

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395922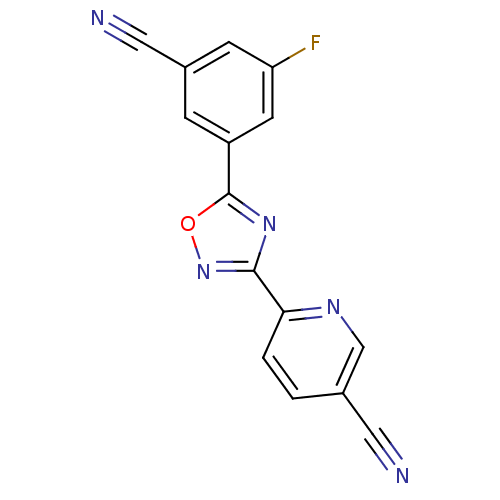 (CHEMBL2164552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]AZD9272 from human mGluR5 expressed in HEK293 cell membranes expressing GLAST after 1 hr by scintillation counter | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395922 (CHEMBL2164552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from human mGluR5 expressed in HEK293 cell membranes expressing GLAST after 1 hr by scintillation counter | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50395923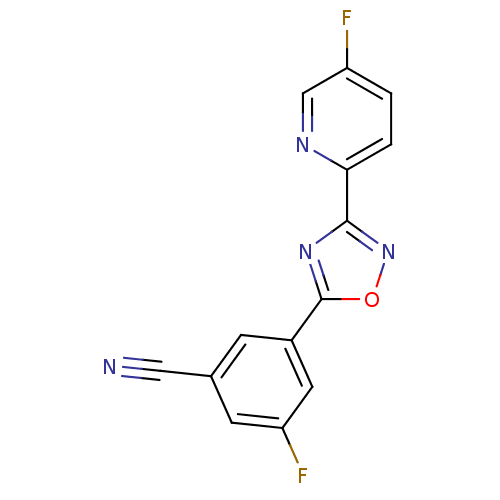 (CHEMBL2164550) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of rat recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracellu... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50395922 (CHEMBL2164552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of rat recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracellu... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395923 (CHEMBL2164550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50390485 (CHEMBL2071601) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human TRPA1 expressed in HEK293 cells assessed as inhibition of Zn2+-induced intracellular