

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171626 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118338 (US8653111, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171614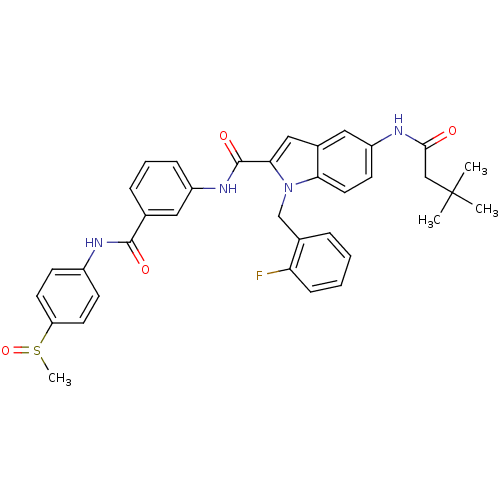 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171633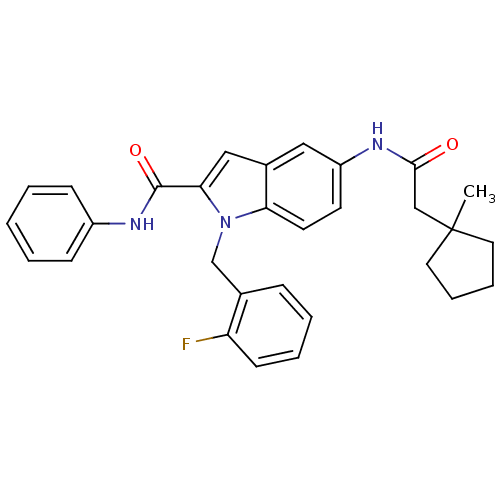 (1-(2-Fluoro-benzyl)-5-[2-(1-methyl-cyclopentyl)-ac...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171611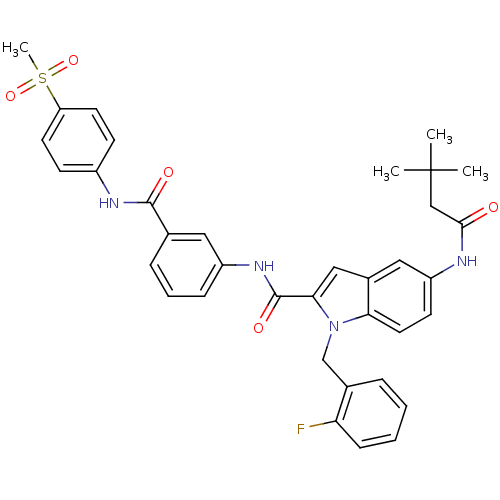 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171630 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171629 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171615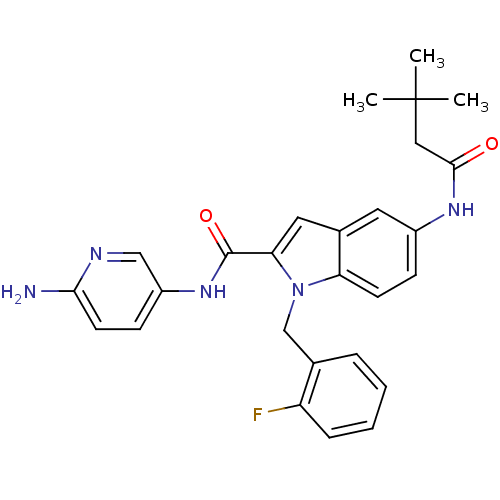 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171613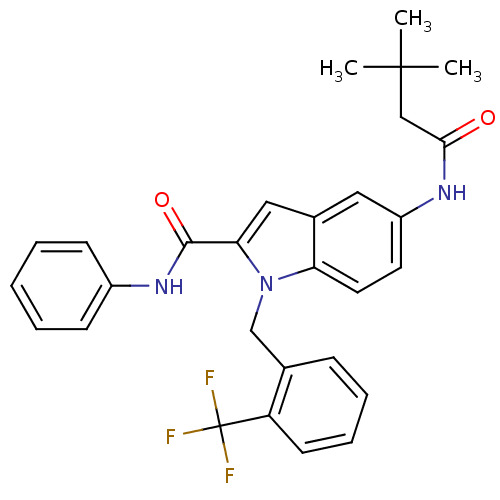 (5-(3,3-Dimethyl-butyrylamino)-1-(2-trifluoromethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3527 (US8524699, 18) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| von Hippel-Lindau disease tumor suppressor (Homo sapiens (Human)) | BDBM171824 (US9085572, 34) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US9085572 (2015) BindingDB Entry DOI: 10.7270/Q2H70DMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118341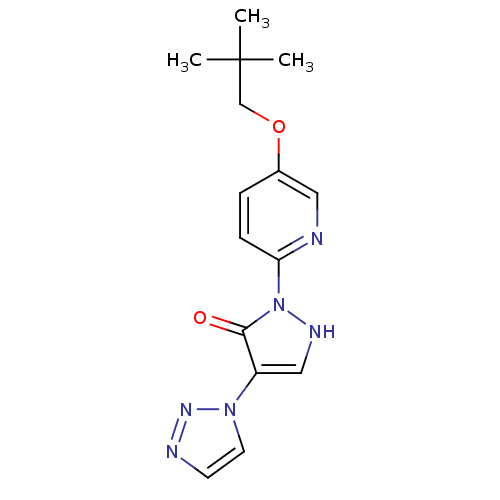 (US8653111, 85) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171643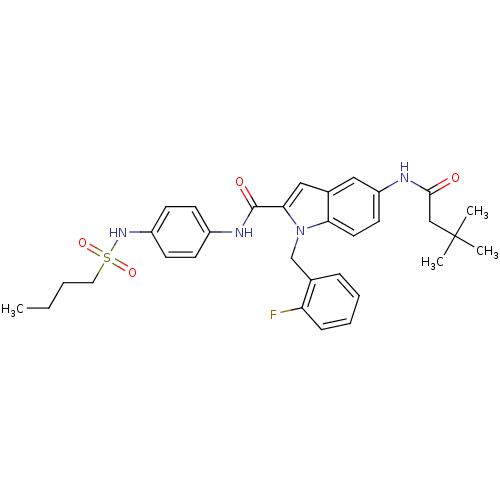 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171628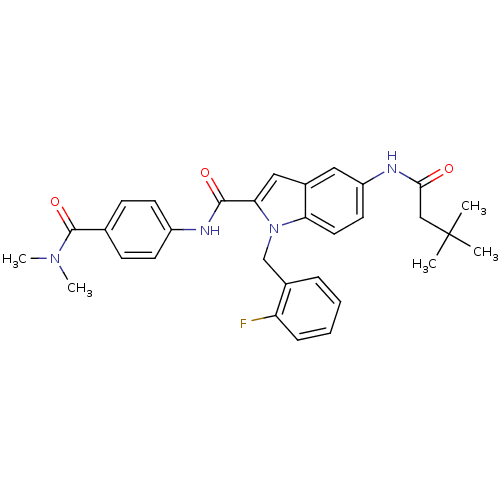 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171637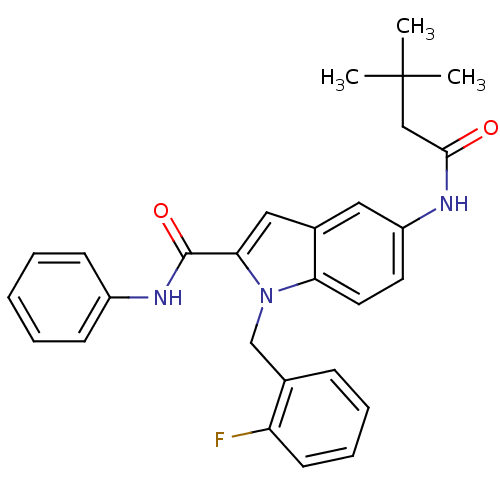 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171624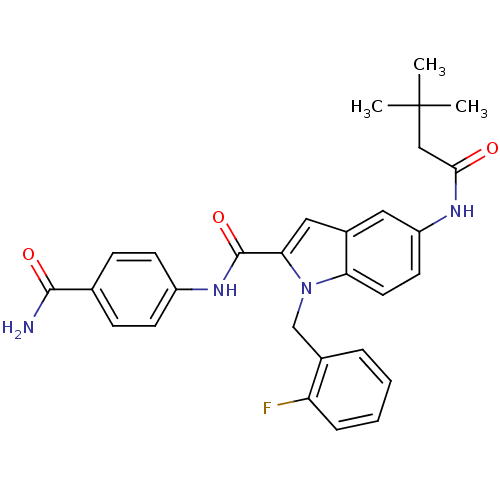 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM109268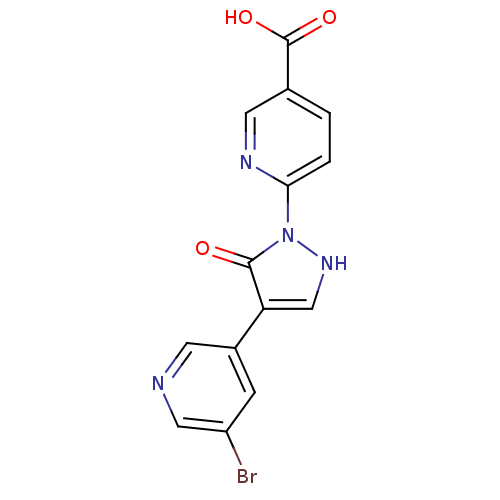 (US8609698, 10) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Inhibition of the Activity of HIF Prolyl Hydroxylase is carried out as described [Oehme F., Jonghaus W., Narouz-Ott L., Huetter J., Flamme I., Anal. ... | US Patent US8609698 (2013) BindingDB Entry DOI: 10.7270/Q2G44NZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171612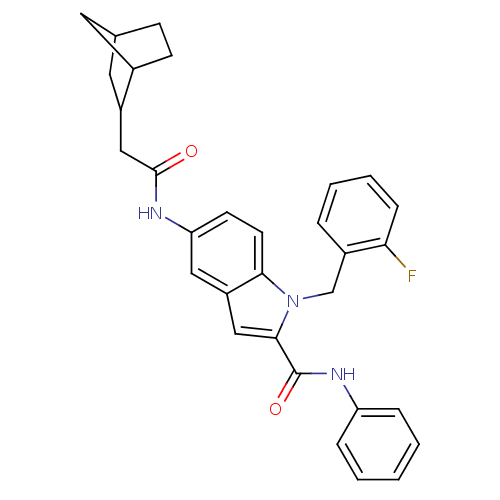 (5-(2-Bicyclo[2.2.1]hept-2-yl-acetylamino)-1-(2-flu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118328 (US8653111, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118344 (US8653111, 103) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118346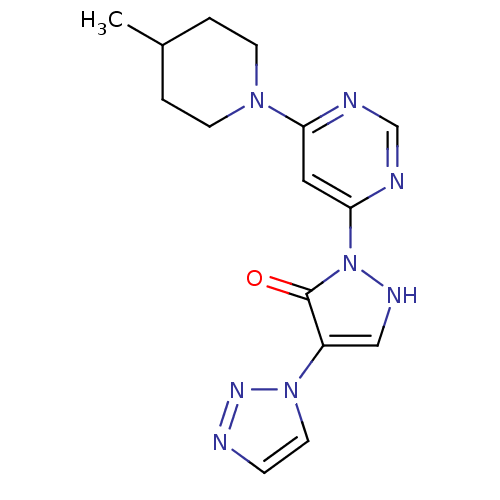 (US8653111, 129) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171631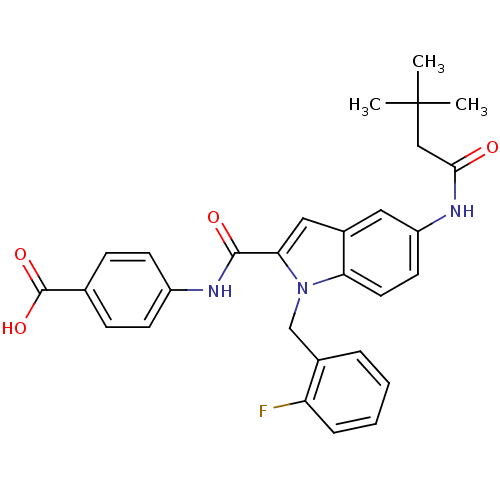 (4-{[5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| von Hippel-Lindau disease tumor suppressor (Homo sapiens (Human)) | BDBM171821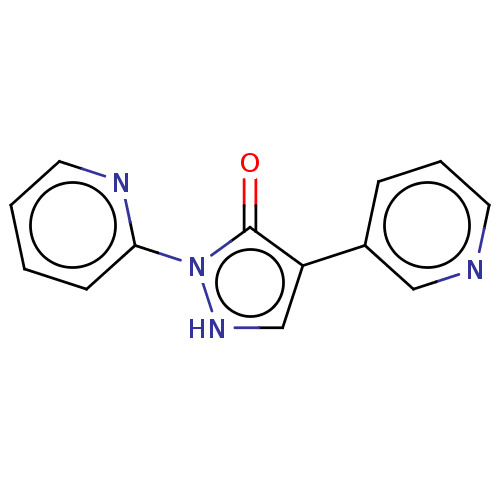 (US9085572, 6) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US9085572 (2015) BindingDB Entry DOI: 10.7270/Q2H70DMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171642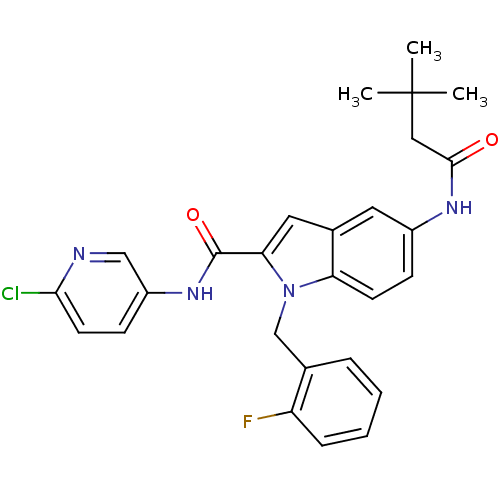 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3543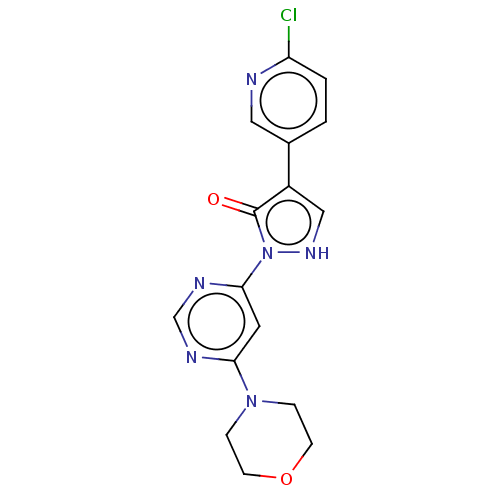 (US8524699, 16) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 480 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118339 (US8653111, 72) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118329 (US8653111, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118331 (US8653111, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171632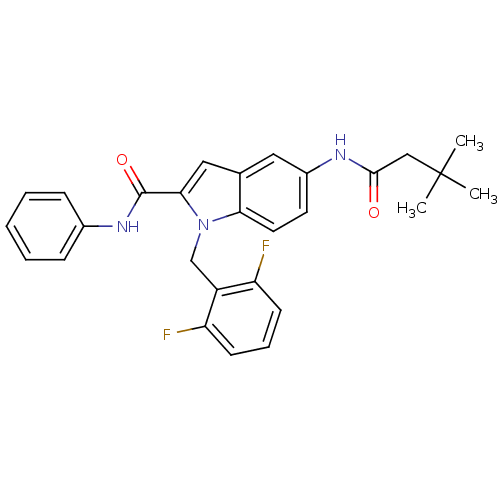 (1-(2,6-Difluoro-benzyl)-5-(3,3-dimethyl-butyrylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171616 (5-(3,3-Dimethyl-thiobutyrylamino)-1-(2-fluoro-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118347 (US8653111, 166) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3555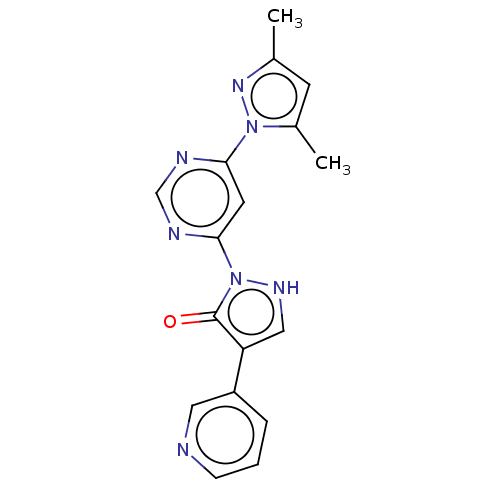 (US8524699, 3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| von Hippel-Lindau disease tumor suppressor (Homo sapiens (Human)) | BDBM171827 (US9085572, 46) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 700 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US9085572 (2015) BindingDB Entry DOI: 10.7270/Q2H70DMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171640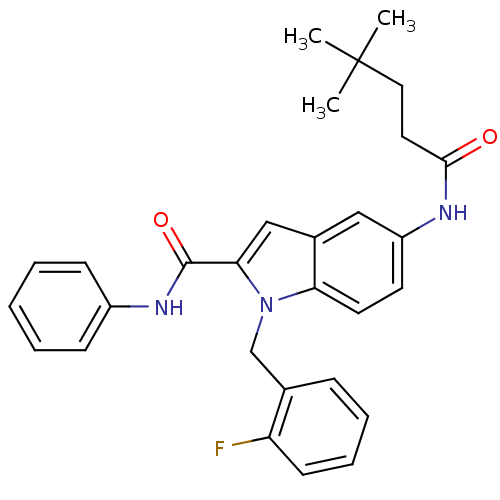 (5-(4,4-Dimethyl-pentanoylamino)-1-(2-fluoro-benzyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118345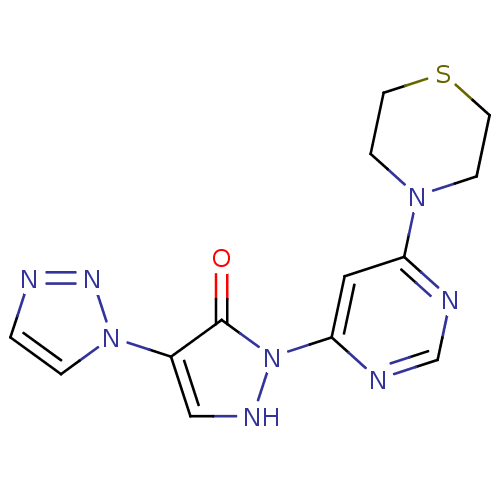 (US8653111, 113) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118330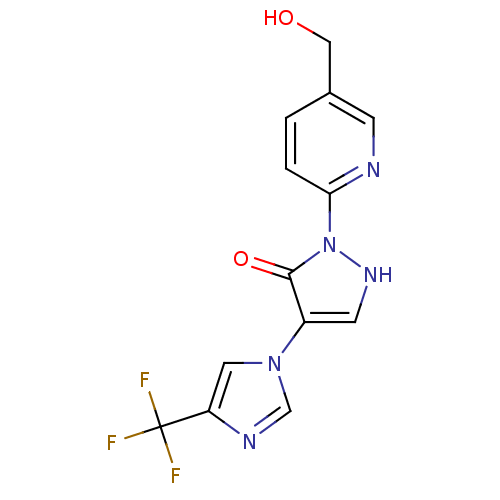 (US8653111, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| von Hippel-Lindau disease tumor suppressor (Homo sapiens (Human)) | BDBM171823 (US9085572, 26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US9085572 (2015) BindingDB Entry DOI: 10.7270/Q2H70DMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118333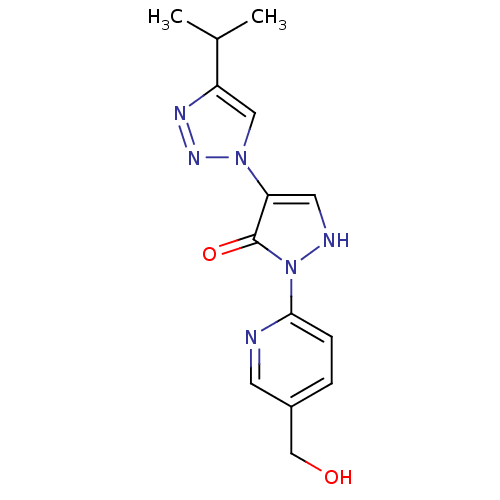 (US8653111, 45) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171638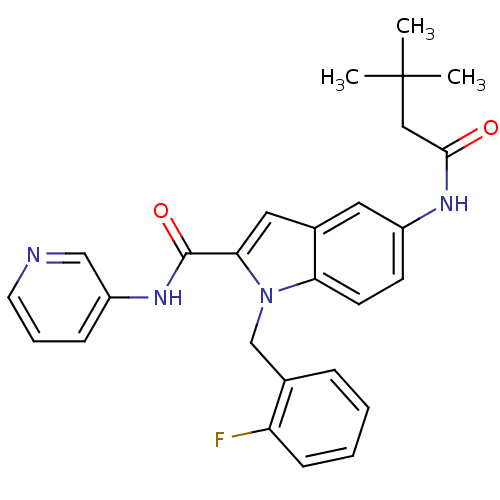 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171621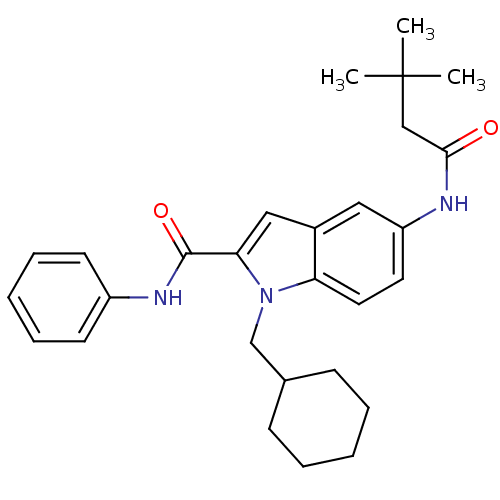 (1-Cyclohexylmethyl-5-(3,3-dimethyl-butyrylamino)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| von Hippel-Lindau disease tumor suppressor (Homo sapiens (Human)) | BDBM171822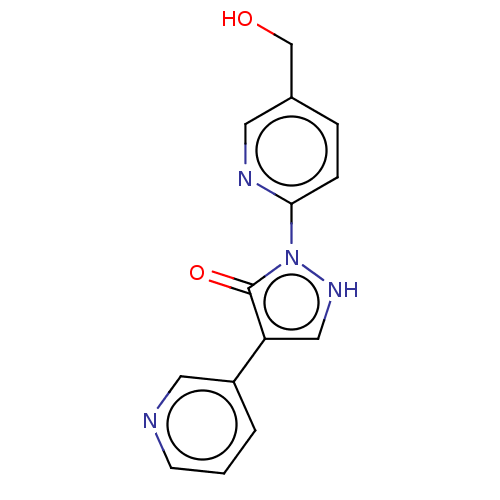 (US9085572, 23) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US9085572 (2015) BindingDB Entry DOI: 10.7270/Q2H70DMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3591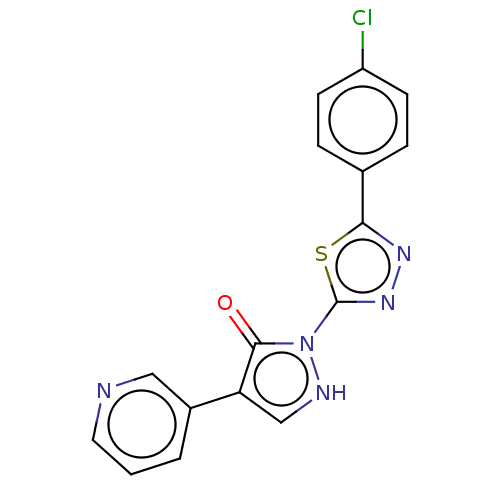 (US8524699, 7) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 880 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3623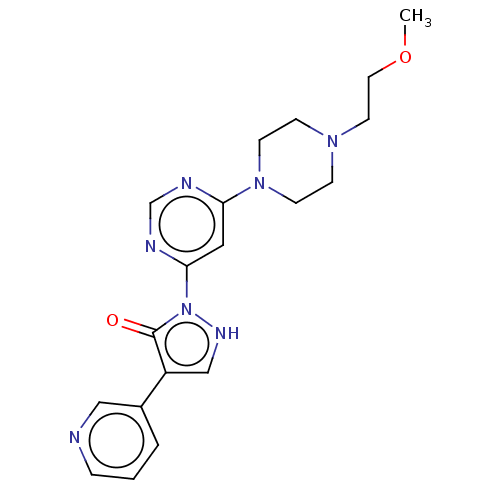 (US8524699, 28) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 890 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM109271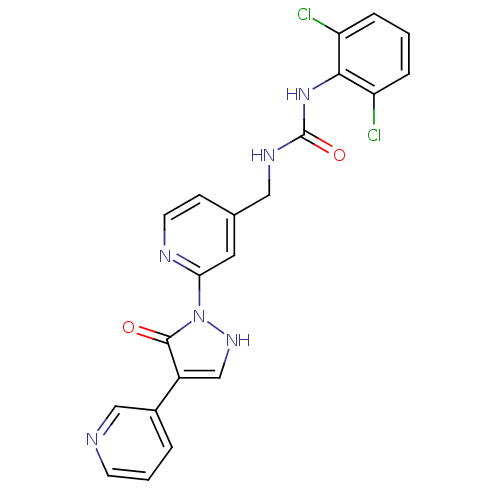 (US8609698, 65) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Inhibition of the Activity of HIF Prolyl Hydroxylase is carried out as described [Oehme F., Jonghaus W., Narouz-Ott L., Huetter J., Flamme I., Anal. ... | US Patent US8609698 (2013) BindingDB