

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29525 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026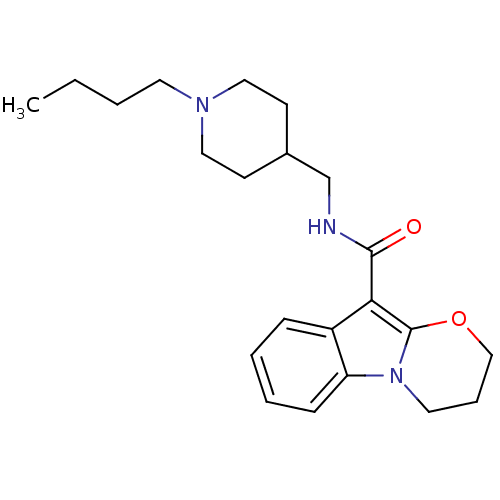 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50492064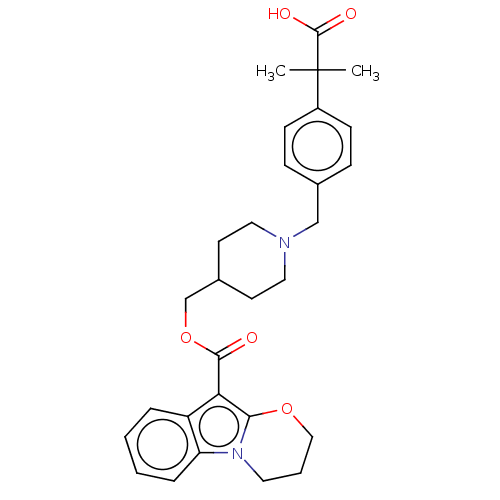 (CHEMBL2391994) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Displacement of [3H]GR113808 from human 5-HT4B receptor expressed in HEK293 cells after 1 hr by liquid scintillation counting analysis | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029662 (3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-ethyl-6-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10908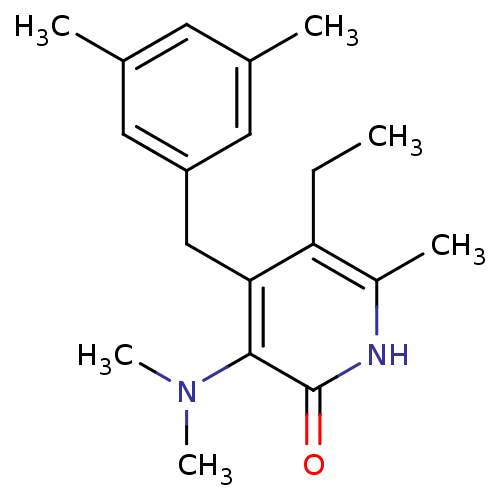 (3-(dimethylamino)-4-[(3,5-dimethylphenyl)methyl]-5...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10903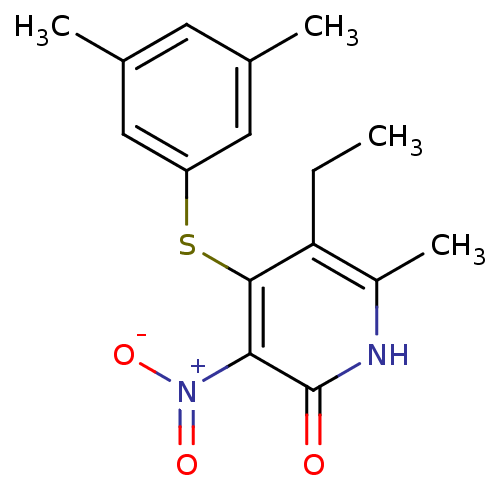 (4-[(3,5-dimethylphenyl)sulfanyl]-5-ethyl-6-methyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029665 (4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029660 (3-Amino-4-(3,5-dimethyl-phenylsulfanyl)-5-methyl-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10906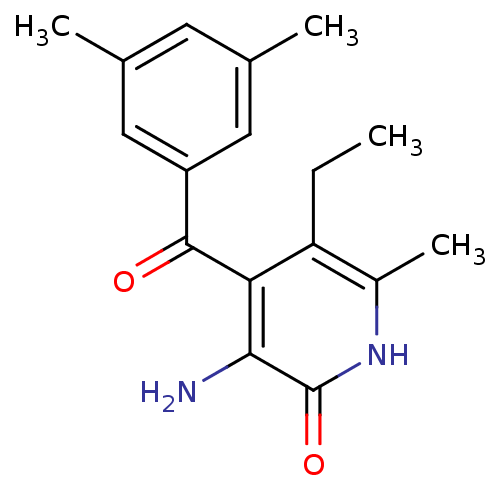 (3-Amino-4-(3,5-dimethylbenzoyl)-5-ethyl-6-methylpy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10905 (3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10907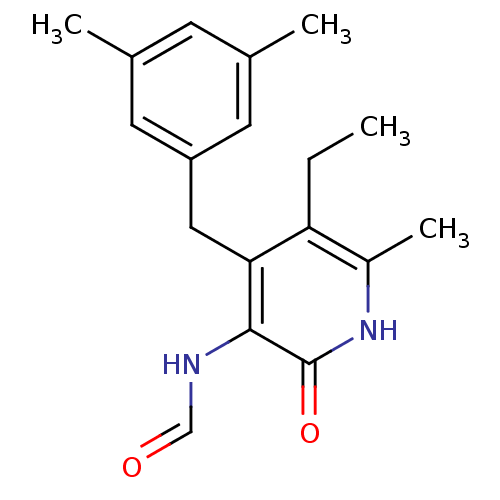 (4-(3,5-Dimethylbenzyl)-5-ethyl-3-formamido-6-methy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM10904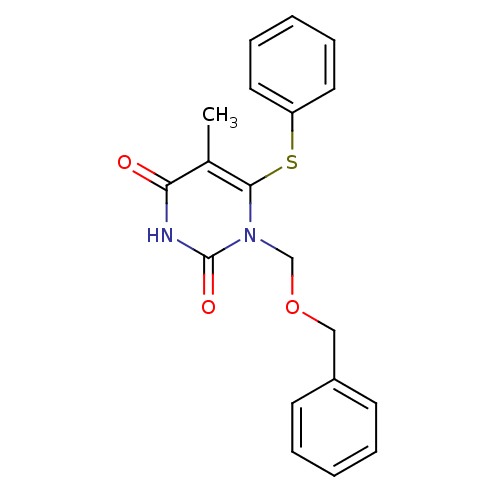 (1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Institut Curie | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 43: 3949-62 (2000) Article DOI: 10.1021/jm0009437 BindingDB Entry DOI: 10.7270/Q2V12315 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM10904 (1-[(benzyloxy)methyl]-5-methyl-6-(phenylsulfanyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029678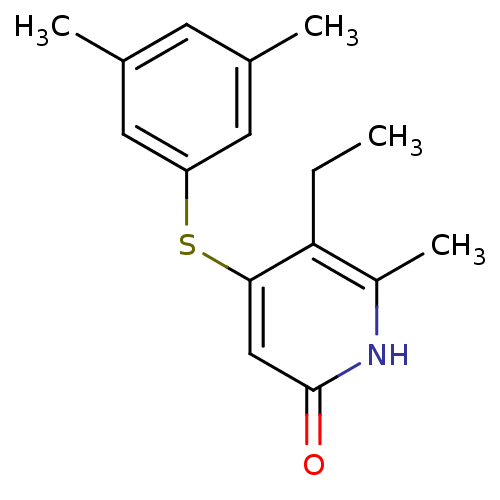 (4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50111322 (CHEMBL274335 | N,N-Dimethyl-N'-(11-nitro-13H-5,13-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Telomerase in telomerase repeat amplification protocol (TRAP) assay | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029663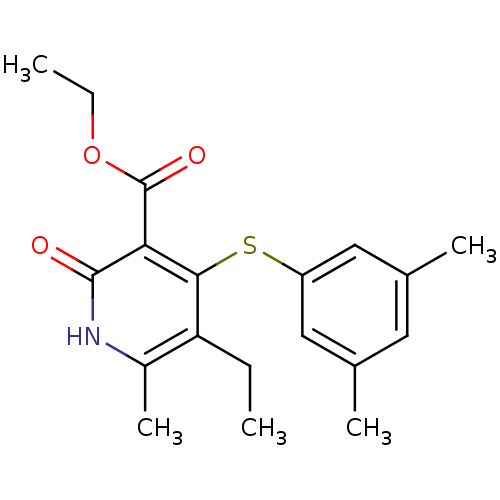 (4-(3,5-Dimethyl-phenylsulfanyl)-5-ethyl-6-methyl-2...