

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148155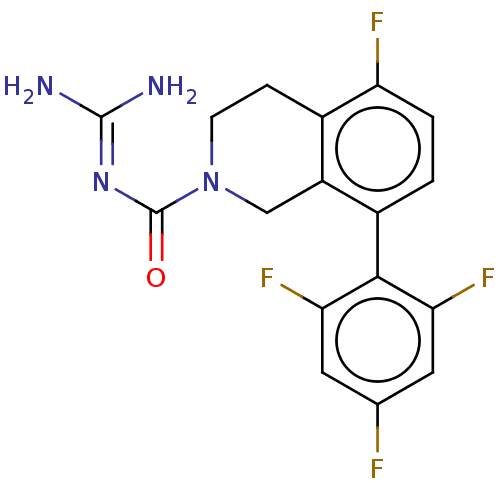 (US8962612, 26) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 0.680 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148163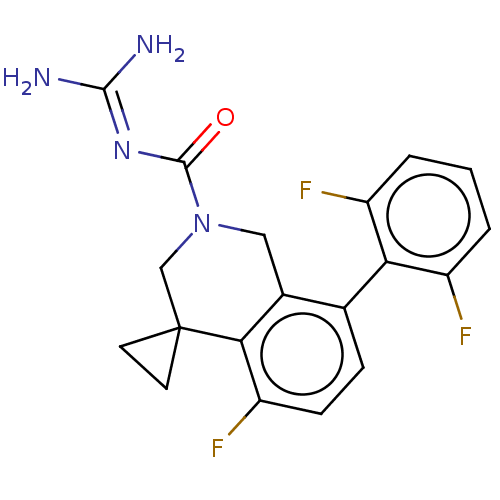 (US8962612, 66) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.75 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148169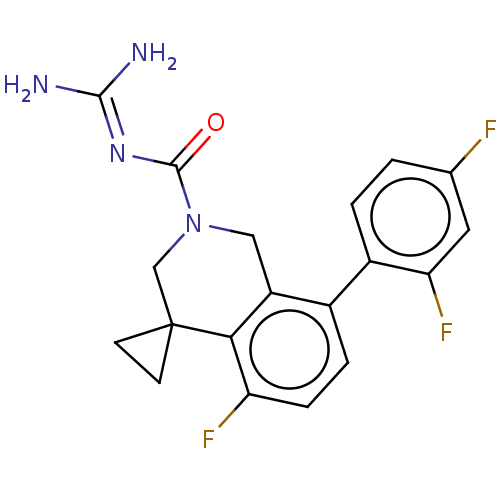 (US8962612, 80) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.850 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148165 (US8962612, 74) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.880 | -53.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148171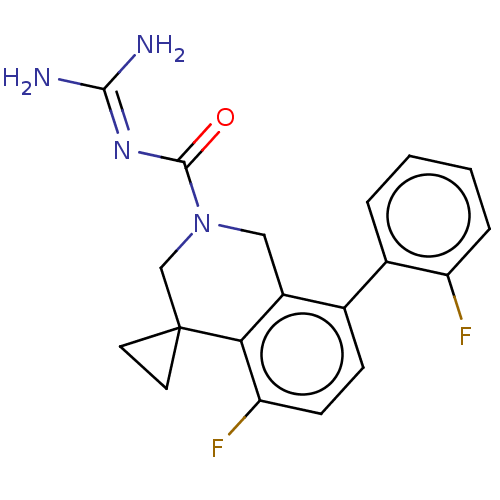 (US8962612, 86) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.950 | -53.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148154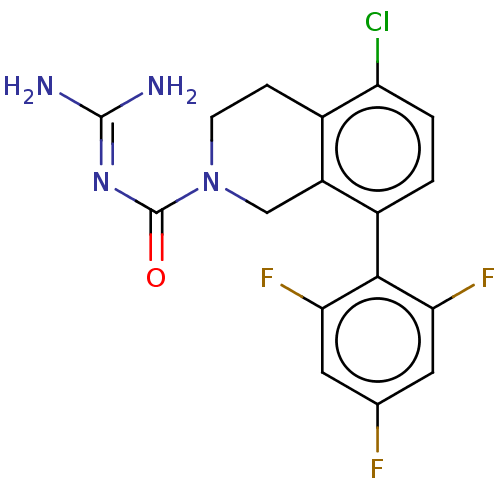 (US8962612, 23) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148156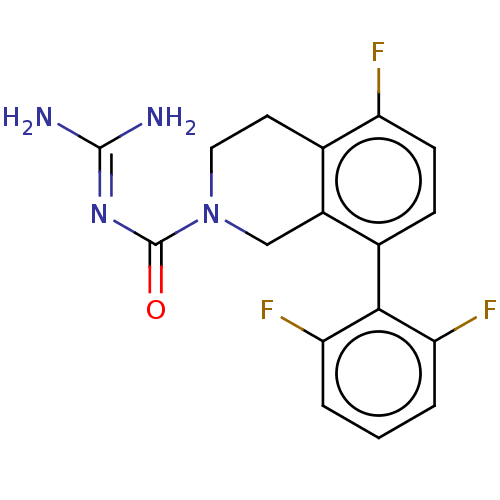 (US8962612, 28) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135790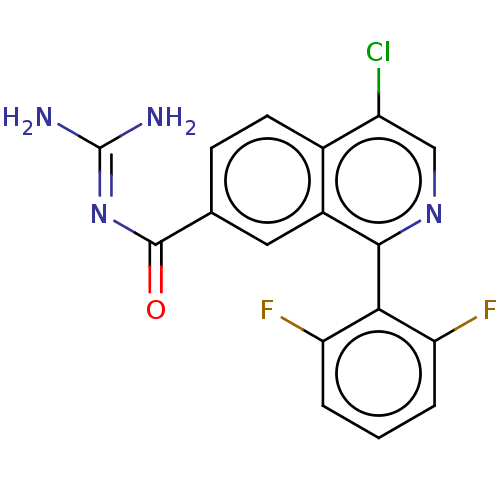 (US8853242, 162) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135780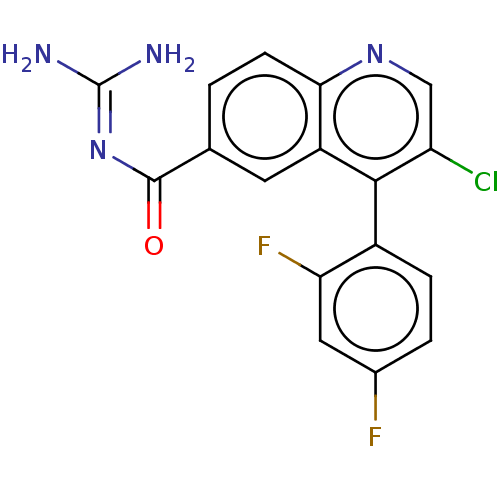 (US8853242, 60) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148167 (US8962612, 78) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -52.