

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127832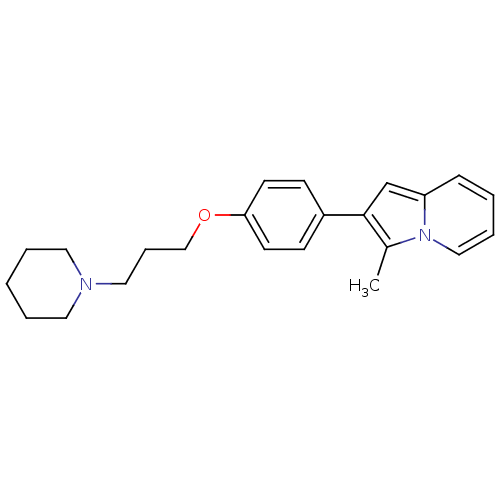 (3-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120543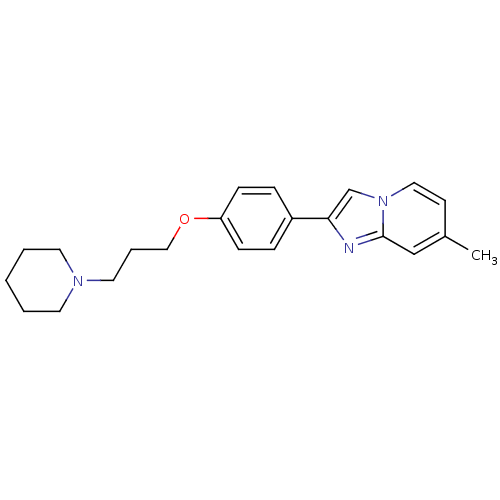 (7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127844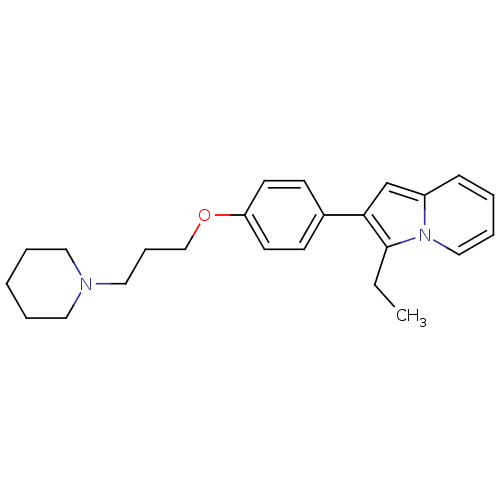 (3-Ethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50120542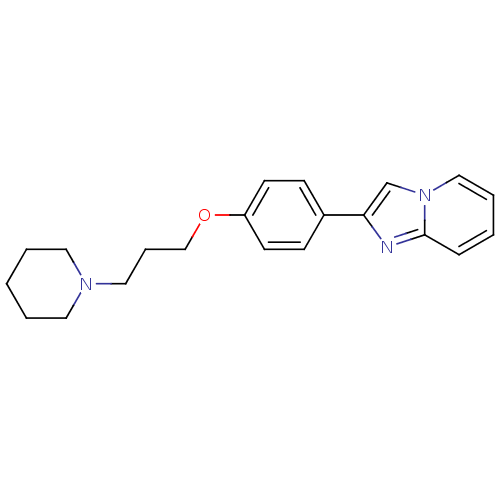 (2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-imidazo[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127831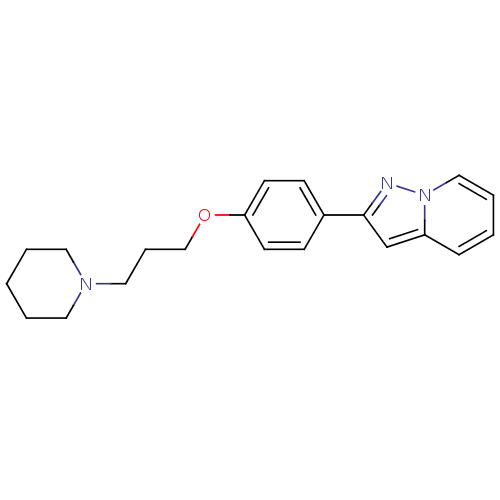 (2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-pyrazolo[1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127838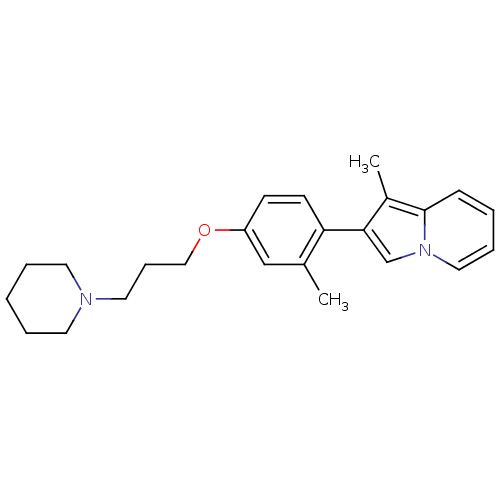 (1-Methyl-2-[2-methyl-4-(3-piperidin-1-yl-propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127841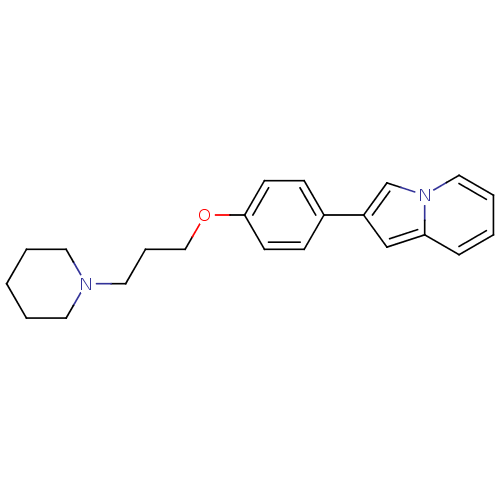 (2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127839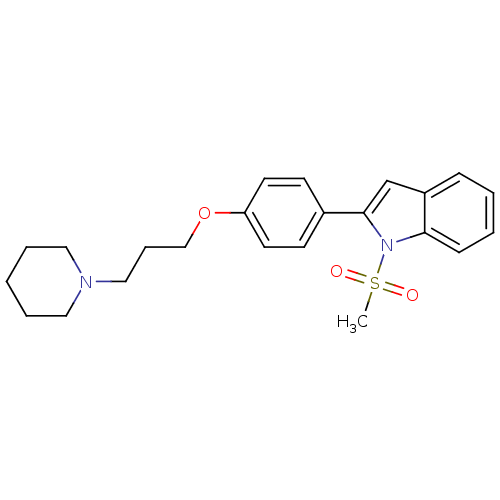 (1-Methanesulfonyl-2-[4-(3-piperidin-1-yl-propoxy)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127834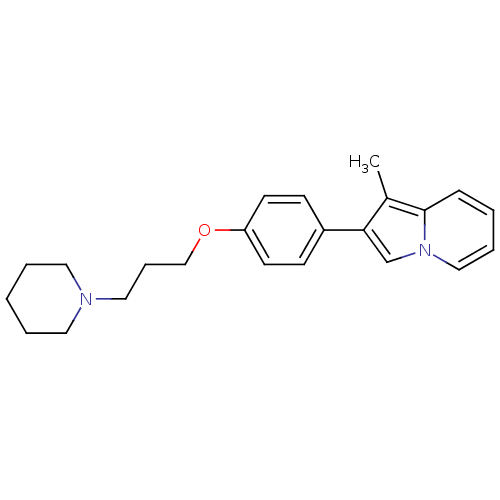 (1-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127840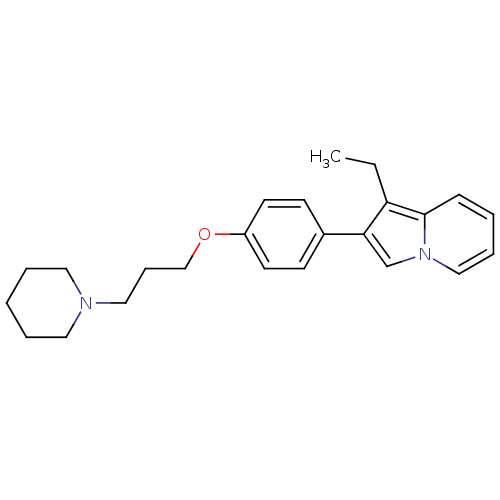 (1-Ethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-in...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127830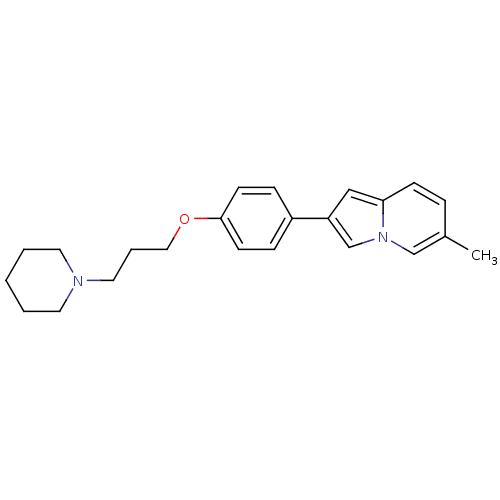 (6-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127835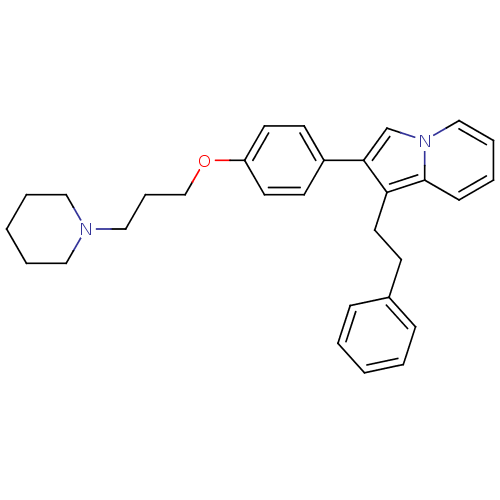 (1-Phenethyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127833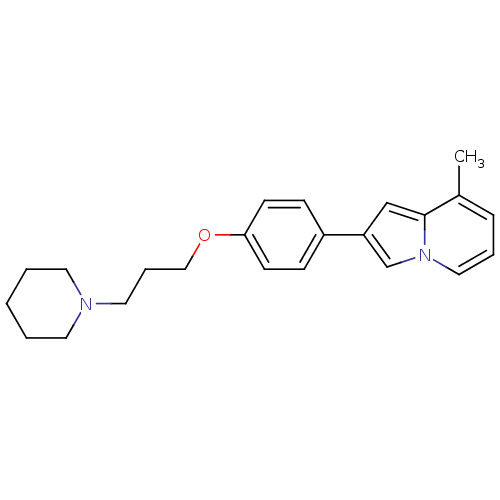 (8-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127847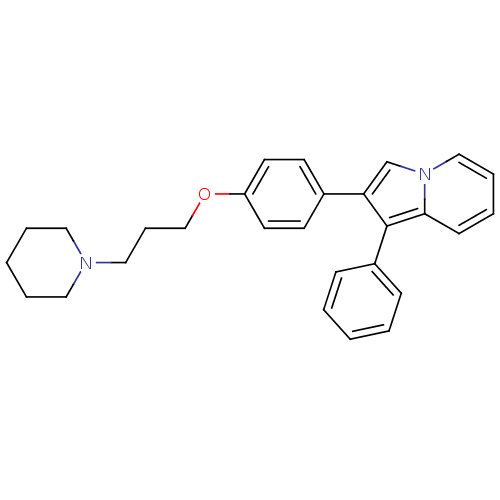 (1-Phenyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127846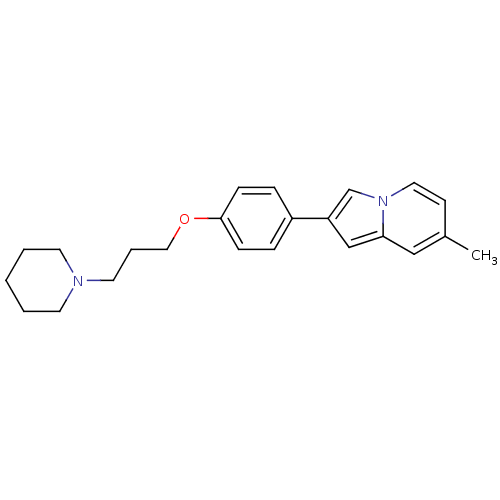 (7-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127843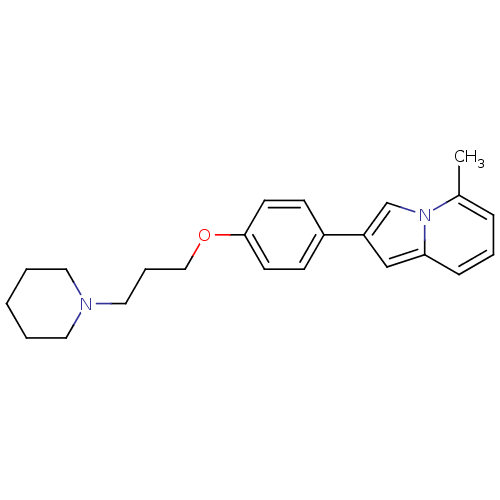 (5-Methyl-2-[4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127837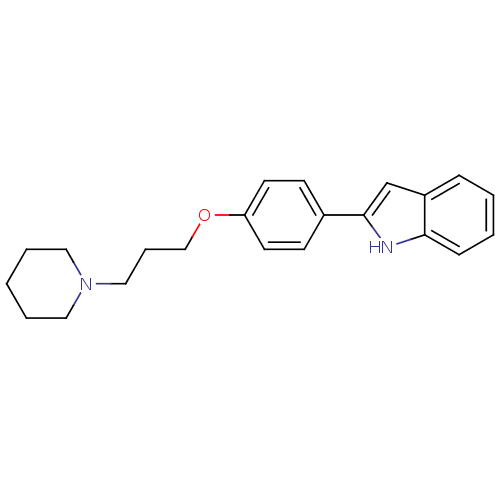 (2-[4-(3-Piperidin-1-yl-propoxy)-phenyl]-1H-indole ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127845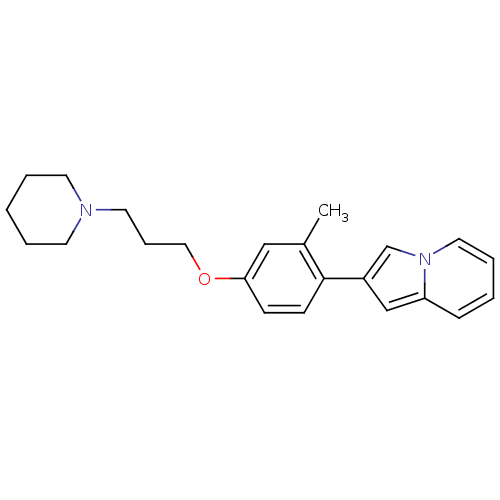 (2-[2-Methyl-4-(3-piperidin-1-yl-propoxy)-phenyl]-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127836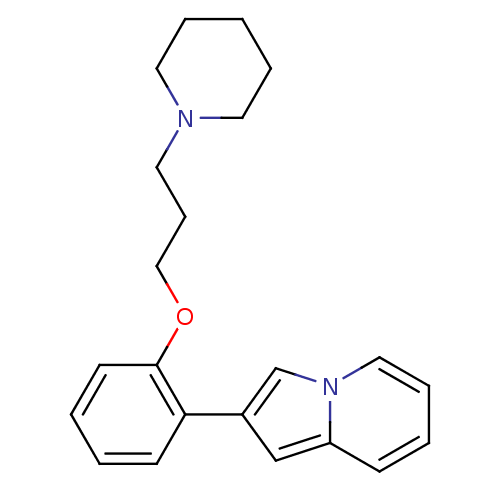 (2-[2-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50127842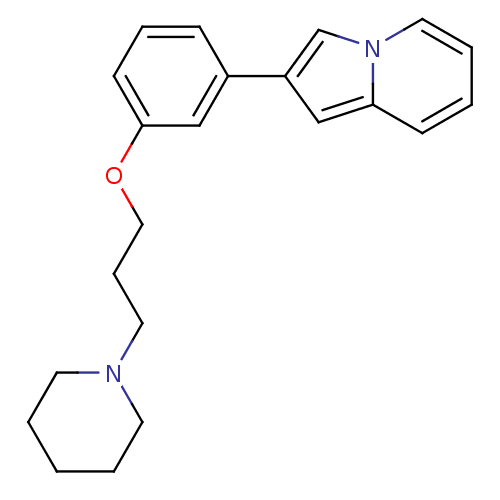 (2-[3-(3-Piperidin-1-yl-propoxy)-phenyl]-indolizine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 308 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development L. L. C. Curated by ChEMBL | Assay Description Binding affinity towards human Histamine H3 receptor using [3H]-N-methyl-histamine as radioligand | Bioorg Med Chem Lett 13: 1767-70 (2003) BindingDB Entry DOI: 10.7270/Q21835V3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163251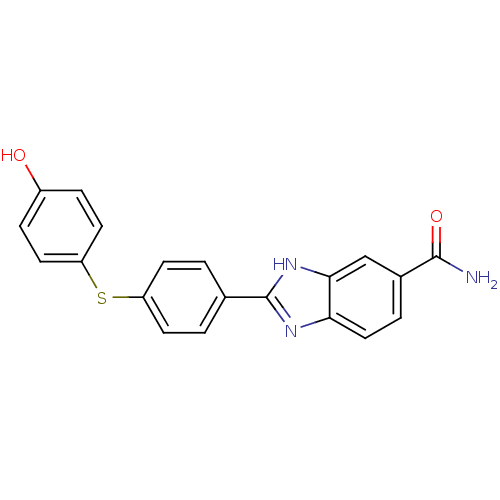 (2-(4-(4-hydroxyphenylthio)phenyl)-1H-benzo[d]imida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human recombinant Chk2 | Bioorg Med Chem Lett 17: 6467-71 (2007) Article