calcium flux by FLIPR assay | Bioorg Med Chem Lett 22: 5485-92 (2012) Article DOI: 10.1016/j.bmcl.2012.07.032 BindingDB Entry DOI: 10.7270/Q2RR20B8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395922 (CHEMBL2164552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13.4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50395922 (CHEMBL2164552) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]AZD9272 from mGluR5 in Sprague-Dawley rat striatum by autoradiographic analysis | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM50005021 (CHEMBL2408786 | US9650336, Example 3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description The cDNA for the soluble part of the human 13-Secretase (AA 1-AA 460) was cloned using the ASP2-Fc10-1-IRES-GFP-neoK mammalian expression vector. The... | US Patent US9650336 (2017) BindingDB Entry DOI: 10.7270/Q23X88QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395942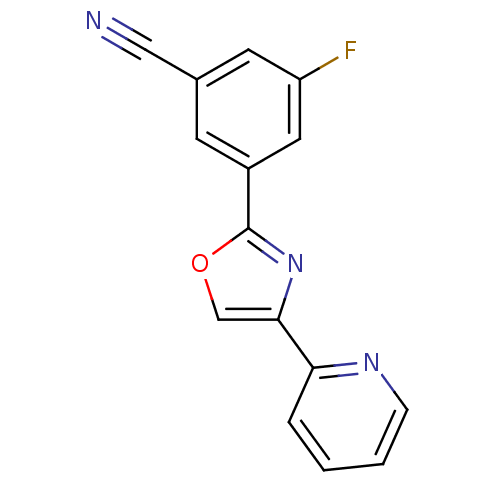 (CHEMBL2164545) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50084137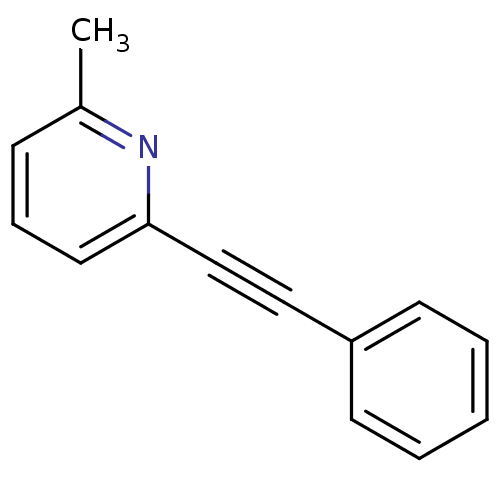 (2-Methyl-6-(phenylethynyl)pyridine | 2-Methyl-6-ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS-Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of glutamate-induced