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase I, thermostable (Thermus aquaticus) | BDBM50111320 (CHEMBL274096 | N'-(10-Methoxy-13H-5,13-diaza-diben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Taq DNA polymerase | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50111320 (CHEMBL274096 | N'-(10-Methoxy-13H-5,13-diaza-diben...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Telomerase in telomerase repeat amplification protocol (TRAP) assay | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50111323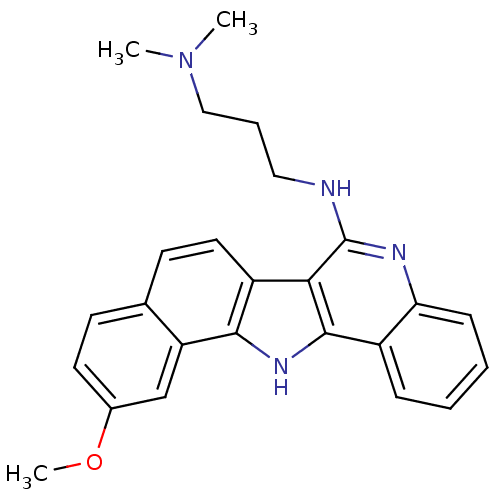 (CHEMBL276202 | N'-(11-Methoxy-13H-5,13-diaza-diben...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Telomerase in telomerase repeat amplification protocol (TRAP) assay | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase I, thermostable (Thermus aquaticus) | BDBM50111323 (CHEMBL276202 | N'-(11-Methoxy-13H-5,13-diaza-diben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Taq DNA polymerase | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029679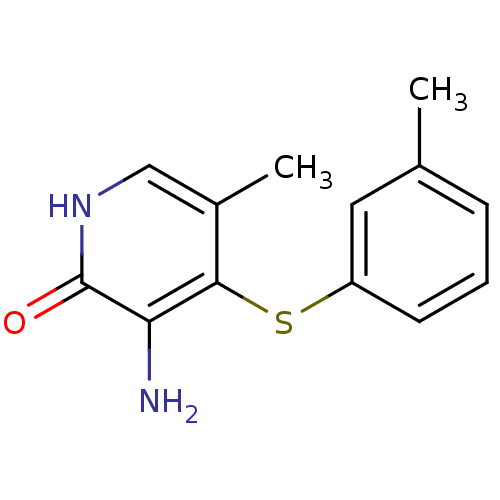 (3-Amino-5-methyl-4-m-tolylsulfanyl-1H-pyridin-2-on...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029671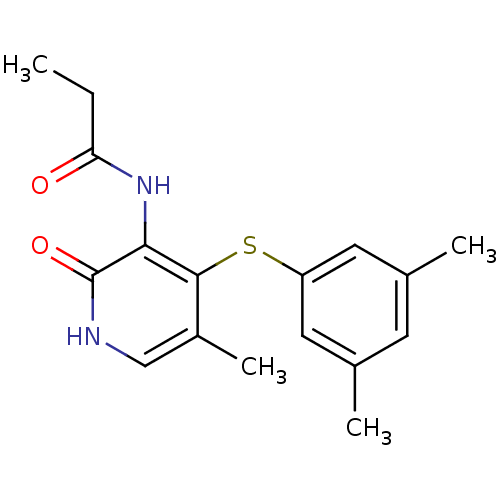 (CHEMBL126931 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029672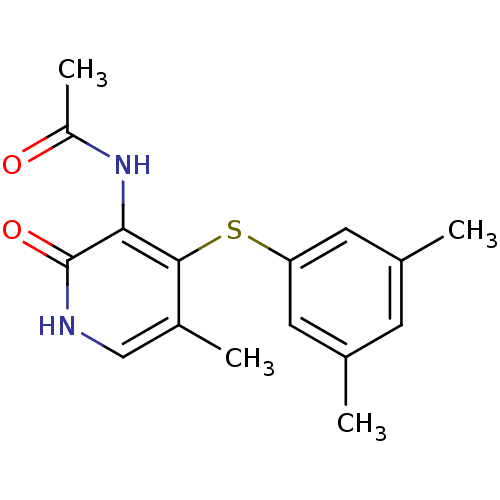 (CHEMBL341880 | N-[4-(3,5-Dimethyl-phenylsulfanyl)-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase I, thermostable (Thermus aquaticus) | BDBM50111322 (CHEMBL274335 | N,N-Dimethyl-N'-(11-nitro-13H-5,13-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Taq DNA polymerase | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50004152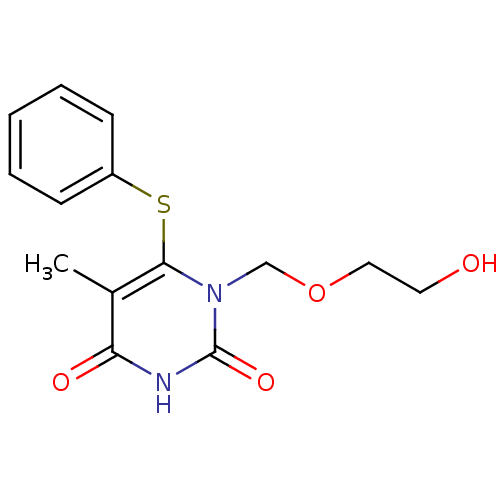 ((HEPT) 1-(2-Hydroxy-ethoxymethyl)-5-methyl-6-pheny...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Telomerase reverse transcriptase (Homo sapiens (Human)) | BDBM50111321 (CHEMBL10763 | N'-(11-Methoxy-13H-5,13-diaza-dibenz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Telomerase in telomerase repeat amplification protocol (TRAP) assay | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029666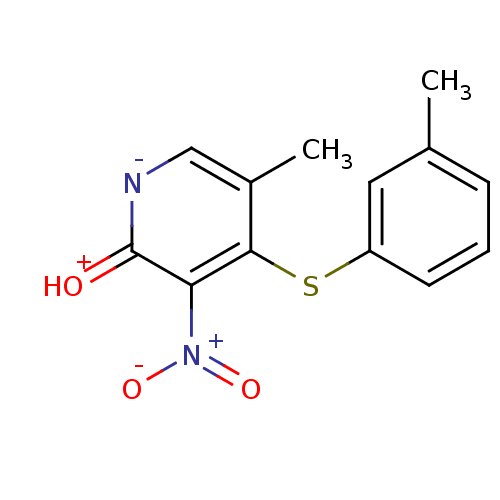 (5-Methyl-3-nitro-4-m-tolylsulfanyl-1H-pyridin-2-on...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase I, thermostable (Thermus aquaticus) | BDBM50111321 (CHEMBL10763 | N'-(11-Methoxy-13H-5,13-diaza-dibenz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Tested for inhibitory activity against Taq DNA polymerase | Bioorg Med Chem Lett 12: 1071-4 (2002) BindingDB Entry DOI: 10.7270/Q2RJ4HS0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50029675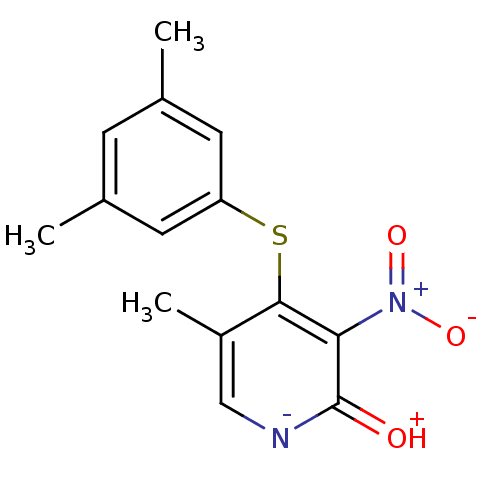 (4-(3,5-Dimethyl-phenylsulfanyl)-5-methyl-3-nitro-1...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