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135785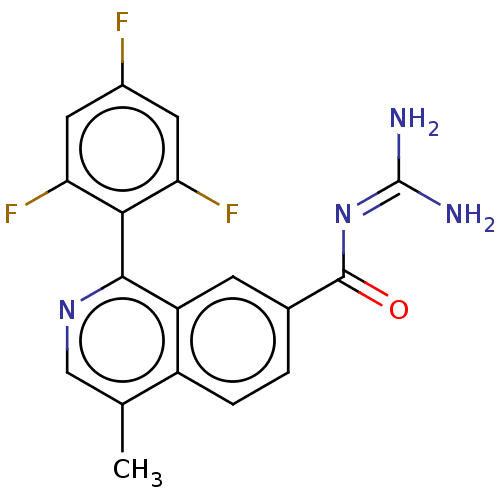 (US8853242, 152) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135783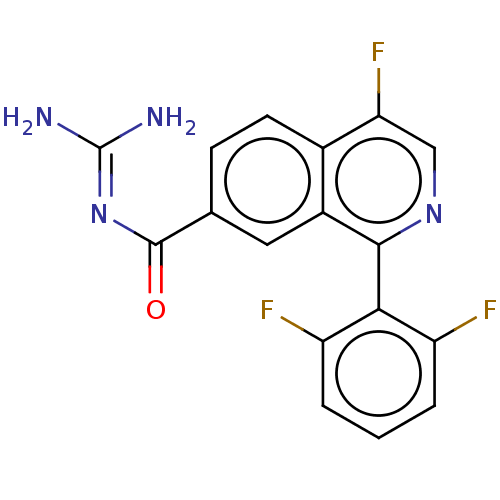 (US8853242, 148) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.40 | -50.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135782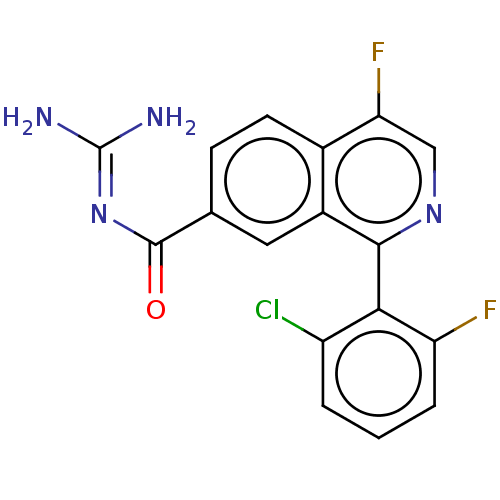 (US8853242, 147) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | -50.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148164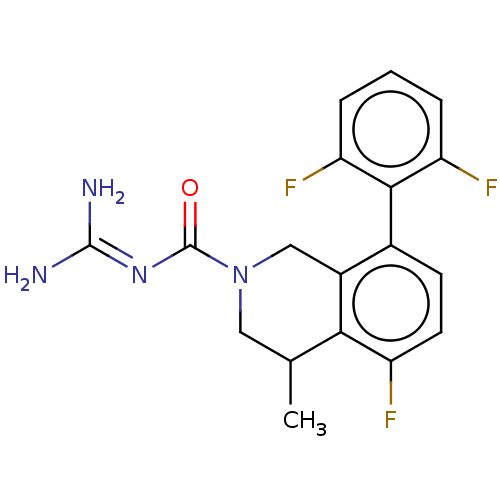 (US8962612, 69) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.60 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135795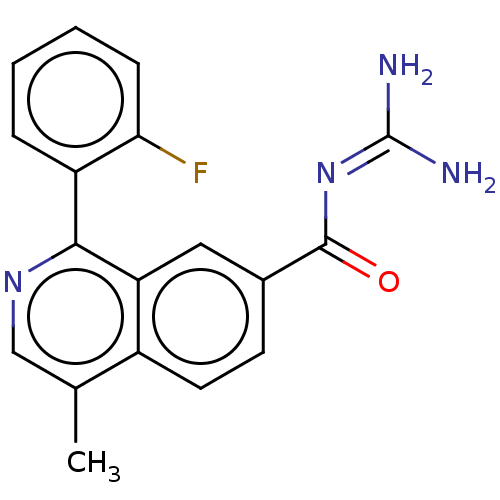 (US8853242, 192) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135792 (US8853242, 170) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | -49.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148162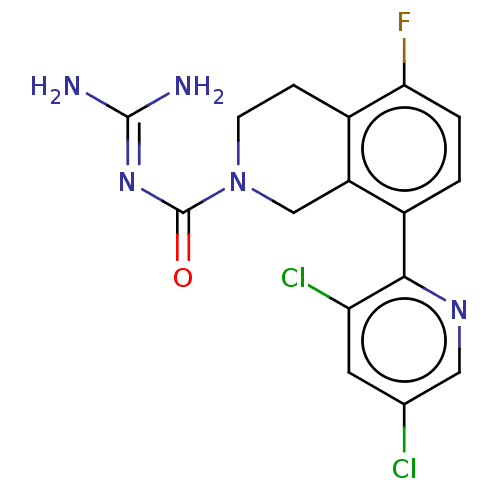 (US8962612, 62) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.90 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135796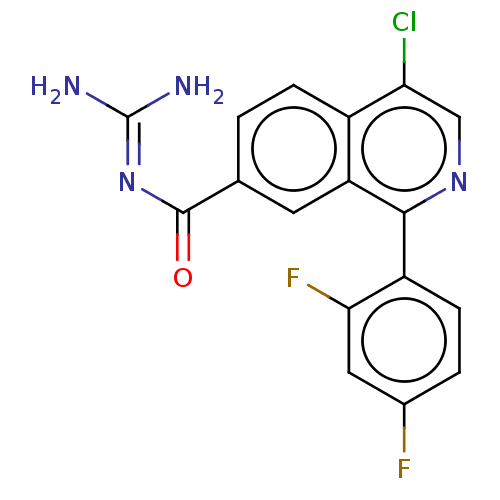 (US8853242, 211) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.30 | -49.