DOI: 10.1016/j.bmcl.2007.09.098 BindingDB Entry DOI: 10.7270/Q2KP81XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163251 (2-(4-(4-hydroxyphenylthio)phenyl)-1H-benzo[d]imida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163246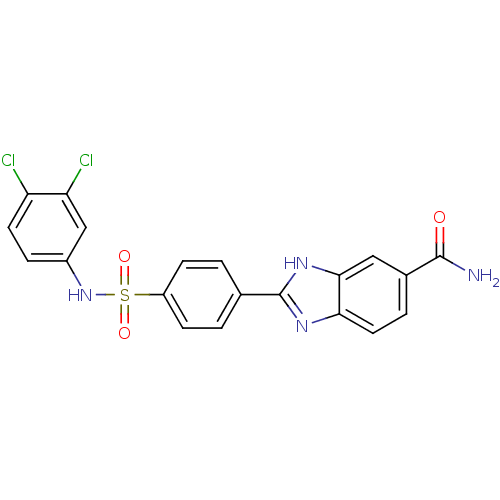 (2-[4-(3,4-Dichloro-phenylsulfamoyl)-phenyl]-1H-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24206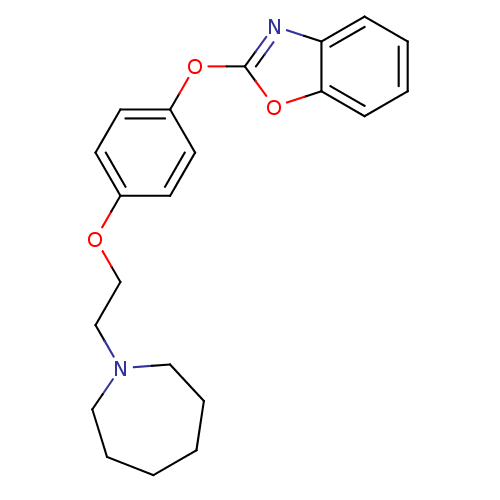 (2-{4-[2-(azepan-1-yl)ethoxy]phenoxy}-1,3-benzoxazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163259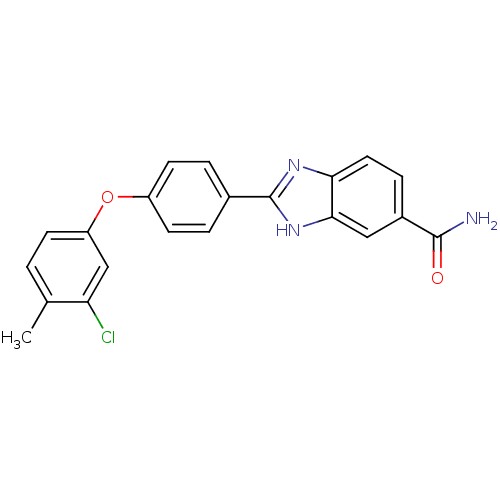 (2-[4-(3-Chloro-4-methyl-phenoxy)-phenyl]-1H-benzoi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163254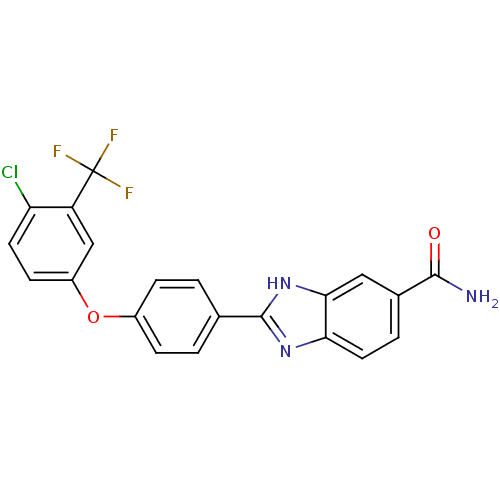 (2-[4-(4-Chloro-3-trifluoromethyl-phenoxy)-phenyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163266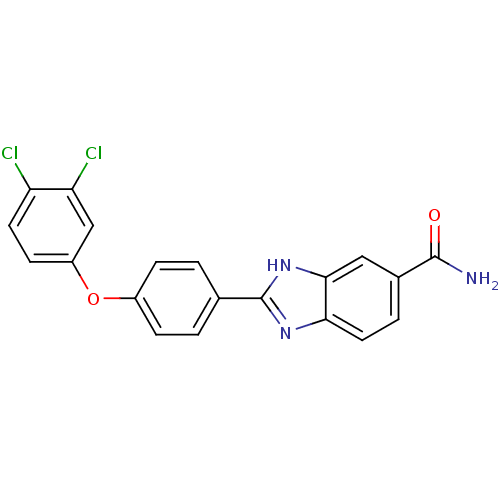 (2-[4-(3,4-Dichloro-phenoxy)-phenyl]-1H-benzoimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24216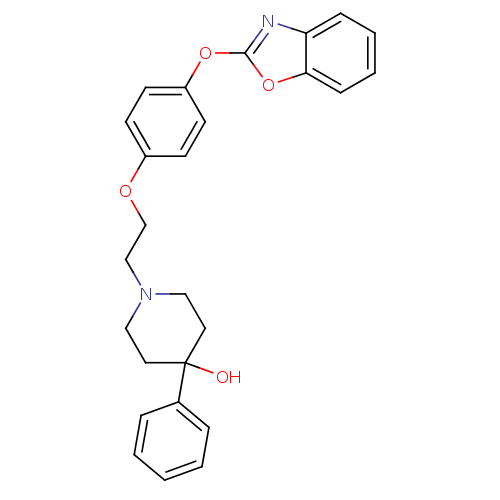 (1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}-4-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163264 (2-[4-(4-Chloro-benzenesulfonyl)-phenyl]-1H-benzoim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24199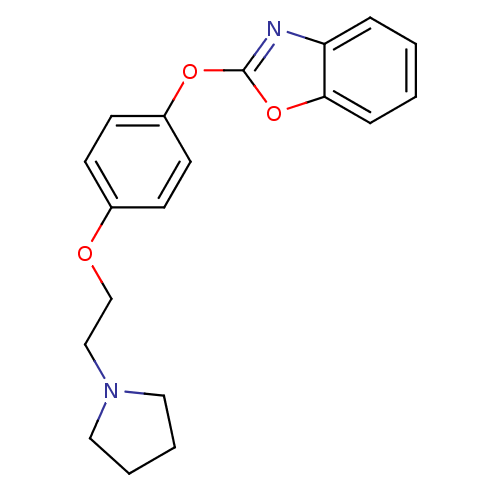 (2-{4-[2-(pyrrolidin-1-yl)ethoxy]phenoxy}-1,3-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24224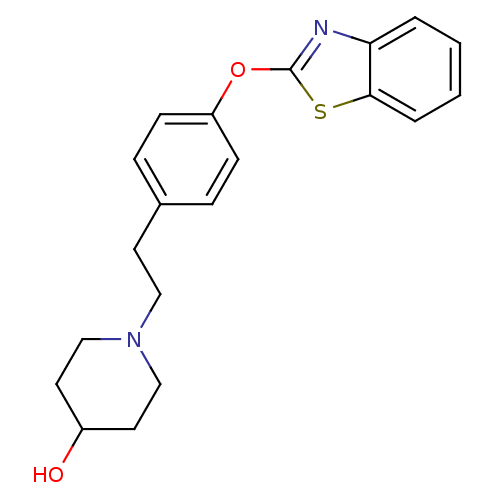 (1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}pip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24238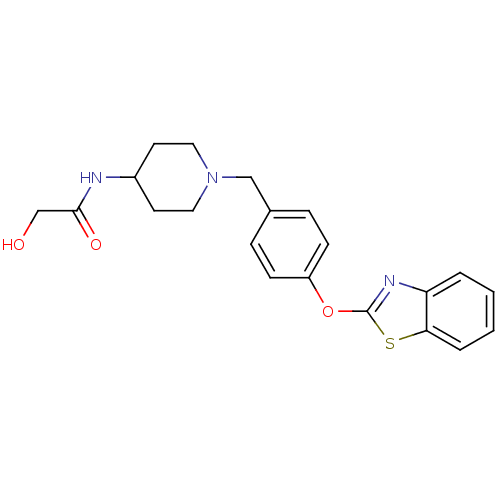 (Benzthiazole compound, 33p | N-(1-{[4-(1,3-benzoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163255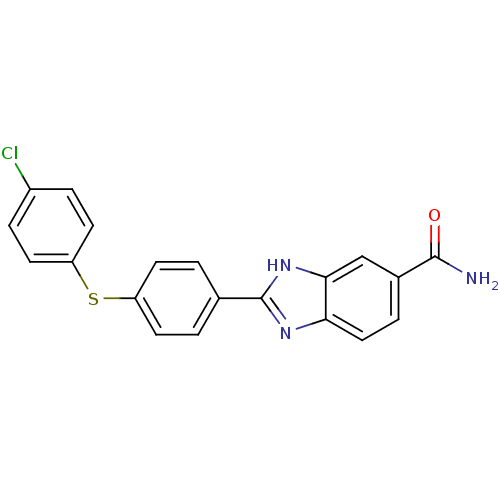 (2-[4-(4-Chloro-phenylsulfanyl)-phenyl]-1H-benzoimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24212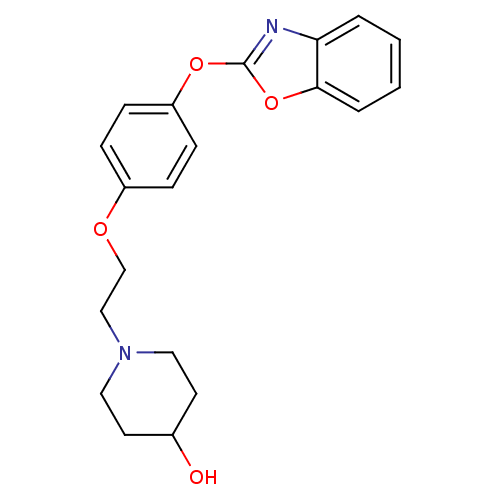 (1-{2-[4-(1,3-benzoxazol-2-yloxy)phenoxy]ethyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163282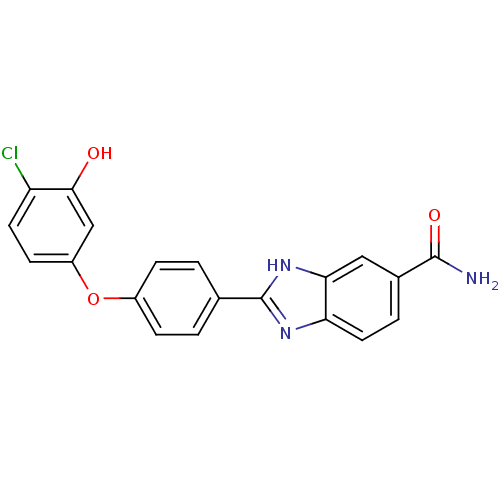 (2-[4-(4-Chloro-3-hydroxy-phenoxy)-phenyl]-1H-benzo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24235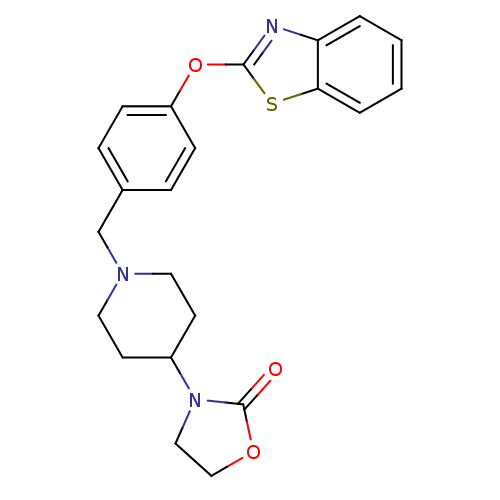 (3-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24203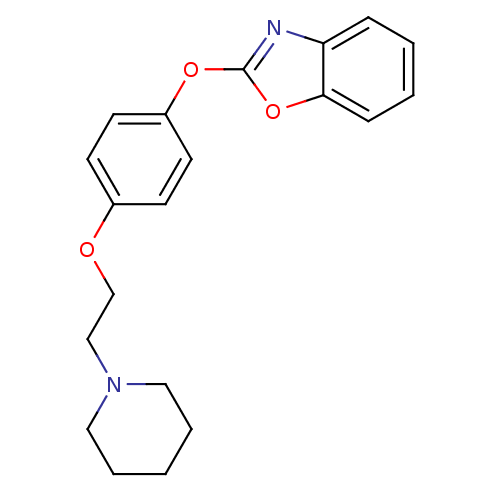 (2-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1,3-benzox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24222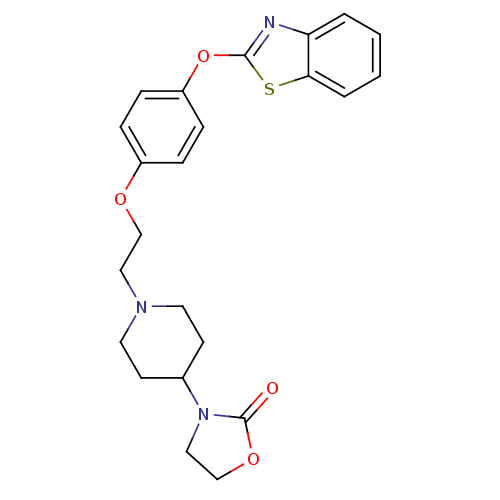 (3-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24239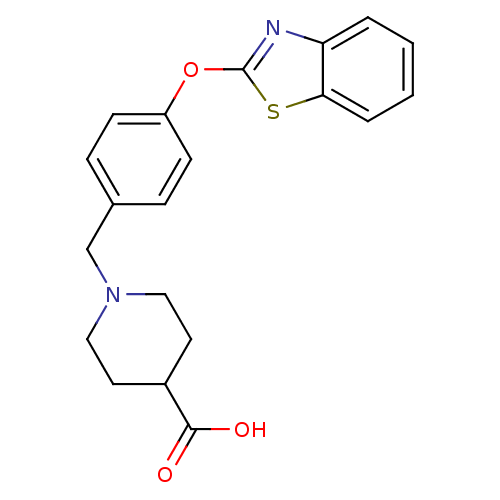 (1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}pipe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24234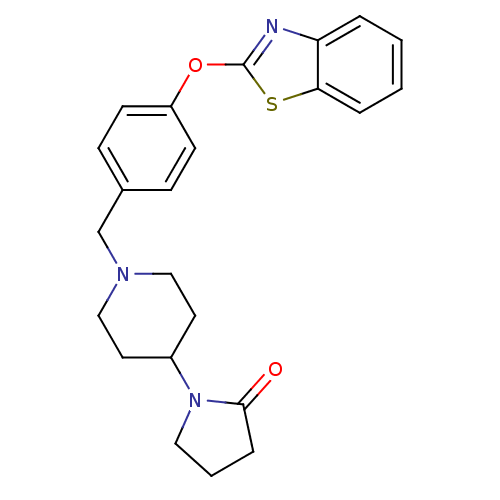 (1-(1-{[4-(1,3-benzothiazol-2-yloxy)phenyl]methyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24236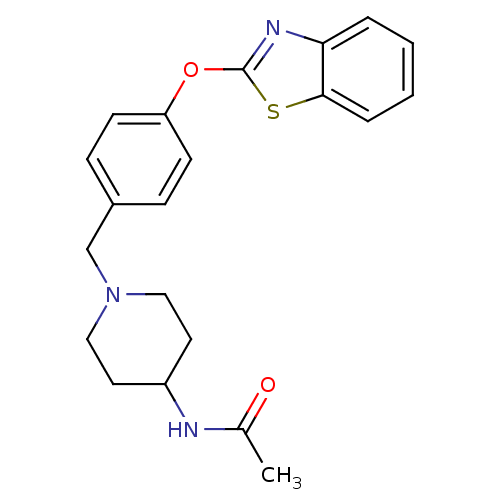 (Benzthiazole compound, 33n | N-(1-{[4-(1,3-benzoth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163250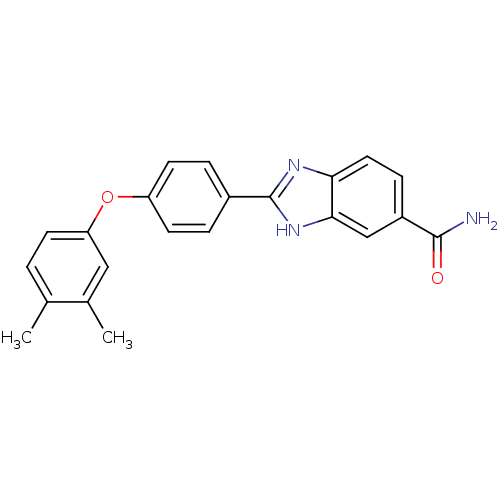 (2-[4-(3,4-Dimethyl-phenoxy)-phenyl]-1H-benzoimidaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163270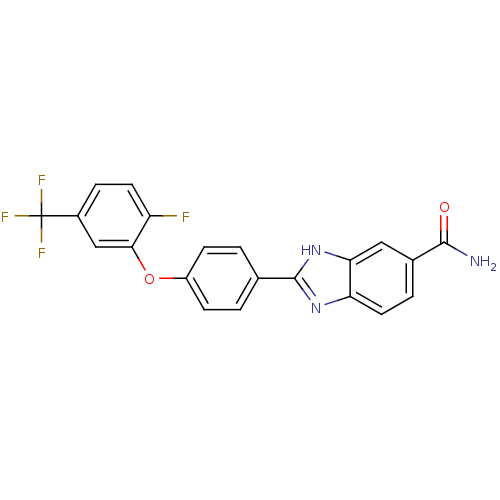 (2-[4-(2-Fluoro-5-trifluoromethyl-phenoxy)-phenyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163278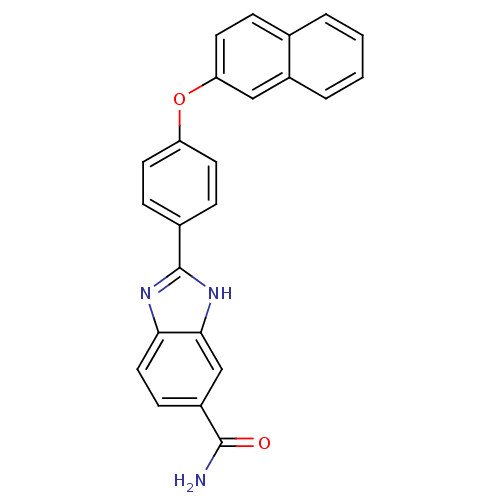 (2-[4-(Naphthalen-2-yloxy)-phenyl]-1H-benzoimidazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24215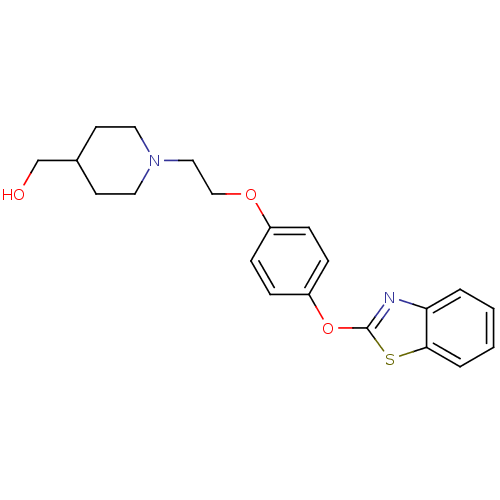 ((1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl}p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24218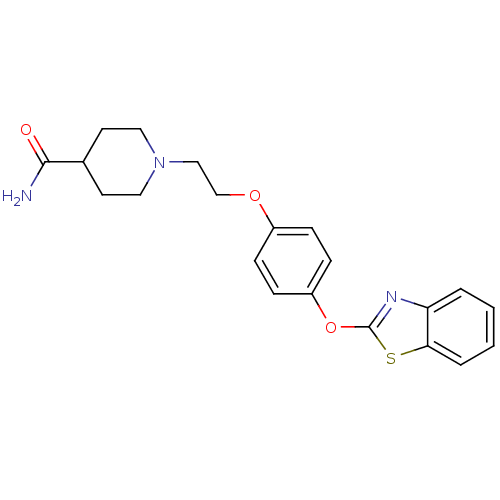 (1-{2-[4-(1,3-benzothiazol-2-yloxy)phenoxy]ethyl}pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24227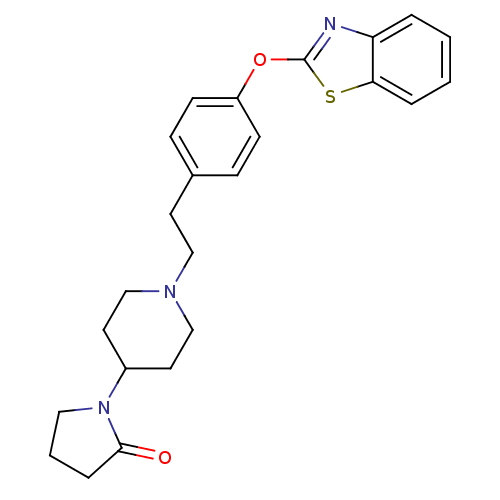 (1-(1-{2-[4-(1,3-benzothiazol-2-yloxy)phenyl]ethyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | 25 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50163269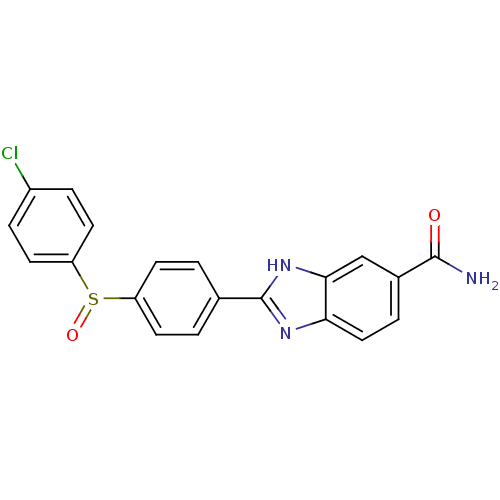 (2-[4-(4-Chloro-benzenesulfinyl)-phenyl]-1H-benzoim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of recombinant human Chk2 kinase | J Med Chem 48: 1873-85 (2005) Article DOI: 10.1021/jm0495935 