

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282079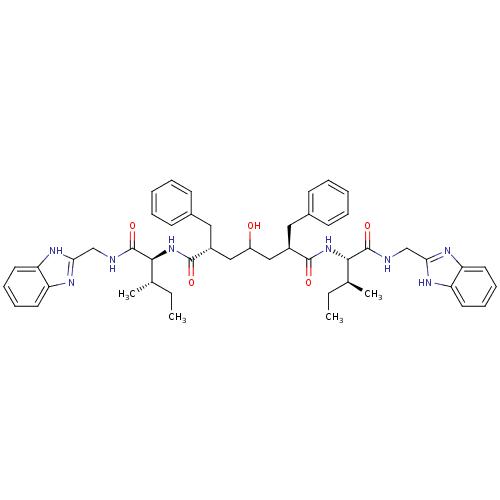 ((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against HIV-1 protease using [125I]-SPA (scintillation proximity assay) | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065621 (CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) | J Med Chem 41: 2806-18 (1998) Article DOI: 10.1021/jm980068d BindingDB Entry DOI: 10.7270/Q29G5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288762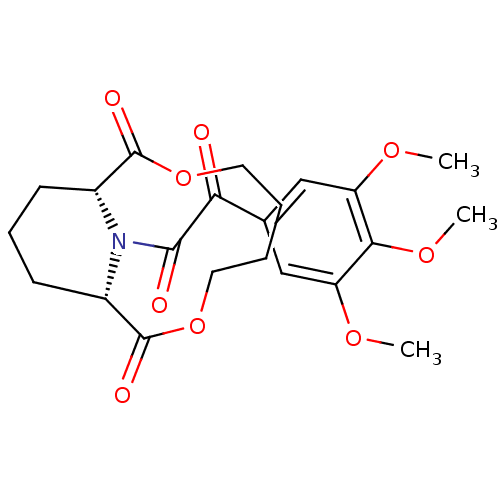 ((1S,10R)-14-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-ace...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288763 ((1S,9R)-5-Benzyloxymethyl-13-[2-oxo-2-(3,4,5-trime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288765 ((1S,9R)-5-(tert-Butyl-dimethyl-silanyloxymethyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288764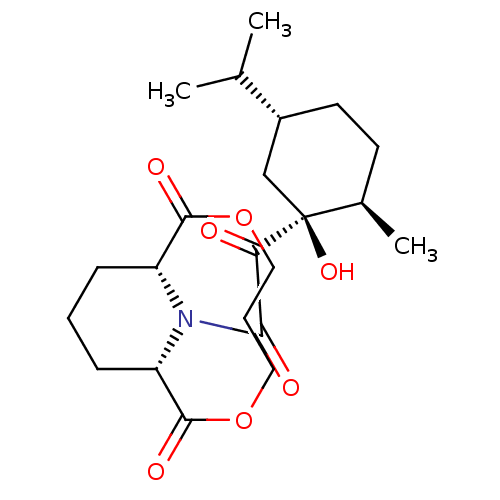 ((1S,9R)-13-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288768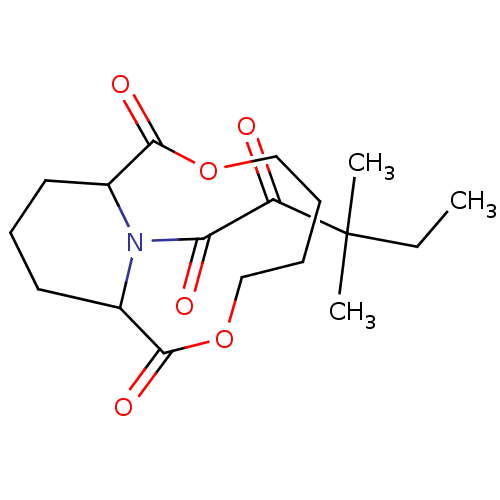 ((1R,10S)-14-(3,3-Dimethyl-2-oxo-pentanoyl)-3,8-dio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288767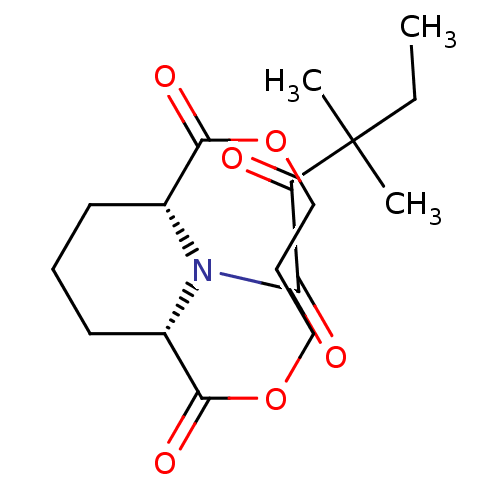 ((1R,9S)-13-(3,3-Dimethyl-2-oxo-pentanoyl)-3,7-diox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288766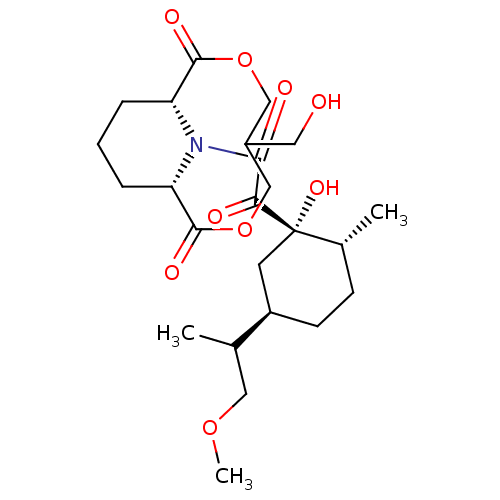 ((1S,9R)-13-{2-[(1S,2R,5R)-1-Hydroxy-5-(2-methoxy-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50288769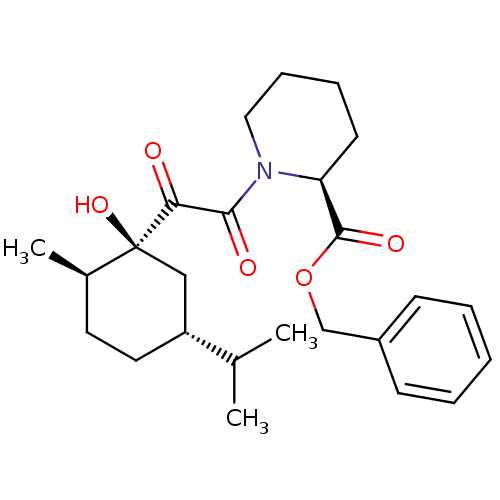 ((S)-1-[2-((1S,2R,5R)-1-Hydroxy-5-isopropyl-2-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against human FKBP-12 rotamase | Bioorg Med Chem Lett 6: 385-390 (1996) Article DOI: 10.1016/0960-894X(96)00032-7 BindingDB Entry DOI: 10.7270/Q2862GFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282079 ((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282083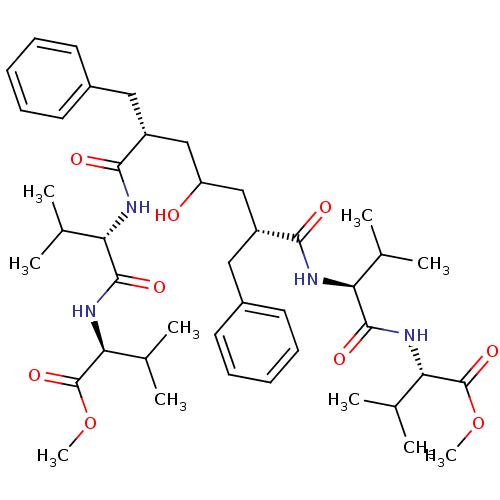 ((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282077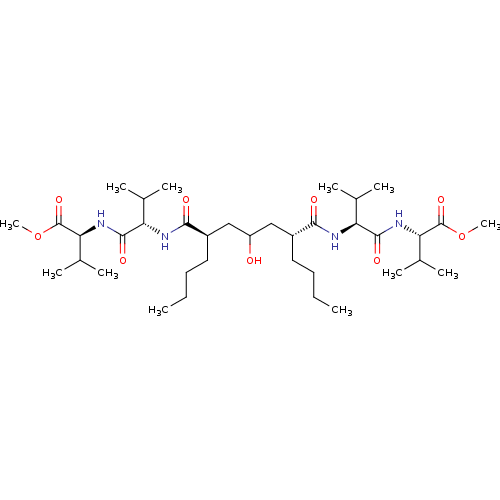 ((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12972 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12973 ((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12974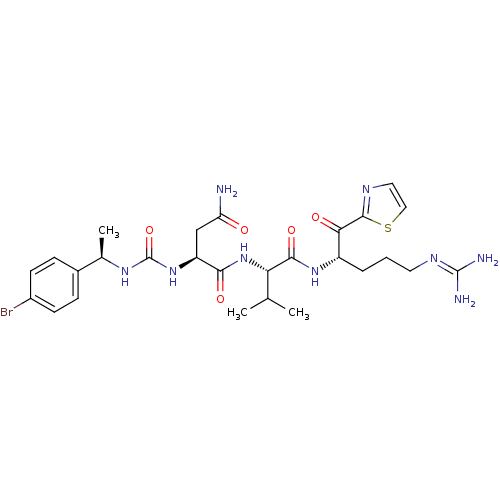 ((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12968 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282076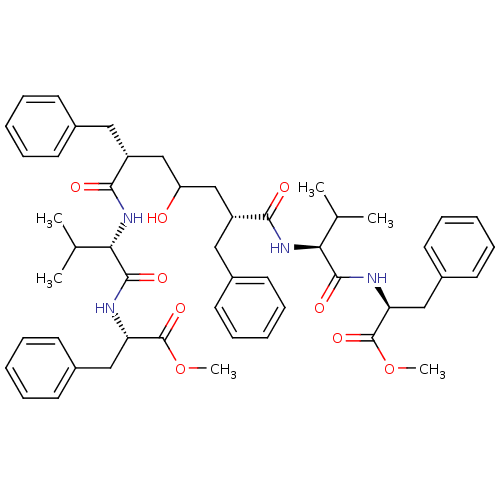 ((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12962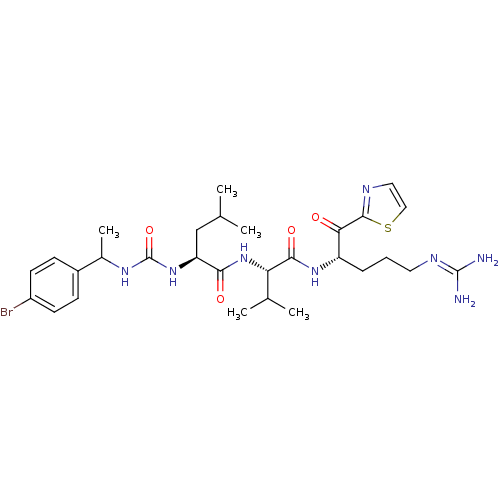 ((2S)-2-({[1-(4-bromophenyl)ethyl]carbamoyl}amino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282087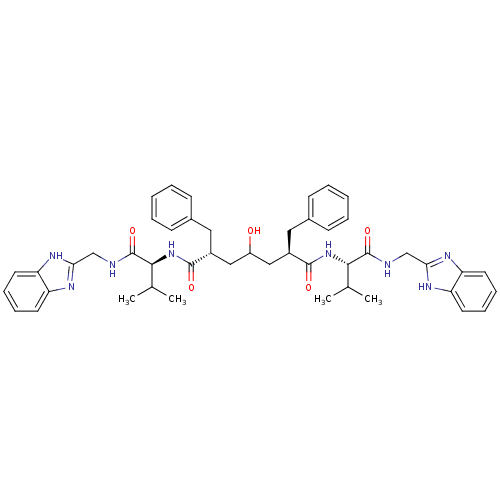 ((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12977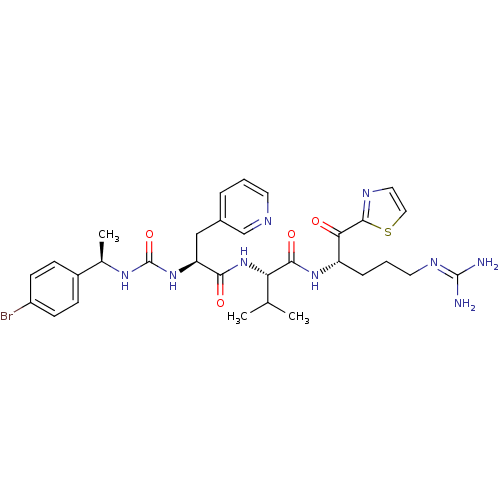 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145722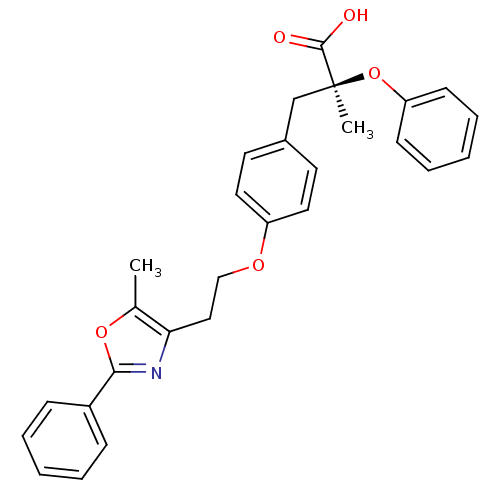 ((S)-2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human Peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 14: 6113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.031 BindingDB Entry DOI: 10.7270/Q2QN67J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12970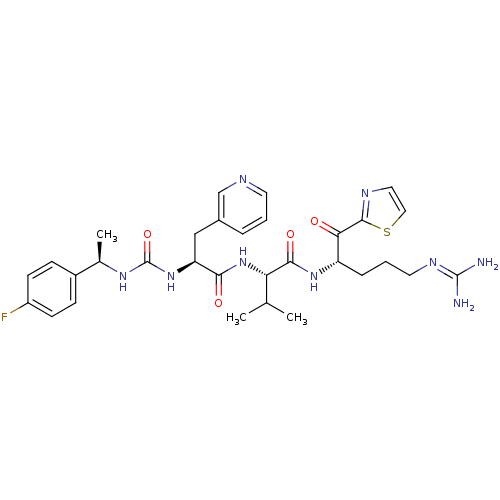 ((2S)-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12975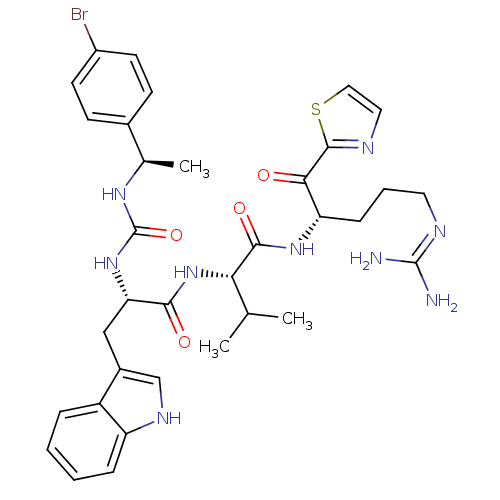 ((2S)-2-[(2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12976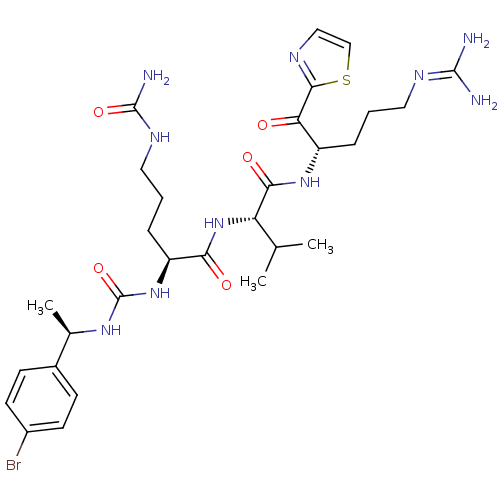 ((2S)-2-({[(1R)-1-(4-bromophenyl)ethyl]carbamoyl}am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282098 (1-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282081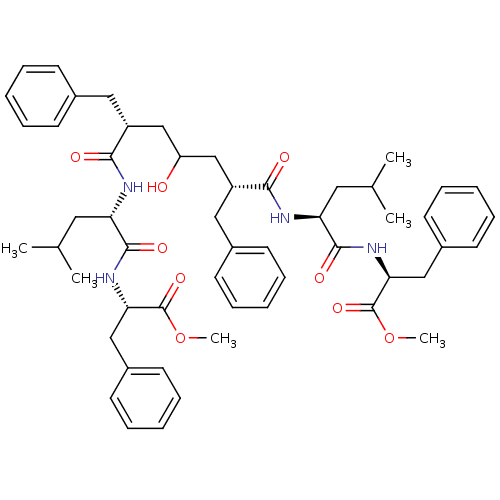 ((S)-2-((S)-2-{(2R,6R)-2-Benzyl-4-hydroxy-6-[(S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282097 ((3S,4aS,8aS)-2-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403197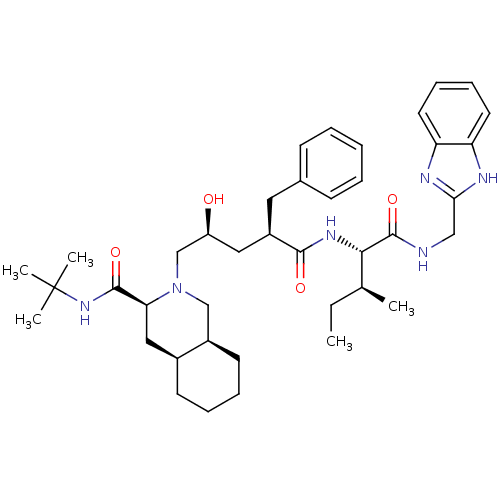 (CHEMBL2115378) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50403198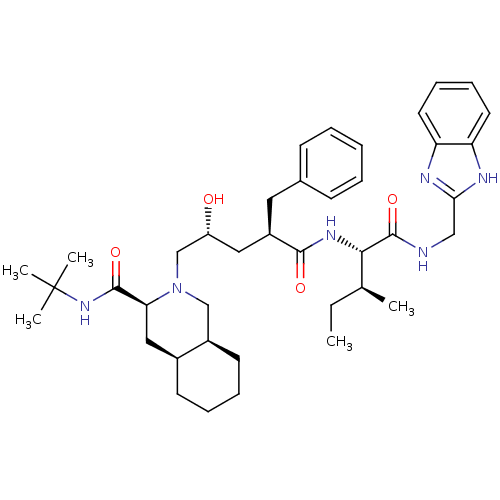 (CHEMBL2114445) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284036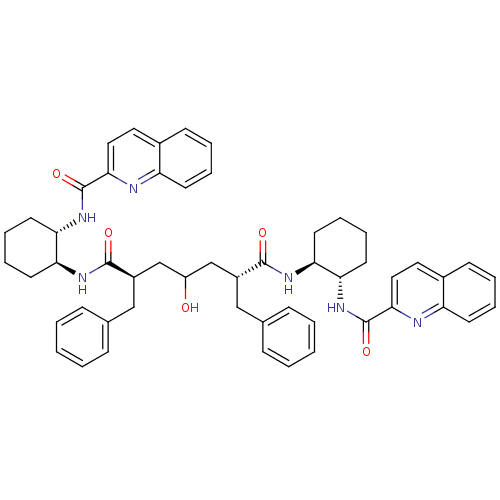 ((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against recombinant HIV-1 protease using 125 I-SPA (scintillation proximity assay) | Bioorg Med Chem Lett 4: 583-588 (1994) Article DOI: 10.1016/S0960-894X(01)80159-1 BindingDB Entry DOI: 10.7270/Q2Q24061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50145712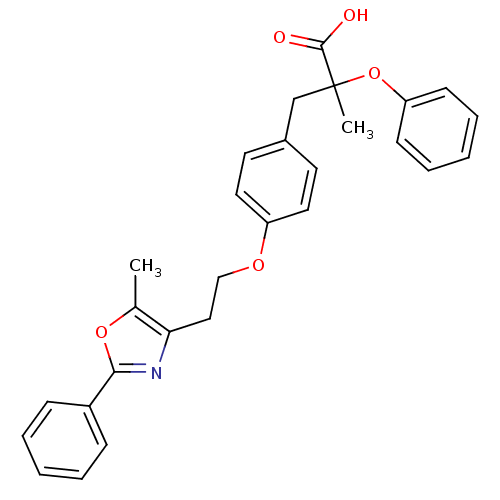 (2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human Peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 14: 6113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.031 BindingDB Entry DOI: 10.7270/Q2QN67J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12963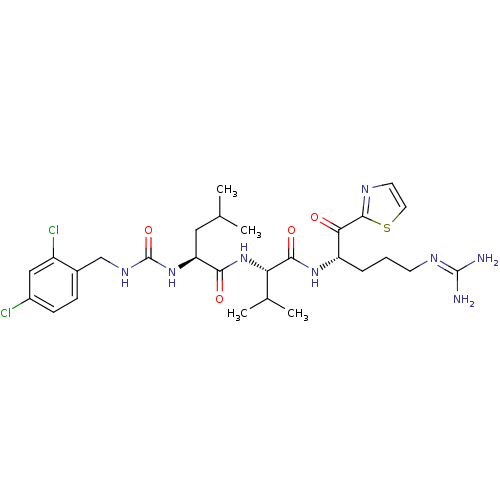 ((2S)-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-oxo-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50284034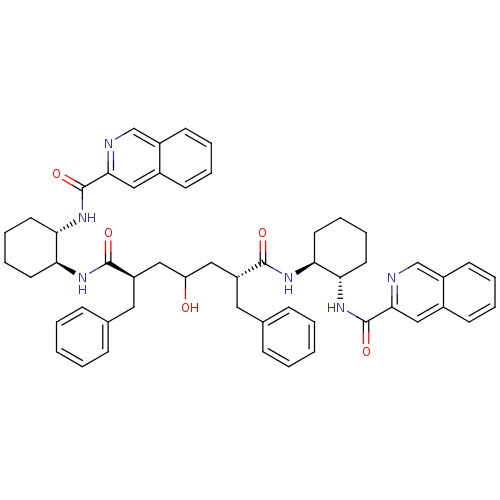 ((2R,6R)-2,6-Dibenzyl-4-hydroxy-heptanedioic acid b...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against recombinant HIV-1 protease using 125 I-SPA (scintillation proximity assay) | Bioorg Med Chem Lett 4: 583-588 (1994) Article DOI: 10.1016/S0960-894X(01)80159-1 BindingDB Entry DOI: 10.7270/Q2Q24061 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12955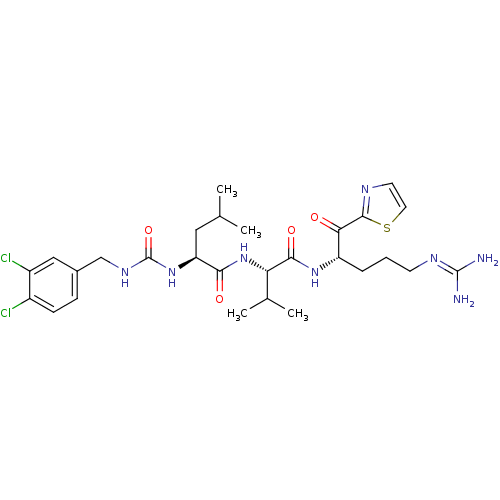 ((2S)-N-[(1S)-1-{[(2S)-5-carbamimidamido-1-oxo-1-(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50156525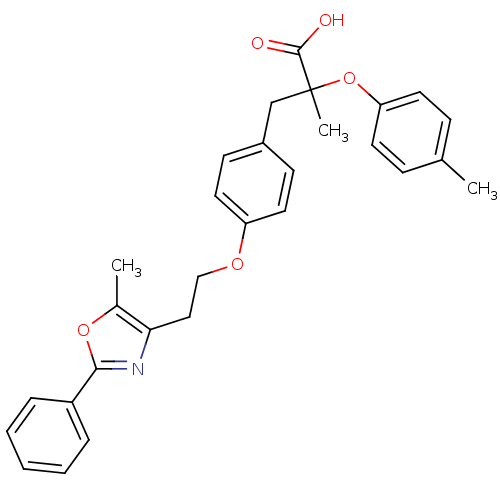 (2-Methyl-3-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human Peroxisome proliferator activated receptor alpha | Bioorg Med Chem Lett 14: 6113-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.031 BindingDB Entry DOI: 10.7270/Q2QN67J1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282095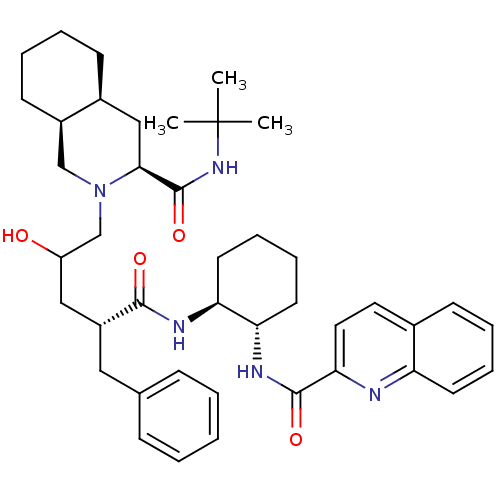 (CHEMBL291155 | Quinoline-2-carboxylic acid {(1S,2S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282103 ((3S,4aS,8aS)-2-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282092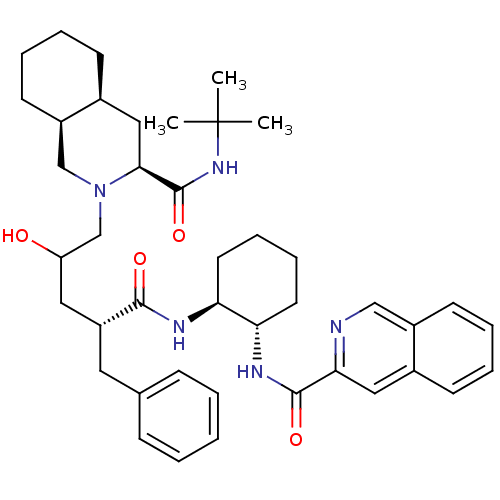 (CHEMBL412878 | Isoquinoline-3-carboxylic acid {(1S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282078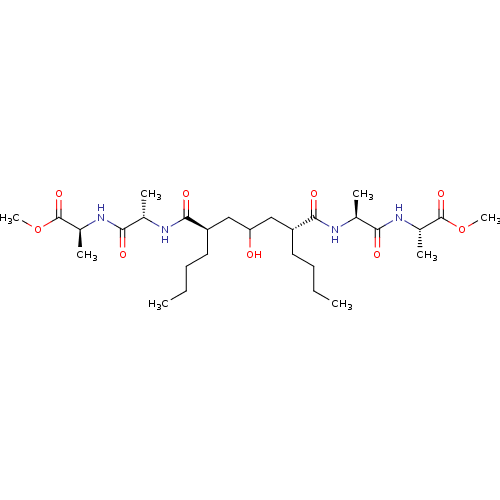 ((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12956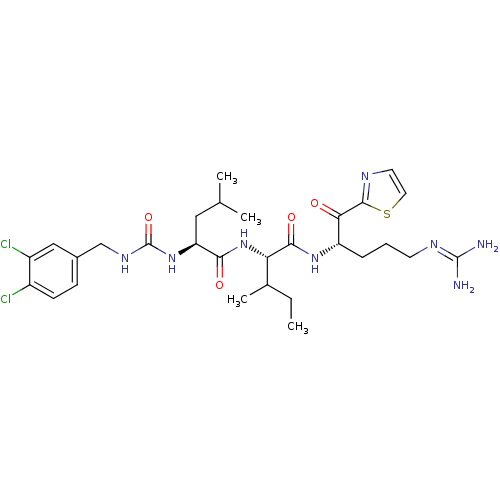 ((2S,3R)-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282091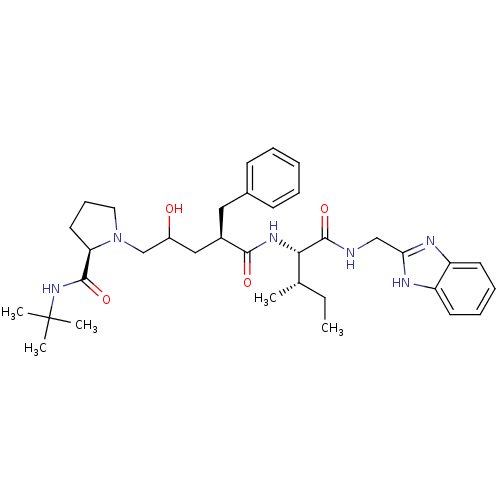 (1-((R)-4-{(1S,2S)-1-[(1H-Benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for inhibitory activity against HIV-1 Protease | Bioorg Med Chem Lett 3: 1595-1600 (1993) Article DOI: 10.1016/S0960-894X(00)80024-4 BindingDB Entry DOI: 10.7270/Q2154GZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50282085 ((S)-2-((S)-2-{(2R,6R)-2-Butyl-4-hydroxy-6-[(S)-1-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease | Bioorg Med Chem Lett 3: 1589-1594 (1993) Article DOI: 10.1016/S0960-894X(00)80023-2 BindingDB Entry DOI: 10.7270/Q24X57QC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM12946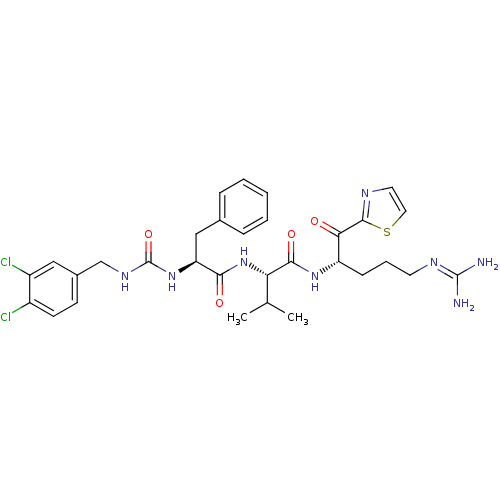 ((2S)-N-[(2S)-5-carbamimidamido-1-oxo-1-(1,3-thiazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Daiichi Asubio Medical Research Laboratories LLC (DAIAMED) | Assay Description Enzyme peptidolytic activities were measured using a fluorogenic reporter group, 7-amido-4-methylcoumarin (AMC). Released AMC was measured at an emis... | J Med Chem 49: 7781-91 (2006) Article DOI: 10.1021/jm060978s BindingDB Entry DOI: 10.7270/Q2SJ1HTM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM439600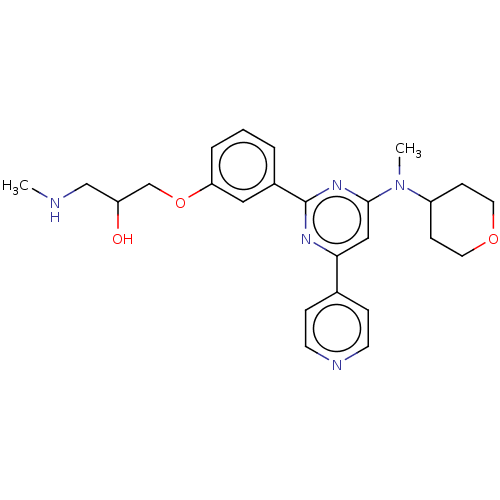 (US10633389, Example 1-1 | US20230279020, Example 1...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% BSG, and 0.002% Tween 20, prepared on the day of use... | US Patent US10633389 (2020) BindingDB Entry DOI: 10.7270/Q29G5QV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM439601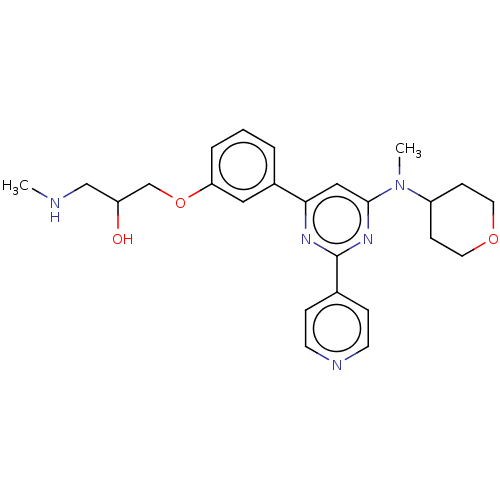 (US10633389, Example 2-1 | US20230279020, Example 2...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% BSG, and 0.002% Tween 20, prepared on the day of use... | US Patent US10633389 (2020) BindingDB Entry DOI: 10.7270/Q29G5QV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM439602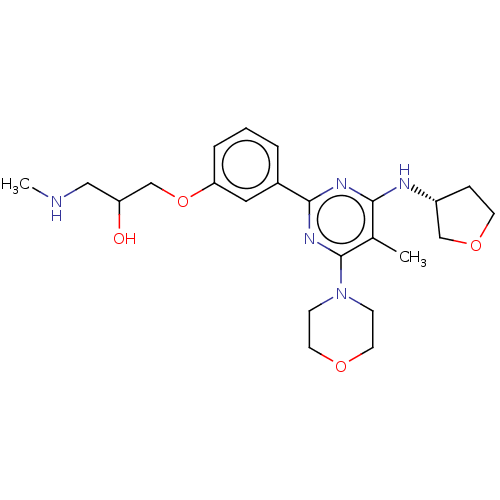 (US10633389, Example 3-1 | US20230279020, Example 3...