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148161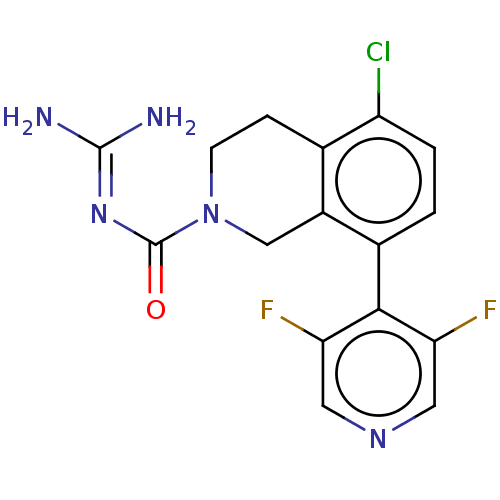 (US8962612, 61) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.5 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148166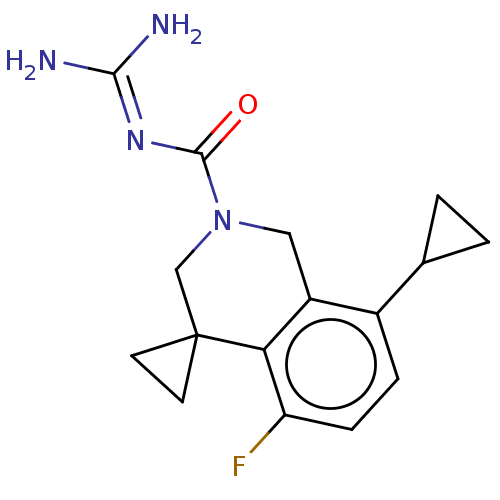 (US8962612, 76) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148157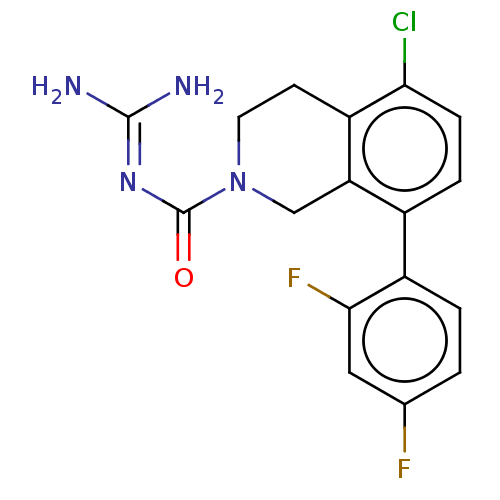 (US8962612, 44) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | -50.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135788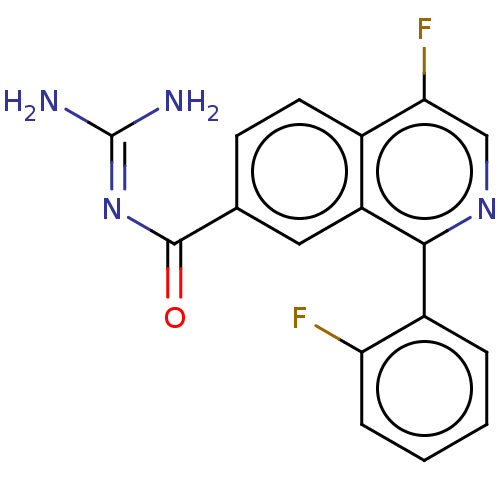 (US8853242, 160) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.30 | -48.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135789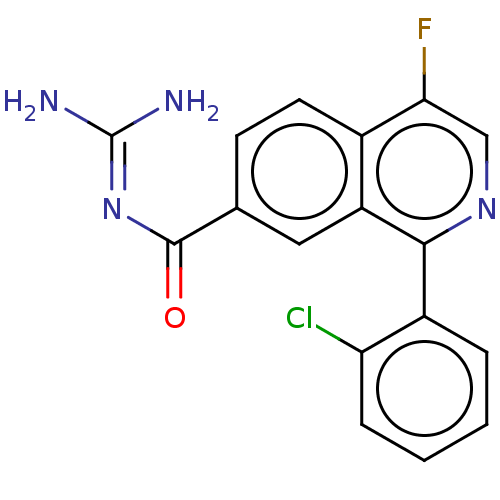 (US8853242, 161) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.40 | -48.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148153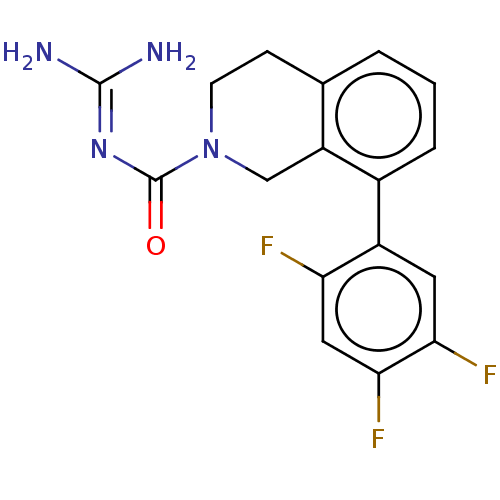 (US8962612, 8) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.60 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148168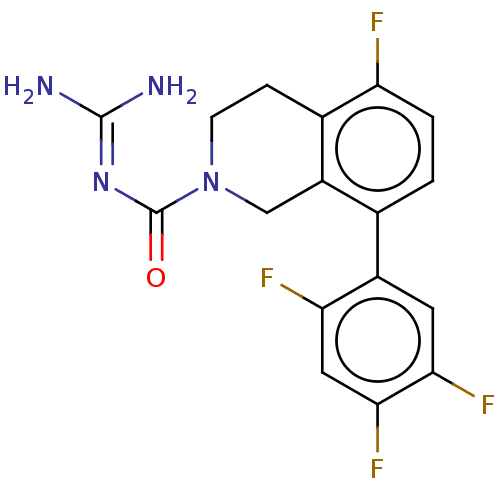 (US8962612, 79) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.70 | -50.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135781 (US8853242, 114) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.70 | -48.