calcium influx in human mGluR5d by FLIPR | Bioorg Med Chem Lett 16: 2467-9 (2006) Article DOI: 10.1016/j.bmcl.2006.01.100 BindingDB Entry DOI: 10.7270/Q22Z1544 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181966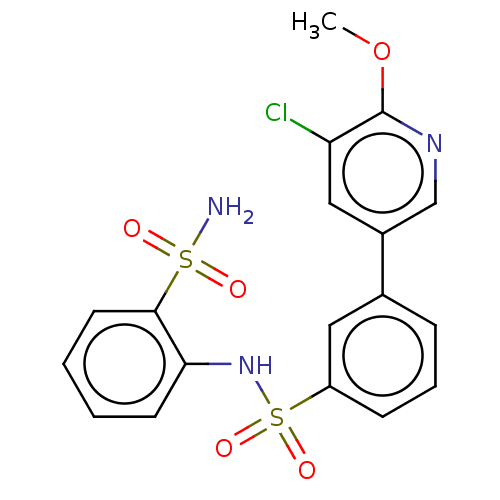 (US9145380, 169) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM50005022 (CHEMBL2408787 | US9650336, Example 1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description The cDNA for the soluble part of the human 13-Secretase (AA 1-AA 460) was cloned using the ASP2-Fc10-1-IRES-GFP-neoK mammalian expression vector. The... | US Patent US9650336 (2017) BindingDB Entry DOI: 10.7270/Q23X88QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395941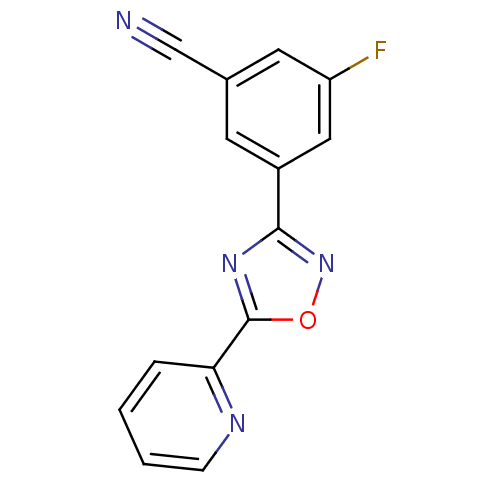 (CHEMBL2164546) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Rattus norvegicus (Rat)) | BDBM50395937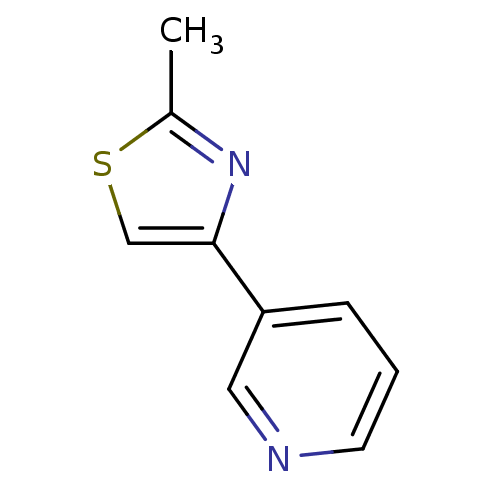 (CHEMBL2164538) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]AZD9272 from mGluR5 in Sprague-Dawley rat striatum by autoradiographic analysis | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391489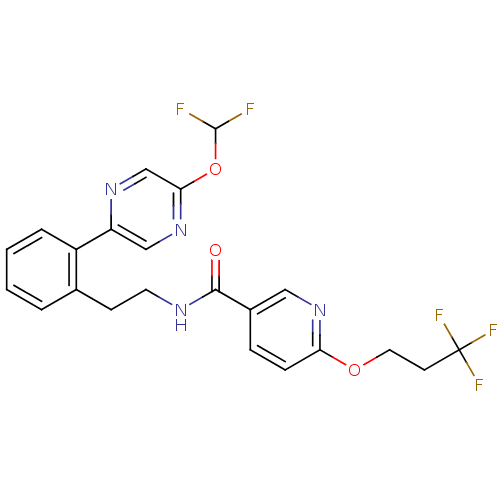 (CHEMBL2147212) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391491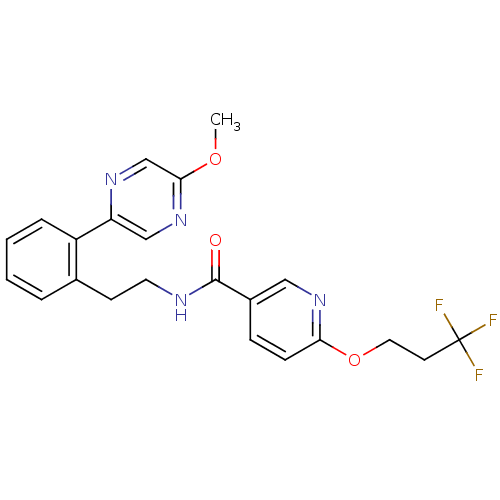 (CHEMBL2147211) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391513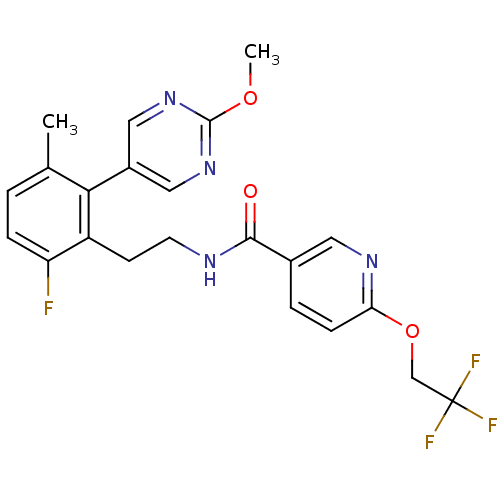 (CHEMBL2147306) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395943 (CHEMBL2164544) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395923 (CHEMBL2164550) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of glutamate-stimulated ... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182037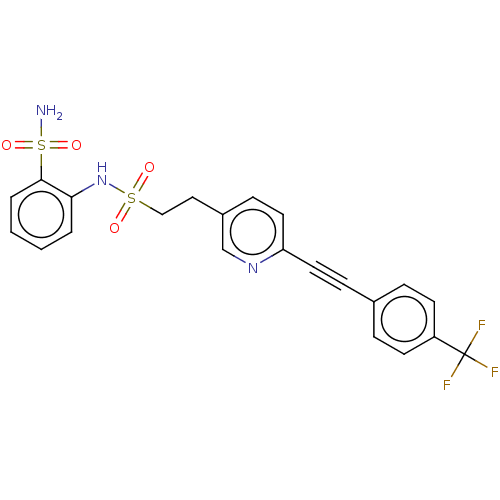 (US9145380, 240) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391483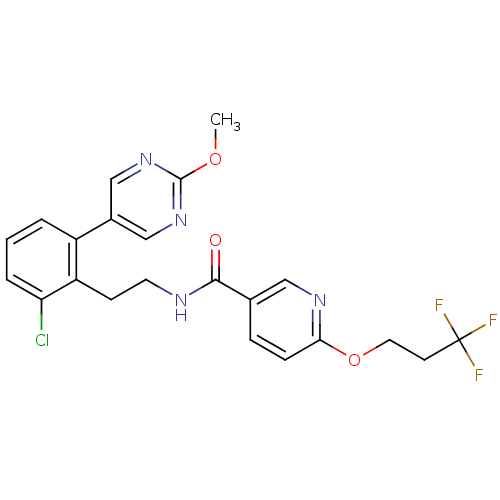 (CHEMBL2147223) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391502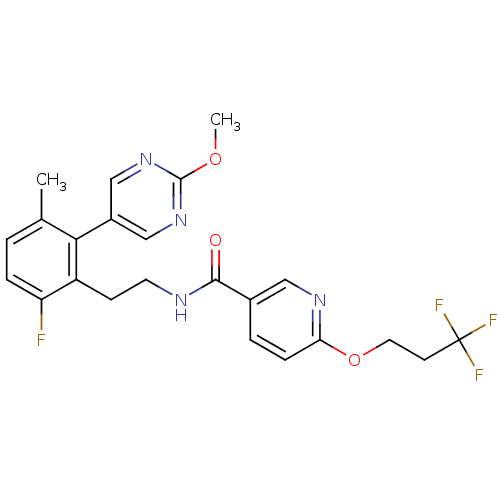 (CHEMBL2147305) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391496 (CHEMBL2147221) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181846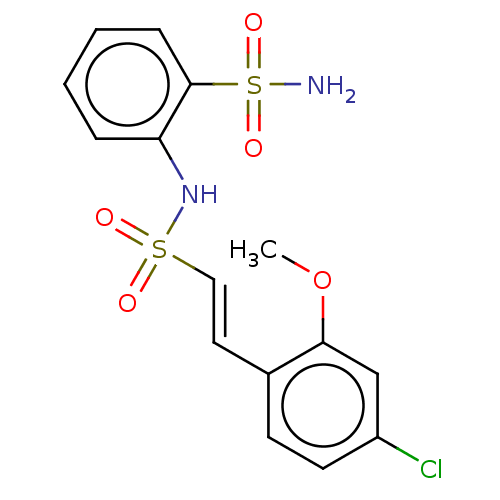 (US9145380, 49) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM307707 (4''-fluoro-6'-(3-fluoropropoxy)-4-[(2H3)methyloxy]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description The cDNA for the soluble part of the human 13-Secretase (AA 1-AA 460) was cloned using the ASP2-Fc10-1-IRES-GFP-neoK mammalian expression vector. The... | US Patent US9650336 (2017) BindingDB Entry DOI: 10.7270/Q23X88QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391501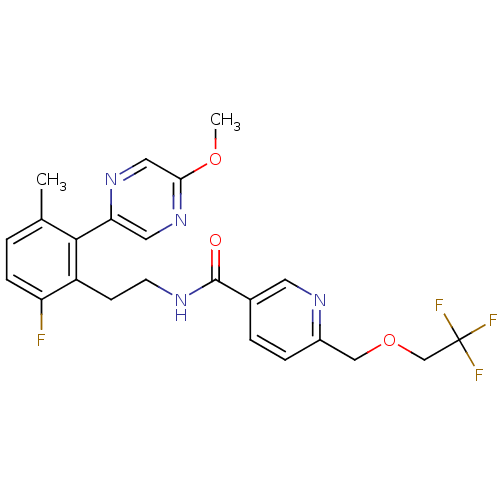 (CHEMBL2147307) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181802 (US9145380, 5) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391492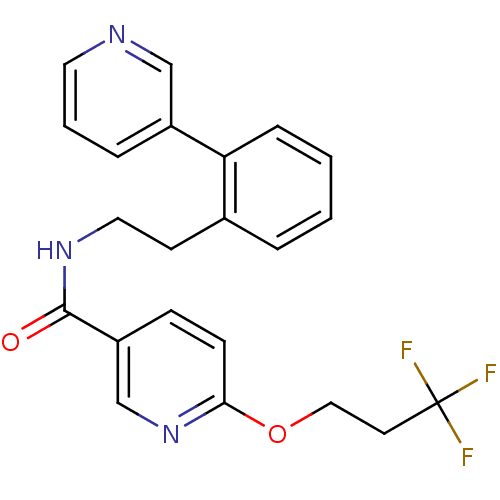 (CHEMBL2147213) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182009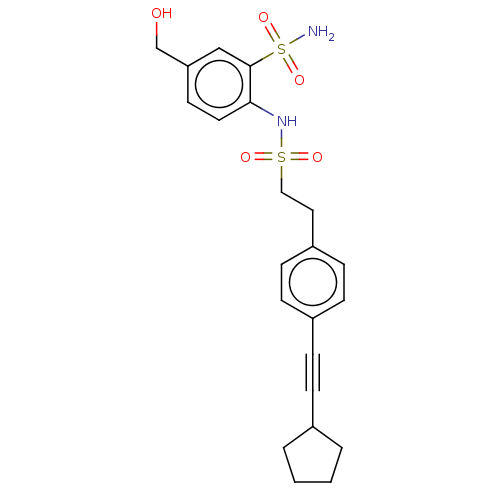 (US9145380, 212) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 41 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50151909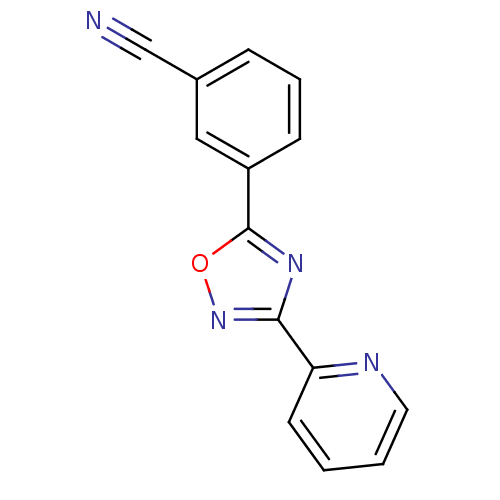 (3-(3-Pyridin-2-yl-[1,2,4]oxadiazol-5-yl)-benzonitr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181815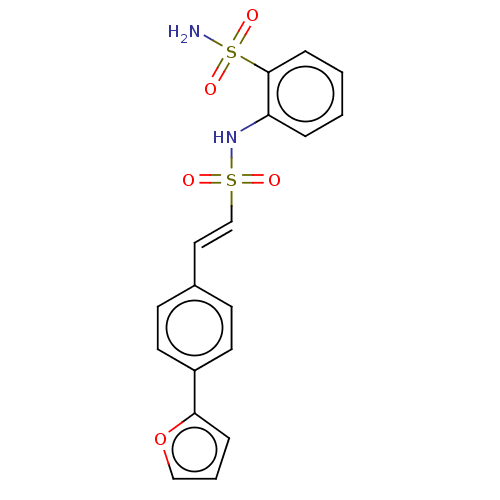 (US9145380, 18) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 44 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391500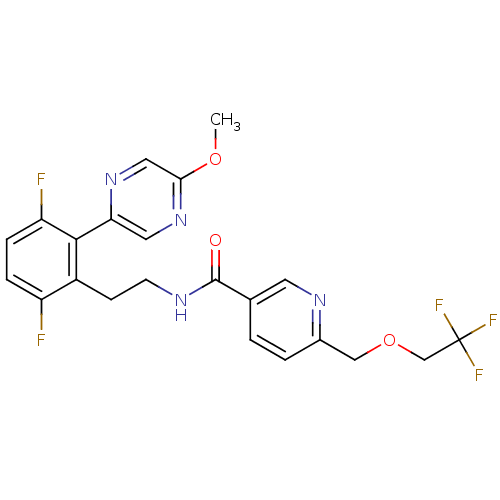 (CHEMBL2147308) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395922 (CHEMBL2164552) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of glutamate-stimulated ... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182005 (US9145380, 208) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 55 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181833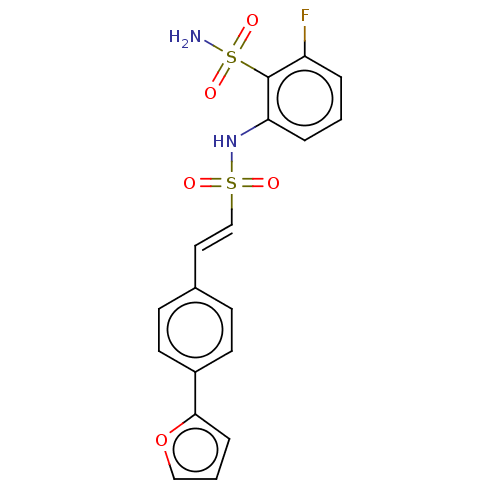 (US9145380, 36) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50395940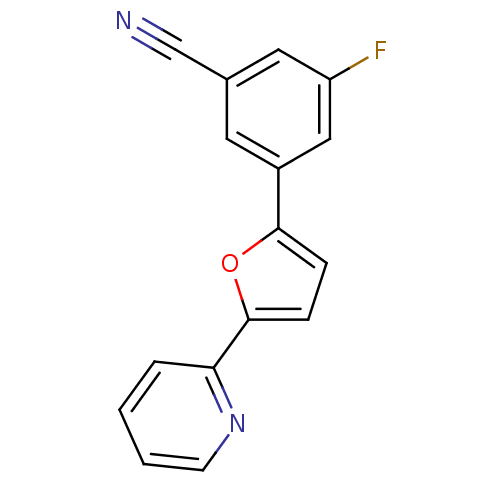 (CHEMBL2164547) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Negative allosteric modulation of human recombinant mGluR5 expressed in HEK293 cells expressing GLAST assessed as inhibition of DHPG-induced intracel... | Bioorg Med Chem Lett 22: 6974-9 (2012) Article DOI: 10.1016/j.bmcl.2012.08.100 BindingDB Entry DOI: 10.7270/Q2BP03WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391493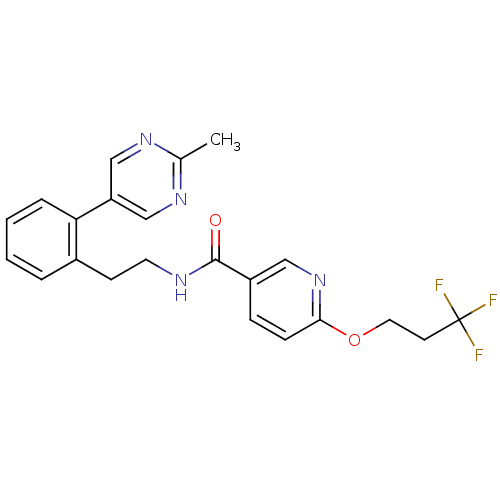 (CHEMBL2147214) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50391490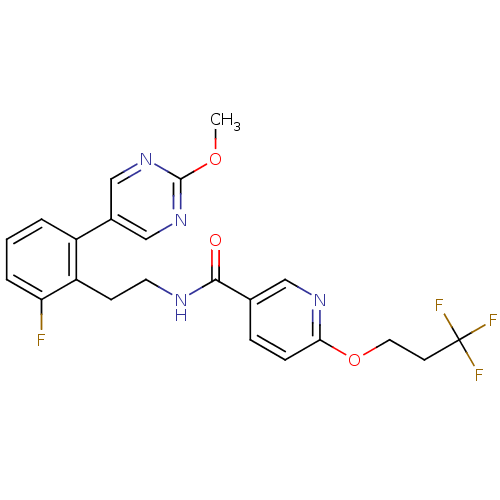 (CHEMBL2147222) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human Nav1.7 expressed in HEK293 cells by whole cell voltage-clamp electrophysiology assay | Bioorg Med Chem Lett 22: 6108-15 (2012) Article DOI: 10.1016/j.bmcl.2012.08.031 BindingDB Entry DOI: 10.7270/Q2B8596S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 [1-460] (Homo sapiens (Human)) | BDBM307702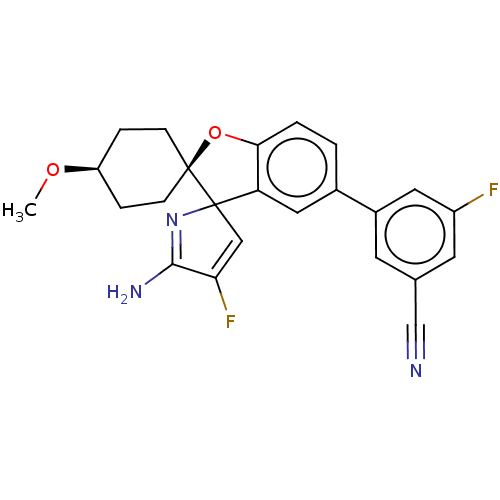 (US9650336, Example 5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description