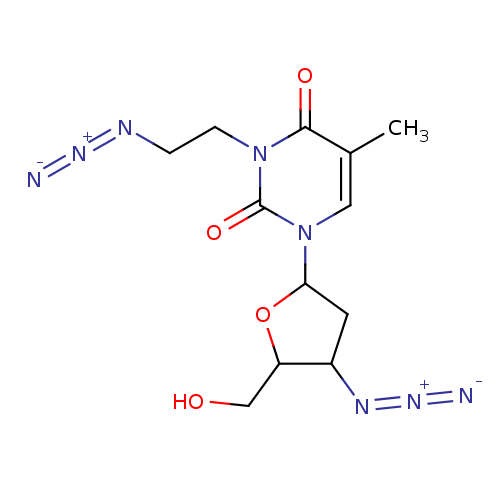 (3-(2-Azido-ethyl)-1-(4-azido-5-hydroxymethyl-tetra...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles CNRS Curated by ChEMBL | Assay Description Reverse transcriptase activity was measured in the culture supernatant, concentration that reduces by 50% the HIV produced in the supernatant. | J Med Chem 40: 1550-8 (1997) Article DOI: 10.1021/jm9600095 BindingDB Entry DOI: 10.7270/Q2TH8KSM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008297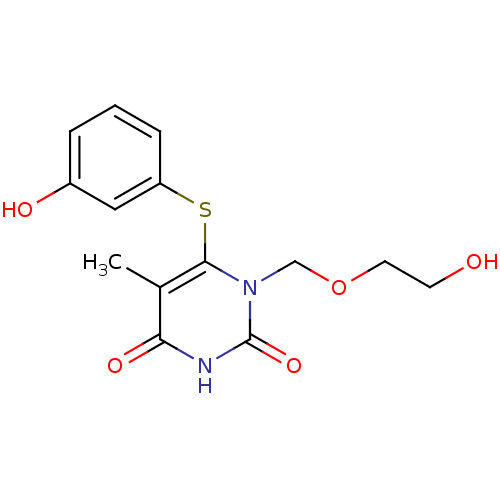 (1-(2-Hydroxy-ethoxymethyl)-6-(3-hydroxy-phenylsulf...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004118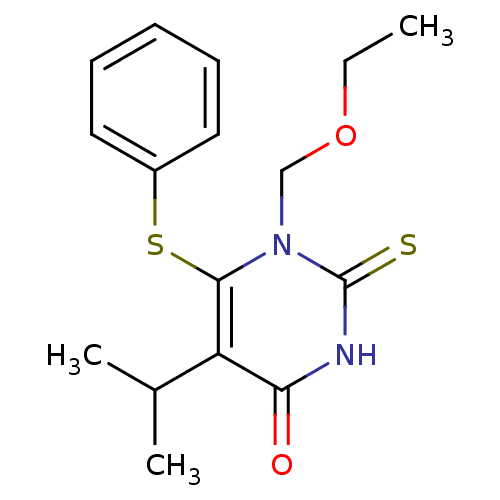 (1-Ethoxymethyl-5-isopropyl-6-phenylsulfanyl-2-thio...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004139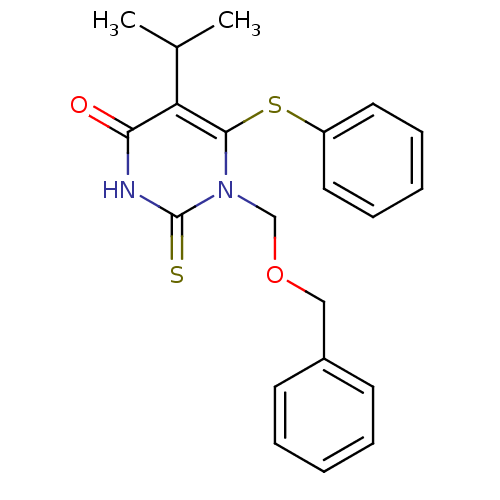 (1-Benzyloxymethyl-5-isopropyl-6-phenylsulfanyl-2-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50032237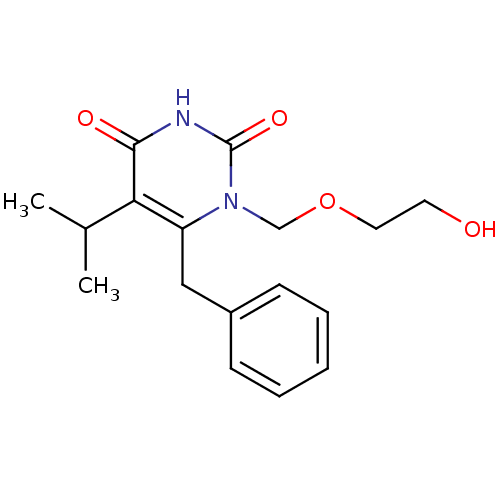 (6-Benzyl-1-(2-hydroxy-ethoxymethyl)-5-isopropyl-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004126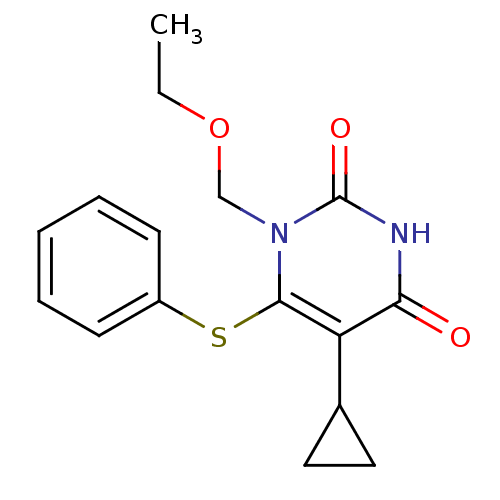 (5-Cyclopropyl-1-ethoxymethyl-6-phenylsulfanyl-1H-p...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50032235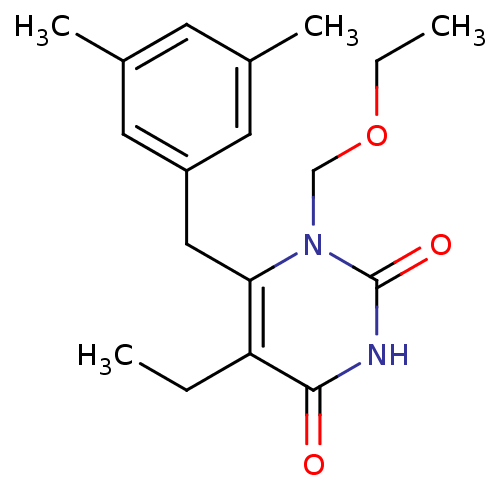 (6-(3,5-Dimethyl-benzyl)-1-ethoxymethyl-5-ethyl-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004138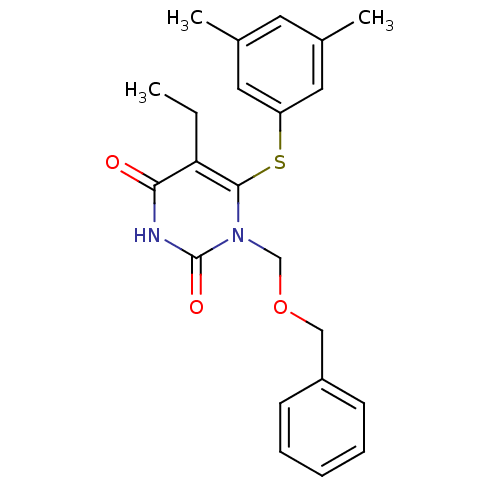 (1-Benzyloxymethyl-6-(3,5-dimethyl-phenylsulfanyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008328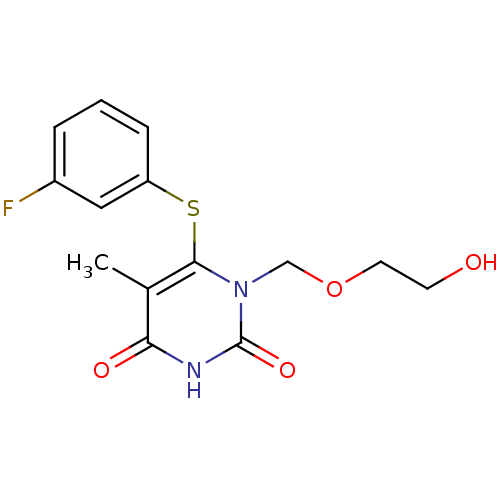 (6-(3-Fluoro-phenylsulfanyl)-1-(2-hydroxy-ethoxymet...