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148160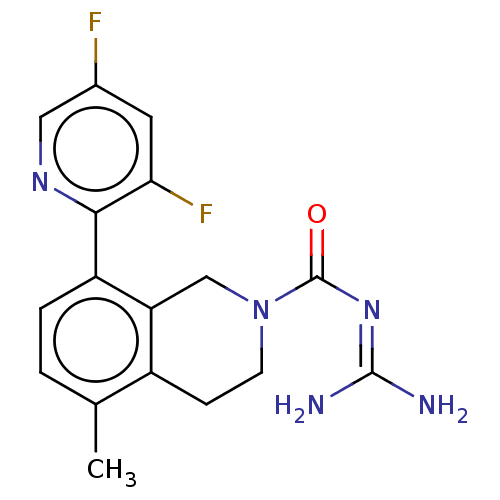 (US8962612, 60) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.90 | -49.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135784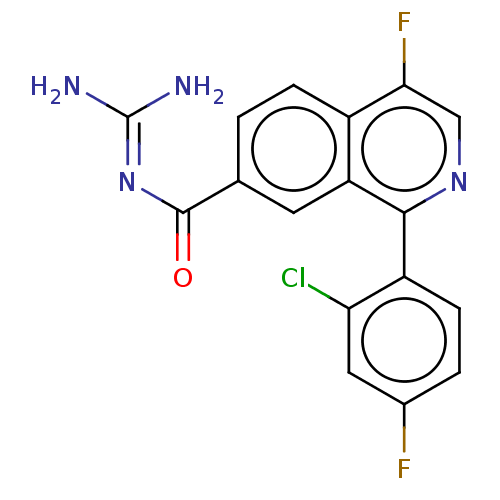 (US8853242, 151) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.10 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148170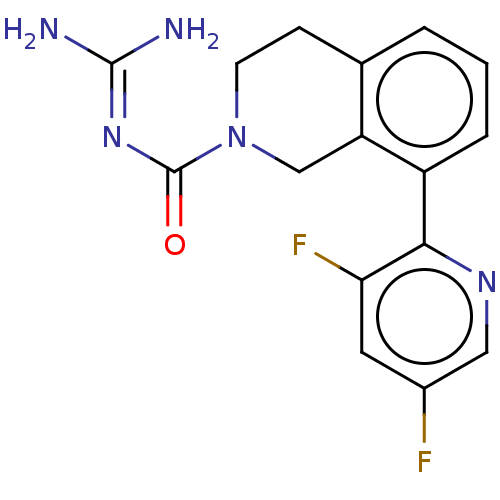 (US8962612, 81) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.20 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148152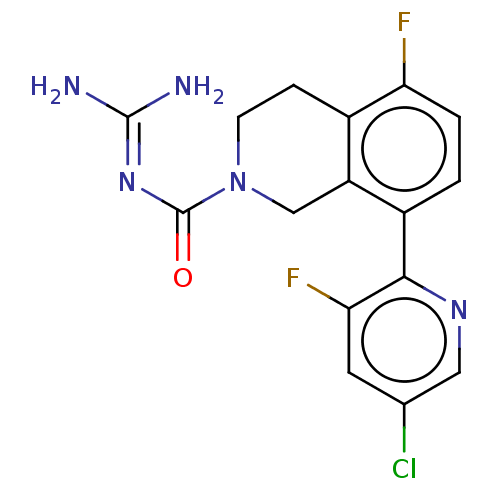 (US8962612, 4) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.30 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135794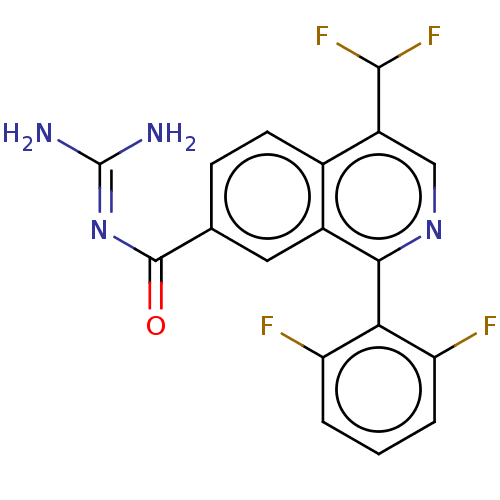 (US8853242, 187) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.60 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135791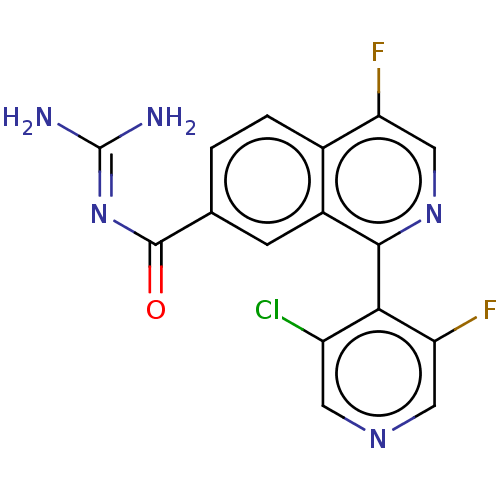 (US8853242, 164) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 4.70 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135786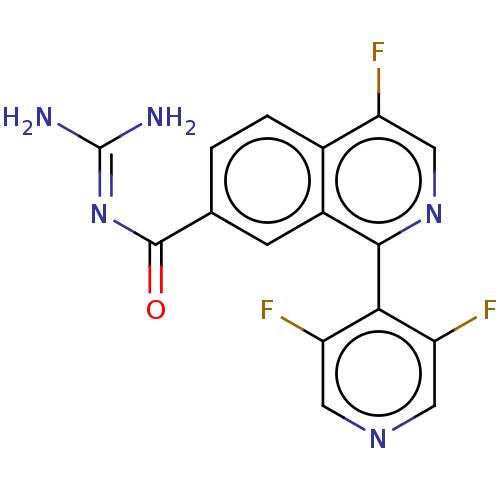 (US8853242, 157) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | 5.30 | -47.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135793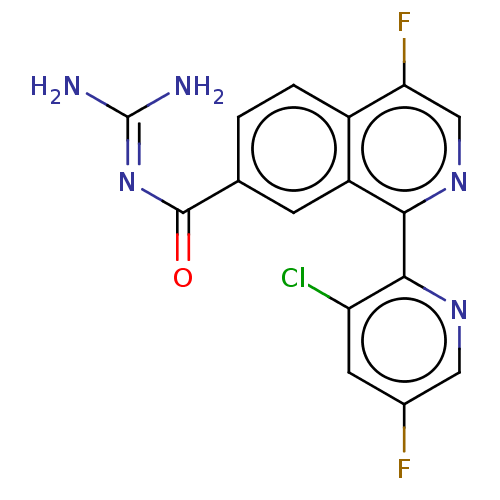 (US8853242, 171) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.20 | -46.