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% BSG, and 0.002% Tween 20, prepared on the day of use... | US Patent US10633389 (2020) BindingDB Entry DOI: 10.7270/Q29G5QV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM439609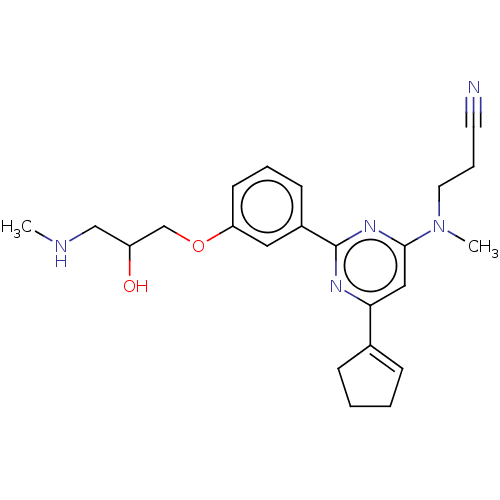 (US10633389, Example 9-1 | US20230279020, Example 9...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% BSG, and 0.002% Tween 20, prepared on the day of use... | US Patent US10633389 (2020) BindingDB Entry DOI: 10.7270/Q29G5QV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-arginine methyltransferase CARM1 (Homo sapiens (Human)) | BDBM439610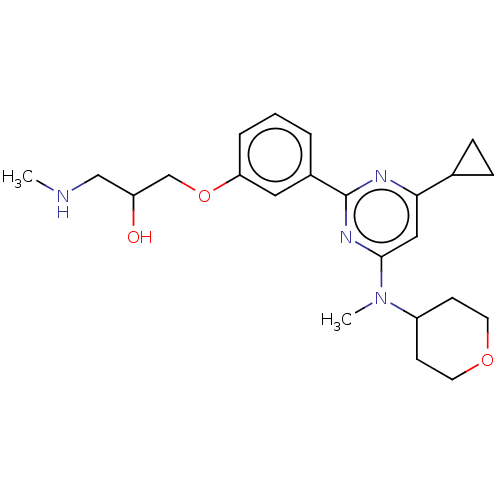 (US10633389, Example 10-1 | US20230279020, Example ...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc. US Patent | Assay Description The assays were all performed in a buffer consisting of 20 mM Bicine (pH=7.6), 1 mM TCEP, 0.005% BSG, and 0.002% Tween 20, prepared on the day of use... | US Patent US10633389 (2020) BindingDB Entry DOI: 10.7270/Q29G5QV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2361 total ) | Next | Last >> |