The cDNA for the soluble part of the human 13-Secretase (AA 1-AA 460) was cloned using the ASP2-Fc10-1-IRES-GFP-neoK mammalian expression vector. The... | US Patent US9650336 (2017) BindingDB Entry DOI: 10.7270/Q23X88QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181803 (US9145380, 6) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181903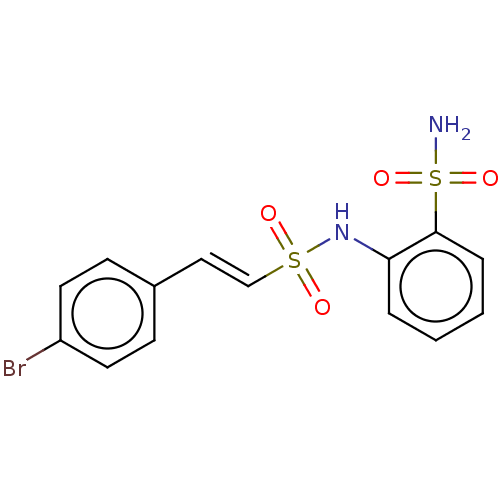 (US9145380, 106) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM182008 (US9145380, 211) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 75 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181822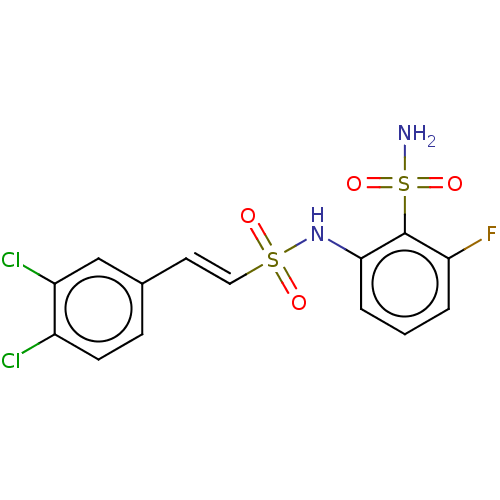 (US9145380, 25) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181801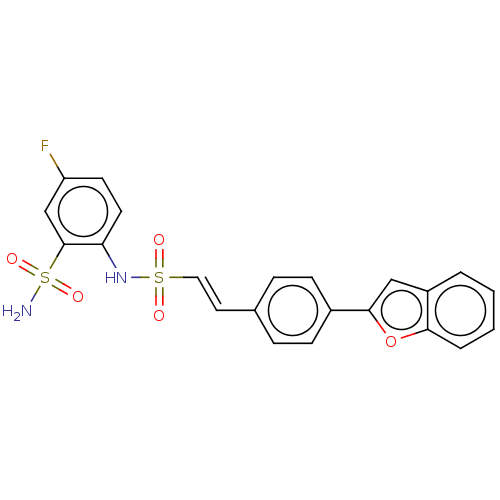 (US9145380, 4) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50182823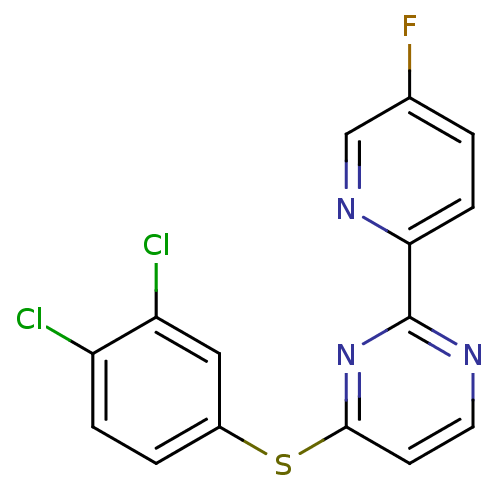 (4-(3,4-dichlorophenylthio)-2-(5-fluoropyridin-2-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS-Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of glutamate-induced calcium influx in human mGluR5d by FLIPR | Bioorg Med Chem Lett 16: 2467-9 (2006) Article DOI: 10.1016/j.bmcl.2006.01.100 BindingDB Entry DOI: 10.7270/Q22Z1544 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181980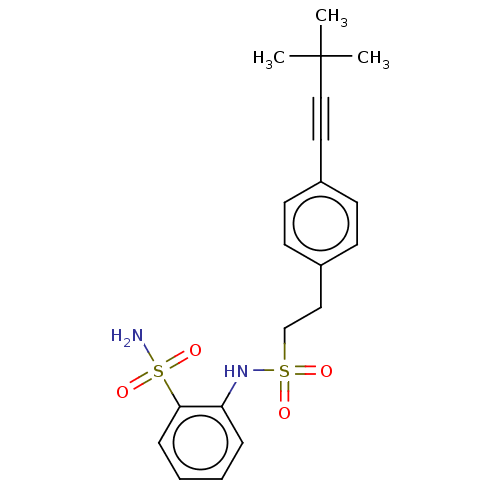 (US9145380, 183) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 81 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181849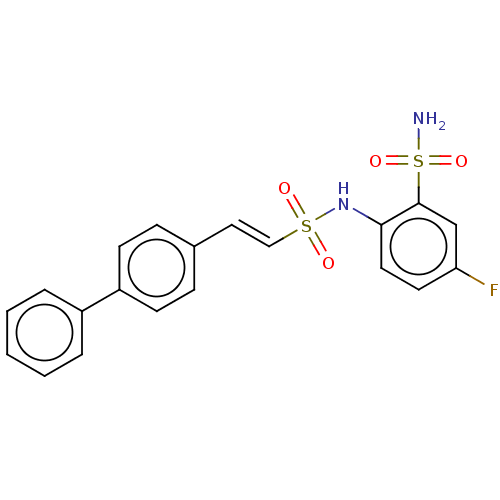 (US9145380, 52) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 84 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50182811 (4-(3,4-dichlorophenylthio)-2-(pyridin-2-yl)pyrimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
NPS-Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of glutamate-induced calcium influx in human mGluR5d by FLIPR | Bioorg Med Chem Lett 16: 2467-9 (2006) Article DOI: 10.1016/j.bmcl.2006.01.100 BindingDB Entry DOI: 10.7270/Q22Z1544 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase 2 (Homo sapiens (Human)) | BDBM181889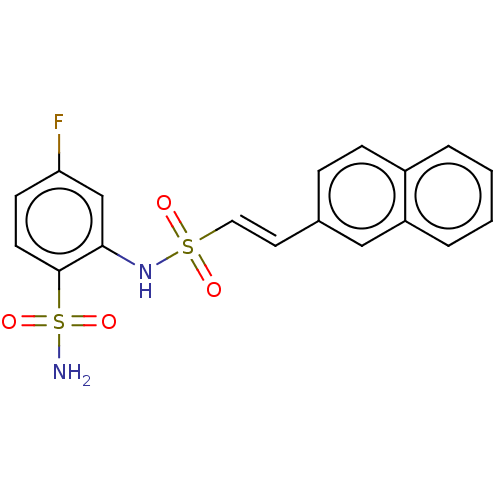 (US9145380, 92) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | 6.8 | n/a |
Astrazeneca AB US Patent | Assay Description A solution of test compound was added to a diluted microsome preparation containing human mPGES-1 and pre-incubated for 15 minutes in potassium phosp... | US Patent US9145380 (2015) BindingDB Entry DOI: 10.7270/Q2XG9PXM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 456 total ) | Next | Last >> |