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148159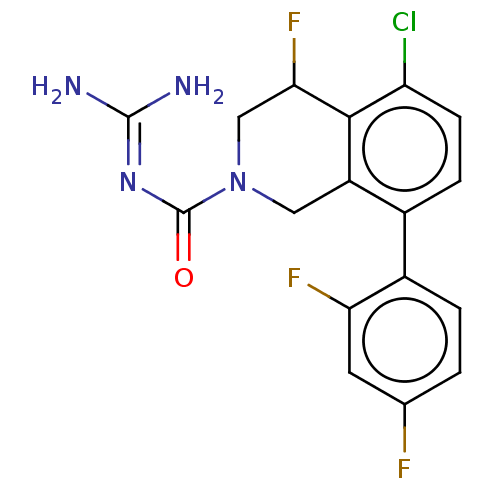 (US8962612, 49) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.60 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM148158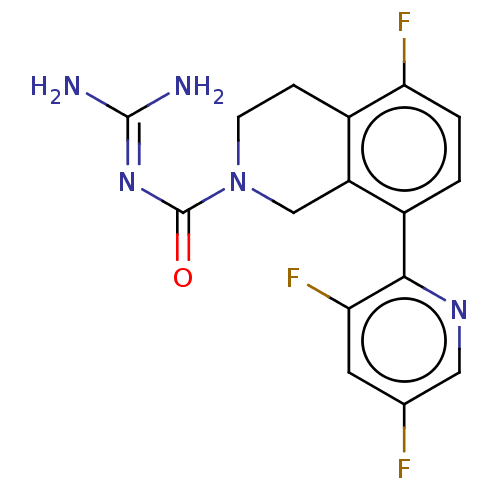 (US8962612, 46) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.90 | -48.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Astellas Pharma Inc. US Patent | Assay Description A test compound and 150 uM of a DMSO solution of 5-carboxamide tryptamine (5-CT) were added to a 96-well plate at 2 ul/well and suspended in the incu... | US Patent US8962612 (2015) BindingDB Entry DOI: 10.7270/Q24Q7SQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135787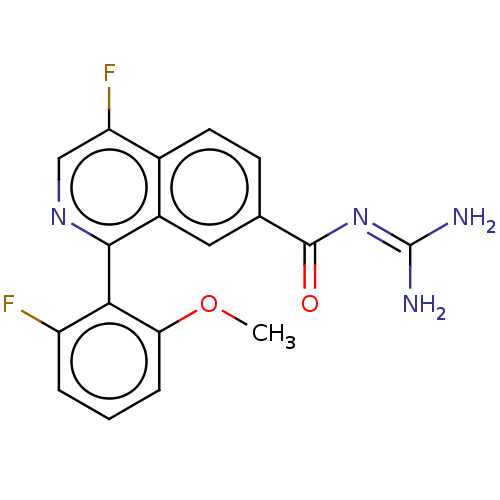 (US8853242, 159) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.10 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 5A (Homo sapiens (Human)) | BDBM135779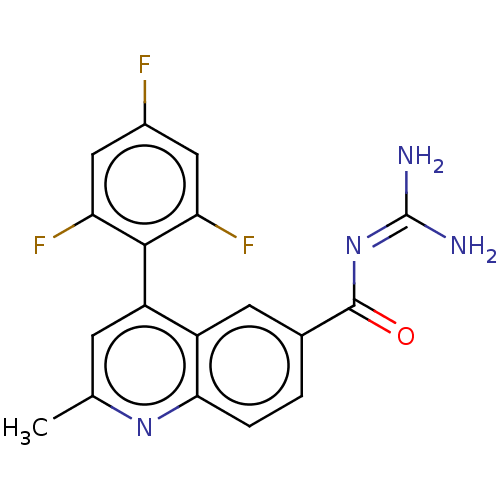 (US8853242, 6) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 13 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Astellas Pharma Inc. US Patent | Assay Description A solution of the compound to be tested and 100 μM 5-CT (5-carboxamidetriptamine) in DMSO was added to a 96-well plate at 2 μl/well, suspen... | US Patent