Entry DOI: 10.7270/Q2G44NZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM109269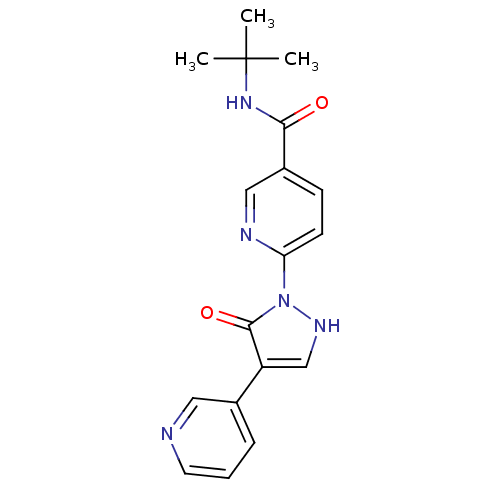 (US8609698, 18) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Inhibition of the Activity of HIF Prolyl Hydroxylase is carried out as described [Oehme F., Jonghaus W., Narouz-Ott L., Huetter J., Flamme I., Anal. ... | US Patent US8609698 (2013) BindingDB Entry DOI: 10.7270/Q2G44NZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3629 (US8524699, 46) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 930 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118337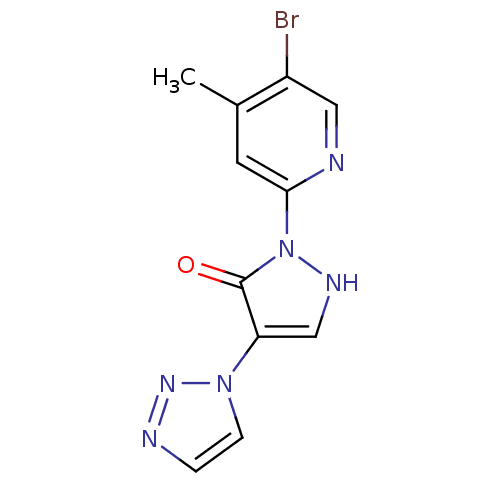 (US8653111, 61) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transmembrane prolyl 4-hydroxylase (Homo sapiens (Human)) | BDBM3643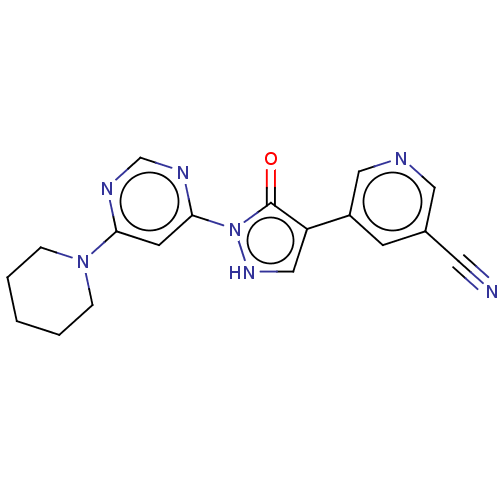 (US8524699, 44) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bayer Intellectual Property GmbH US Patent | Assay Description In Vitro assay determination of the activity and selectivity of HIF Prolyl 4-hydroxylase inhibitors. | US Patent US8524699 (2013) BindingDB Entry DOI: 10.7270/Q2TB15J4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50171641 (5-(3,3-Dimethyl-butyrylamino)-1-(2-fluoro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER HealthCare AG Curated by ChEMBL | Assay Description Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate | Bioorg Med Chem Lett 15: 4201-5 (2005) Article DOI: 10.1016/j.bmcl.2005.06.085 BindingDB Entry DOI: 10.7270/Q2H994R9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl hydroxylase EGLN2 (Homo sapiens (Human)) | BDBM118336 (US8653111, 60) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer Intellectual Property GmbH US Patent | Assay Description Hydroxylated HIF bonds specifically to the von Hippel-Lindau protein-elongin B-elongin C complex (VBC complex). This interaction occurs only if HIF i... | US Patent US8653111 (2014) BindingDB Entry DOI: 10.7270/Q2GH9GMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 83 total ) | Next | Last >> |