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004149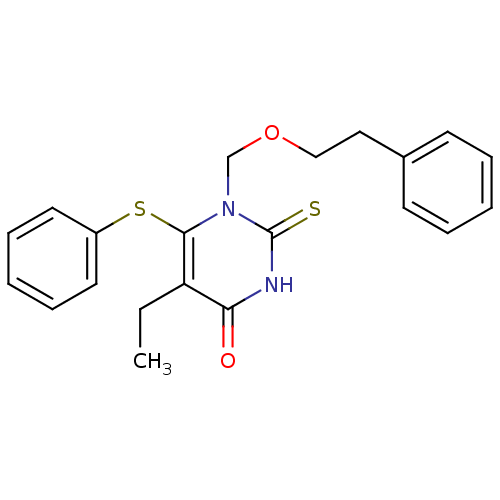 (5-Ethyl-1-phenethyloxymethyl-6-phenylsulfanyl-2-th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 91 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50011000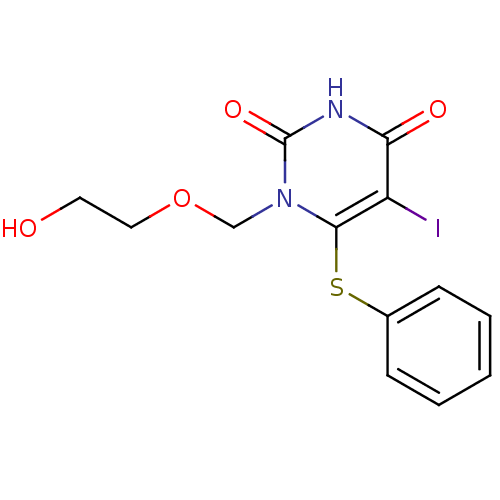 (1-(2-Hydroxy-ethoxymethyl)-5-iodo-6-phenylsulfanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004131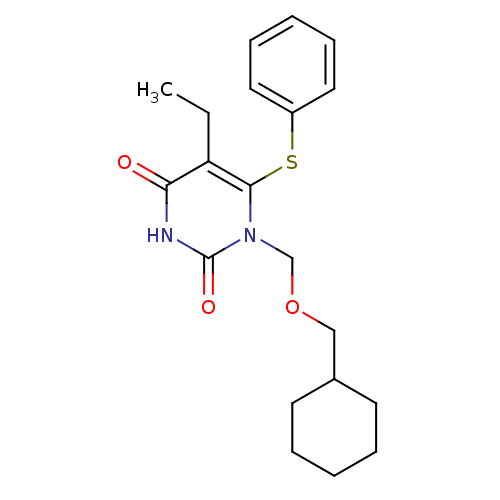 (1-Cyclohexylmethoxymethyl-5-ethyl-6-phenylsulfanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008329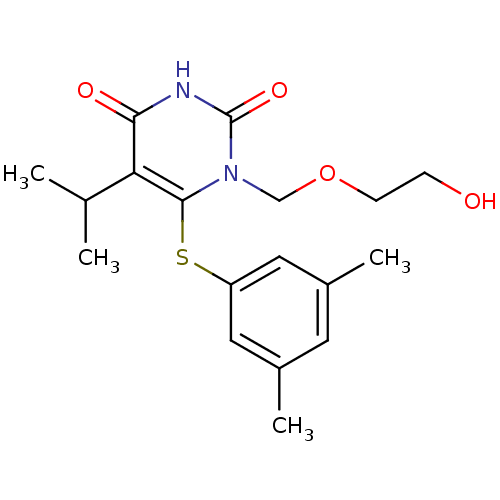 (6-(3,5-Dimethyl-phenylsulfanyl)-1-(2-hydroxy-ethox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008318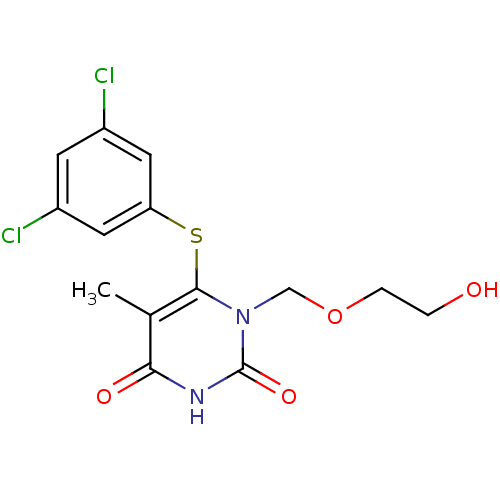 (6-(3,5-Dichloro-phenylsulfanyl)-1-(2-hydroxy-ethox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008333 (1-(2-Hydroxy-ethoxymethyl)-5-isopropyl-6-phenylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50061691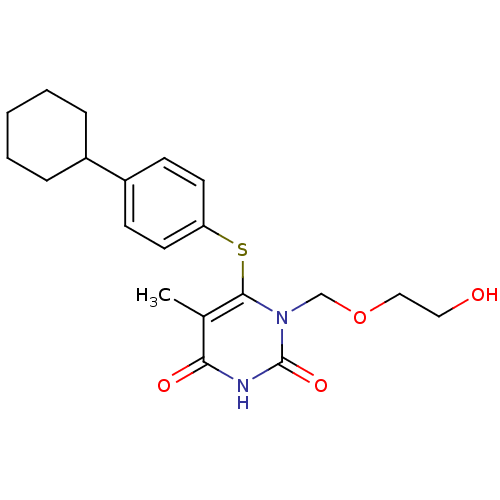 (6-(4-Cyclohexyl-phenylsulfanyl)-1-(2-hydroxy-ethox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50061701 (1-(2-Hydroxy-ethoxymethyl)-5-isopropyl-6-phenylsul...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50032242 (6-Benzyl-5-ethyl-1-(2-hydroxy-ethoxymethyl)-1H-pyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008325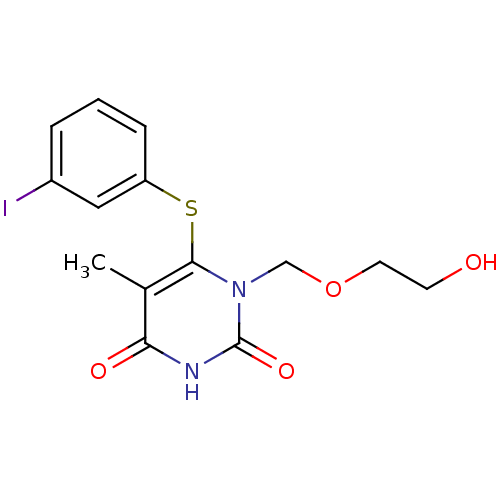 (1-(2-Hydroxy-ethoxymethyl)-6-(3-iodo-phenylsulfany...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004143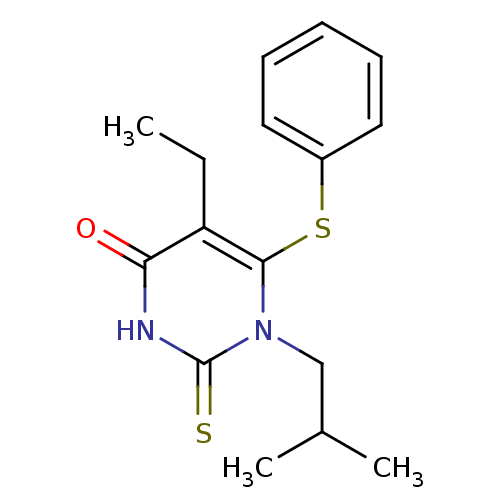 (5-Ethyl-1-isobutyl-6-phenylsulfanyl-2-thioxo-2,3-d...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004125 (6-(3,5-Dichloro-phenylsulfanyl)-1-ethoxymethyl-5-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 13 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008341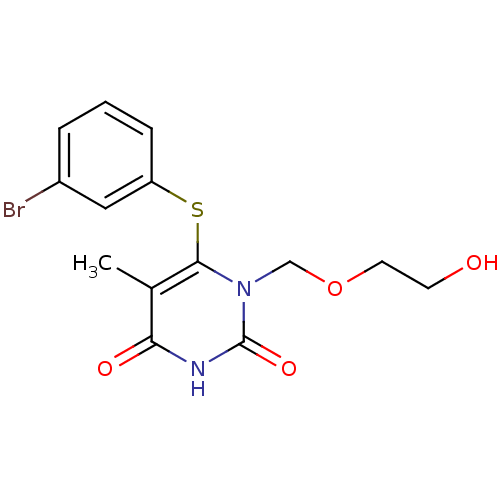 (6-(3-Bromo-phenylsulfanyl)-1-(2-hydroxy-ethoxymeth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004121 (1-Benzyloxymethyl-5-ethyl-6-phenylsulfanyl-2-thiox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004145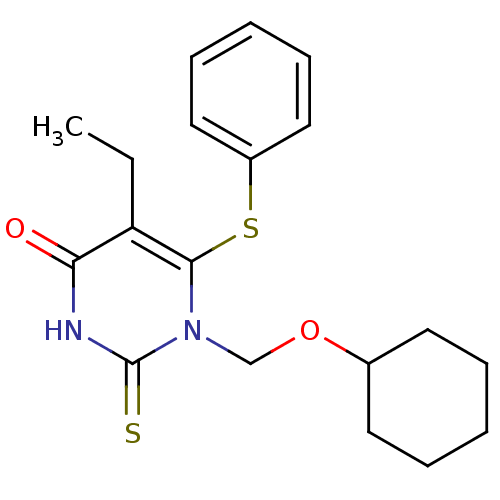 (1-Cyclohexyloxymethyl-5-ethyl-6-phenylsulfanyl-2-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004150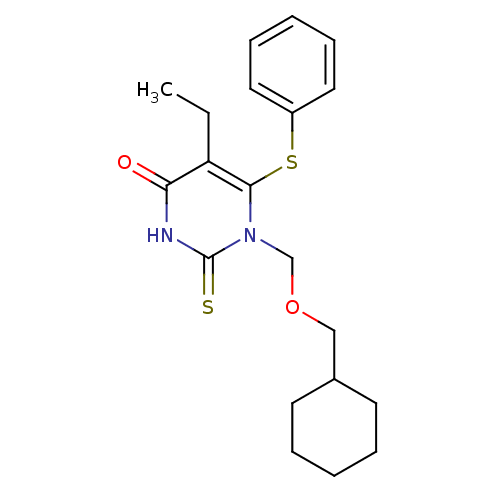 (1-Cyclohexylmethoxymethyl-5-ethyl-6-phenylsulfanyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 350 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008305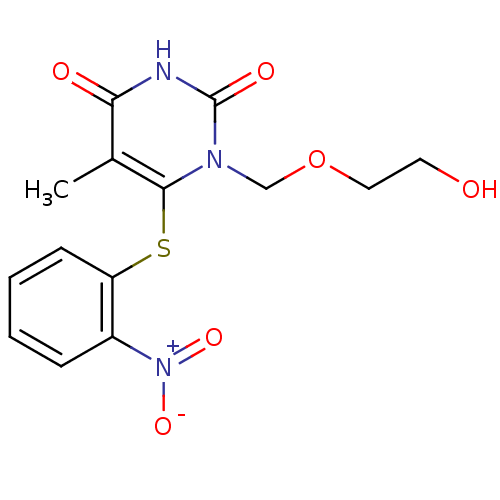 (1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-(2-nitro-phe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40E+5 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004148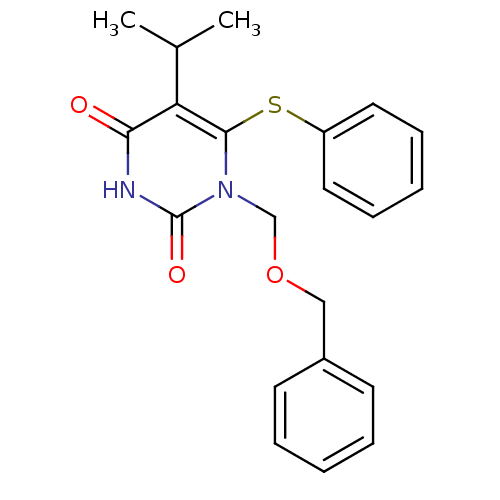 (1-Benzyloxymethyl-5-isopropyl-6-phenylsulfanyl-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50008299 (6-(3,5-Dichloro-phenylsulfanyl)-5-ethyl-1-(2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50061693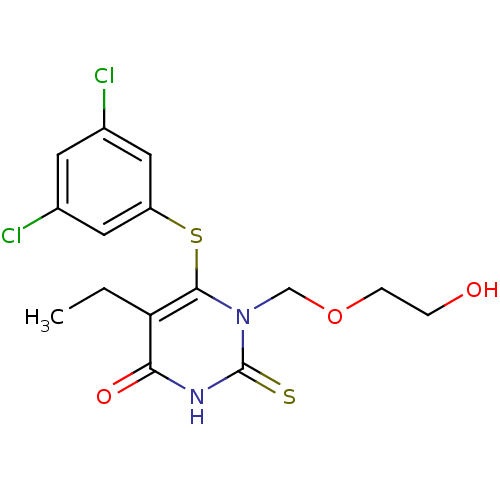 (6-(3,5-Dichloro-phenylsulfanyl)-5-ethyl-1-(2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50058314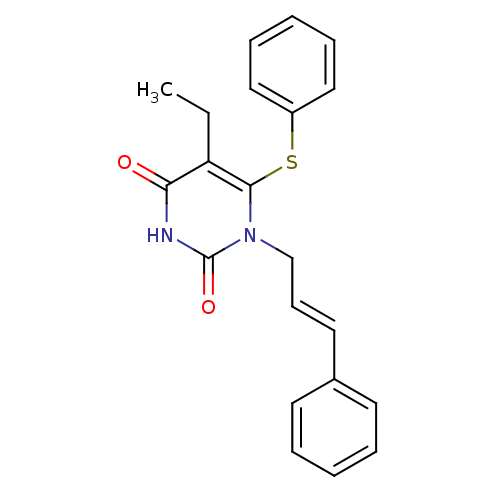 (5-Ethyl-1-((E)-3-phenyl-allyl)-6-phenylsulfanyl-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50058327 (5-Ethyl-6-phenylsulfanyl-1-((E)-3-thiophen-2-yl-al...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Université d'Orléans Curated by ChEMBL | Assay Description Inhibitory concentration against HIV-1 reverse transcriptase | J Med Chem 40: 4257-64 (1998) Article DOI: 10.1021/jm970110p BindingDB Entry DOI: 10.7270/Q2WS8SC3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 149 total ) | Next | Last >> |