US8853242 (2014) BindingDB Entry DOI: 10.7270/Q2NP2346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50520336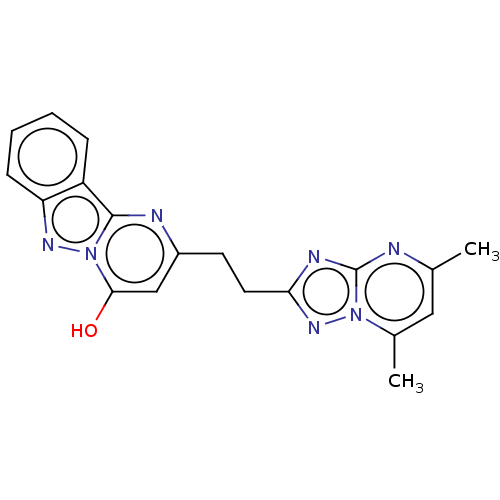 (CHEMBL4470042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE10A using cAMP as substrate preincubated for 30 mins followed by substrate addition and measured after 60 mins by HTRF detecti... | Bioorg Med Chem 27: 3692-3706 (2019) Article DOI: 10.1016/j.bmc.2019.07.010 BindingDB Entry DOI: 10.7270/Q2MK6H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50521882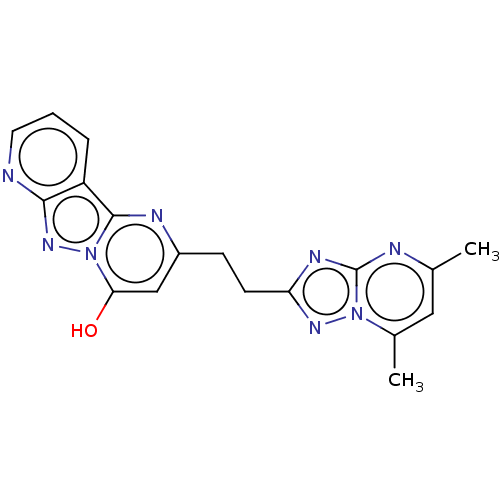 (CHEMBL4469155) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE10A using cAMP as substrate preincubated for 30 mins followed by substrate addition and measured after 60 mins by HTRF detecti... | Bioorg Med Chem 27: 3692-3706 (2019) Article DOI: 10.1016/j.bmc.2019.07.010 BindingDB Entry DOI: 10.7270/Q2MK6H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50521887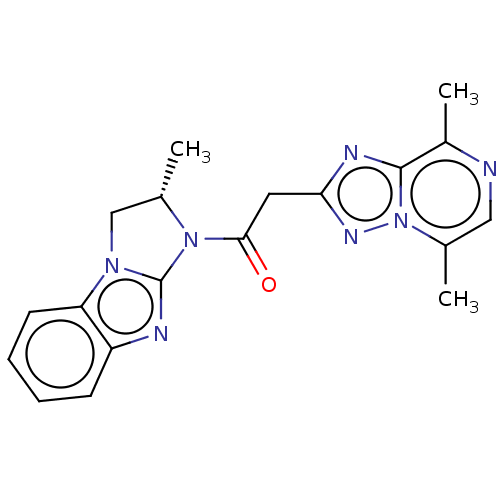 (CHEMBL4459939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE10A using cAMP as substrate preincubated for 30 mins followed by substrate addition and measured after 60 mins by HTRF detecti... | Bioorg Med Chem 27: 3692-3706 (2019) Article DOI: 10.1016/j.bmc.2019.07.010 BindingDB Entry DOI: 10.7270/Q2MK6H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50149871 ((R)-2-((3-(biphenyl-4-yloxy)-3-(4-fluorophenyl)pro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]glycine from GlyT1 in rat C6 glioma cells incubated for 30 mins prior to substrate addition measured after 10 mins by scintillati... | J Med Chem 56: 5744-56 (2014) Article DOI: 10.1021/jm400383w BindingDB Entry DOI: 10.7270/Q2D79CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50243945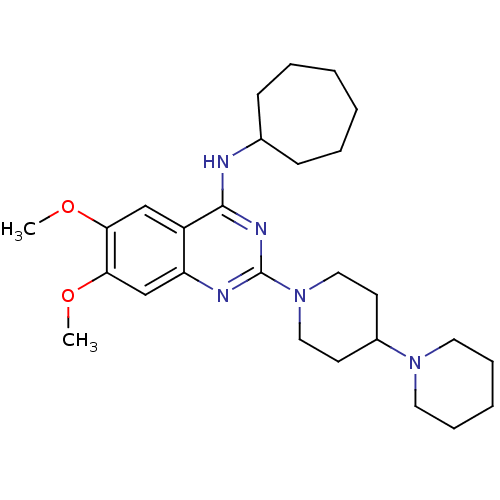 (2-(1,4'-Bipiperidine-1'-yl)-N-cycloheptyl-6,7-dime...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265669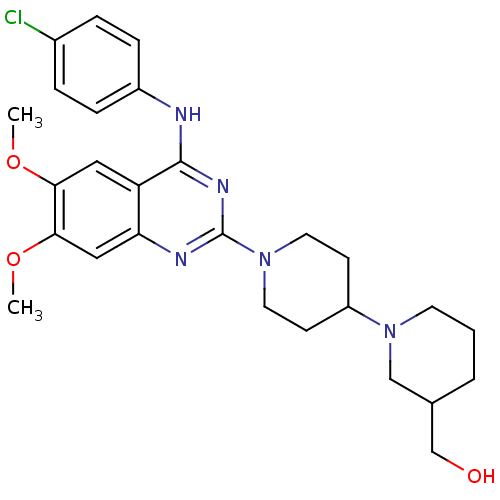 ((1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinaz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50244380 (CHEMBL487635 | N-Cycloheptyl-6,7-dimethoxy-2-(4-py...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced [35S]GTPgammaS binding | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Mus musculus) | BDBM50265742 (CHEMBL521737 | {1'-[4-(Cycloheptylamino)-6,7-dimet...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at mouse CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium- and chloride-dependent glycine transporter 1 (attus norvegicus (Rat)) | BDBM50102692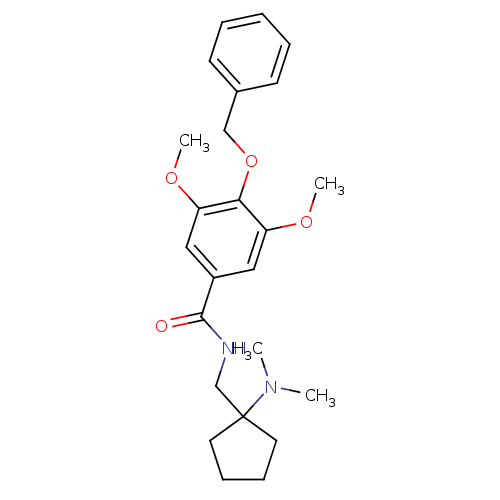 (4-Benzyloxy-N-(1-dimethylamino-cyclopentylmethyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Displacement of [3H]glycine from GlyT1 in rat C6 glioma cells incubated for 30 mins prior to substrate addition measured after 10 mins by scintillati... | J Med Chem 56: 5744-56 (2014) Article DOI: 10.1021/jm400383w BindingDB Entry DOI: 10.7270/Q2D79CVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50265669 ((1'-{4-[(4-Chlorophenyl)amino]-6,7-dimethoxyquinaz...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50245036 (CHEMBL486840 | N-(4-Chlorophenyl)-6,7-dimethoxy-2-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Antagonist activity at human CCR4 receptor expressed in mouse B300-19 cells assessed as CCL22-induced chemotaxis | Bioorg Med Chem 17: 64-73 (2008) Article DOI: 10.1016/j.bmc.2008.11.020 BindingDB Entry DOI: 10.7270/Q21836BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50521879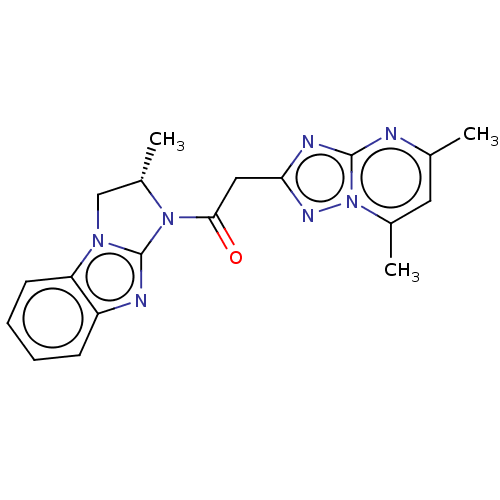 (CHEMBL4559232) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Astellas Pharma Inc. Curated by ChEMBL | Assay Description Inhibition of human PDE10A using cAMP as substrate preincubated for 30 mins followed by substrate addition and measured after 60 mins by HTRF detecti... | Bioorg Med Chem 27: 3692-3706 (2019) Article DOI: 10.1016/j.bmc.2019.07.010 BindingDB Entry DOI: 10.7270/Q2MK6H9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 191 total ) | Next | Last >> |