BindingDB Entry DOI: 10.7270/Q2FQ9W4V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk2 (Homo sapiens (Human)) | BDBM50225014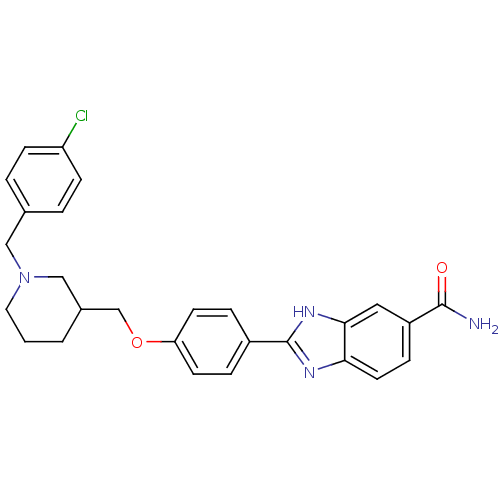 (2-(4-((1-(4-chlorobenzyl)piperidin-3-yl)methoxy)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human recombinant Chk2 | Bioorg Med Chem Lett 17: 6467-71 (2007) Article DOI: 10.1016/j.bmcl.2007.09.098 BindingDB Entry DOI: 10.7270/Q2KP81XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene A-4 hydrolase (Homo sapiens (Human)) | BDBM24201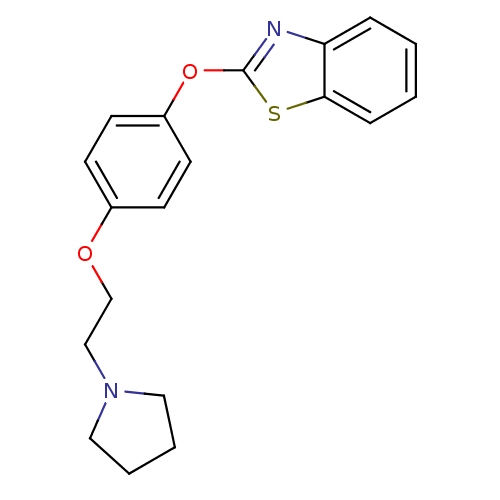 (2-{4-[2-(pyrrolidin-1-yl)ethoxy]phenoxy}-1,3-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Johnson & Johnson Pharmaceutical | Assay Description Recombinant human LTA4H was incubated with various concentrations of test compound for 10 min at room temperature in assay buffer, and the substrate,... | J Med Chem 51: 4150-69 (2008) Article DOI: 10.1021/jm701575k BindingDB Entry DOI: 10.7270/Q2GB22CX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 288 total ) | Next | Last >> |