URA 1387 CNRS Curated by ChEMBL | Assay Description Inhibition of HIV-1 reverse transcriptase(RT) | J Med Chem 38: 4679-86 (1995) BindingDB Entry DOI: 10.7270/Q2QF8RWD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50492064 (CHEMBL2391994) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Drug Discovery Laboratory AS Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in HEK293 cells by whole-cell patch-clamp technique | Eur J Med Chem 64: 629-37 (2013) Article DOI: 10.1016/j.ejmech.2013.03.060 BindingDB Entry DOI: 10.7270/Q27947NT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408880 (CHEMBL58213) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408882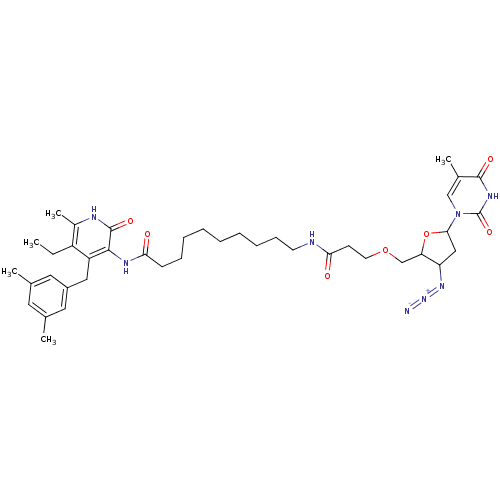 (CHEMBL59514) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408883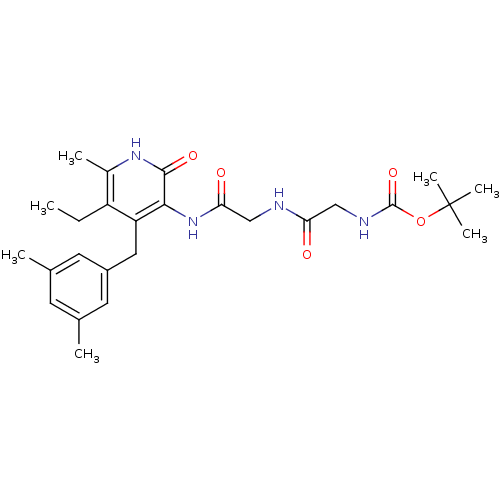 (CHEMBL58497) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408884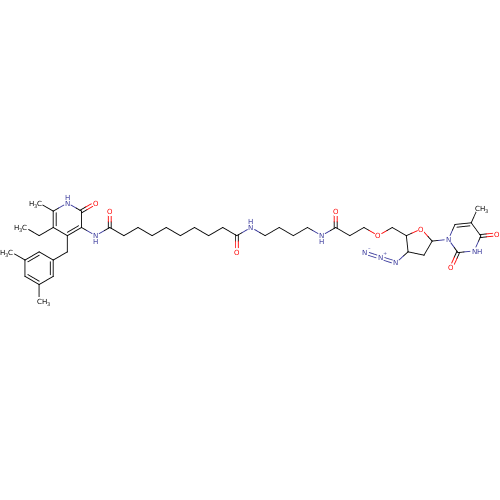 (CHEMBL301685) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408885 (CHEMBL57313) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408886 (CHEMBL57929) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408887 (CHEMBL418294) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408888 (CHEMBL417227) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408889 (CHEMBL57161) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408890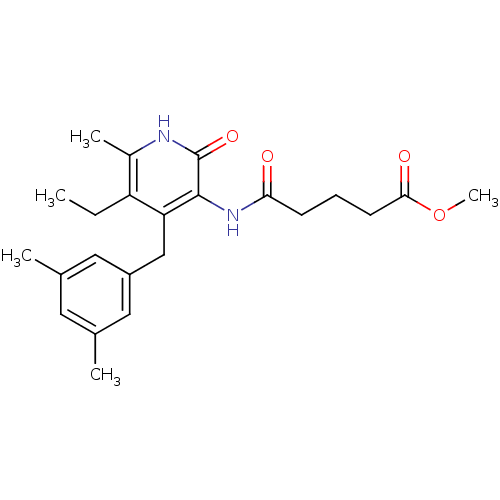 (CHEMBL57301) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408891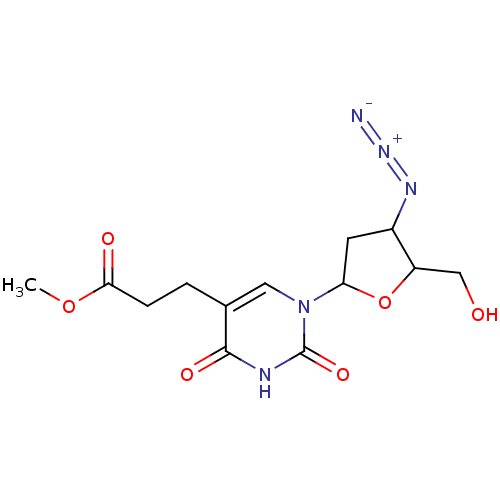 (CHEMBL58891) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408892 (CHEMBL418118) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408893 (CHEMBL58914) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408894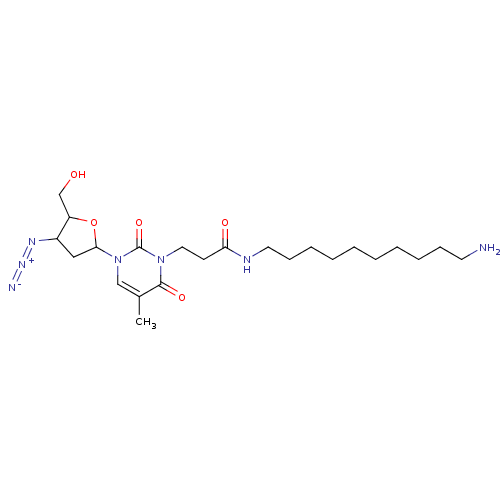 (CHEMBL292392) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408895 (CHEMBL440482) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408896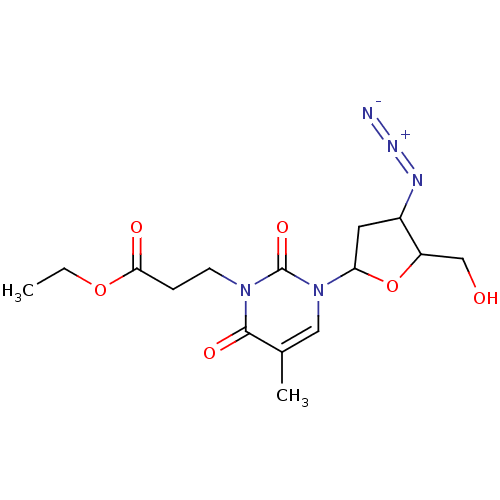 (CHEMBL56328) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408897 (CHEMBL262003) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM10905 (3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408899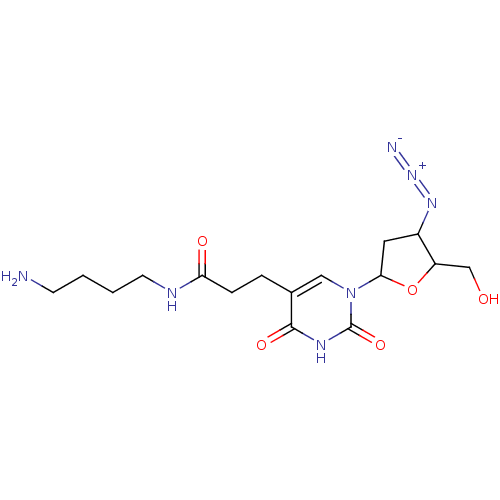 (CHEMBL300607) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |