

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304417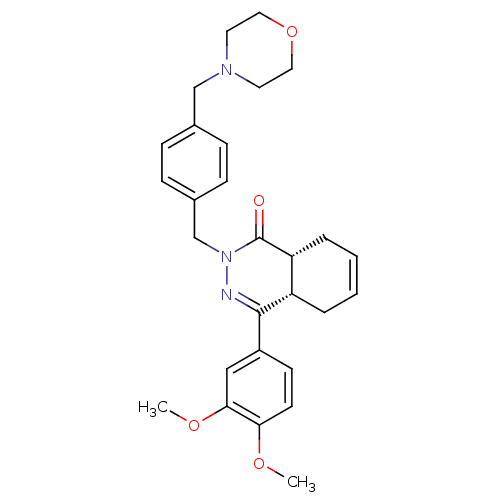 (CHEMBL593656 | CHEMBL593877 | cis-(+/-)-4-(3,4-Dim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304410 (CHEMBL594108 | cis-2-[(E)-4-(1H-Imidazol-1-yl)but-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304411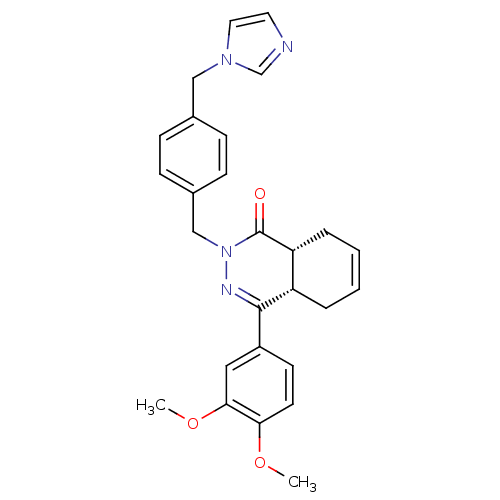 (CHEMBL594109 | cis-2-{4-[(1H-Imidazol-1-yl)methyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304417 (CHEMBL593656 | CHEMBL593877 | cis-(+/-)-4-(3,4-Dim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304417 (CHEMBL593656 | CHEMBL593877 | cis-(+/-)-4-(3,4-Dim...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304412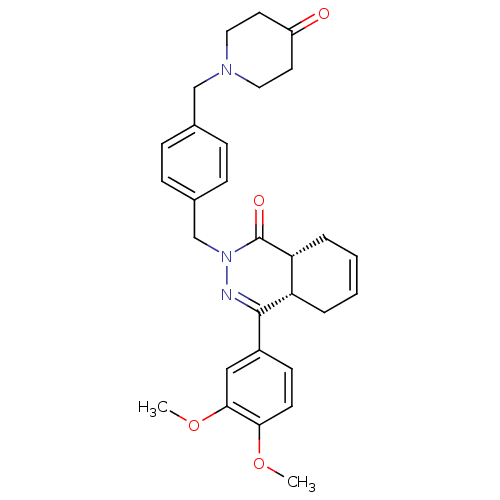 (CHEMBL595064 | cis-4-(3,4-Dimethoxyphenyl)-2-{4-[(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506948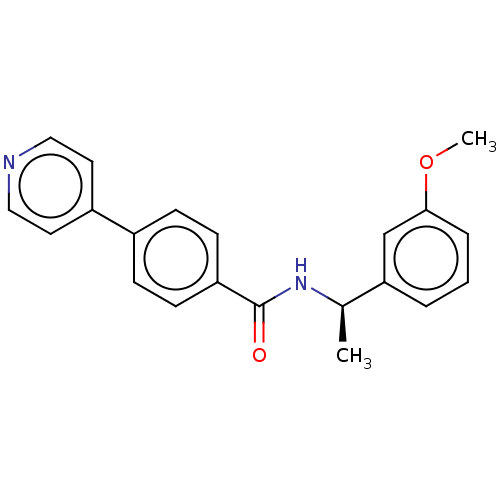 (CHEMBL4448806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of ROCK2 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50506948 (CHEMBL4448806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of PKG1A (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506948 (CHEMBL4448806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of ROCK1 (unknown origin) incubated for 2 hrs by AbbVie kinase panel assay | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506950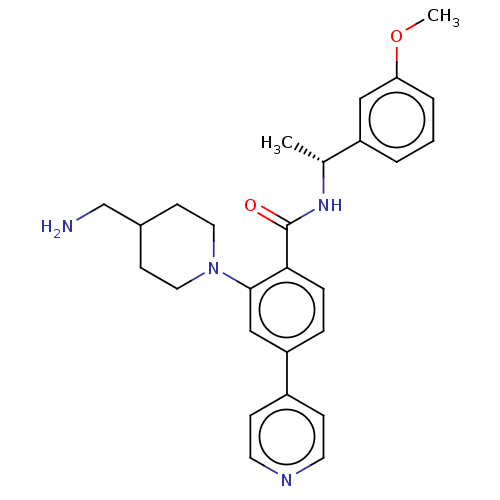 (CHEMBL4434688) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304423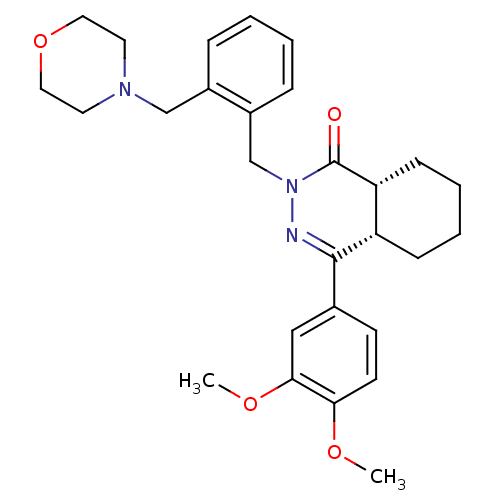 (CHEMBL595272 | cis-4-(3,4-Dimethoxyphenyl)-2-[4-(m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506946 (CHEMBL4472858) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304413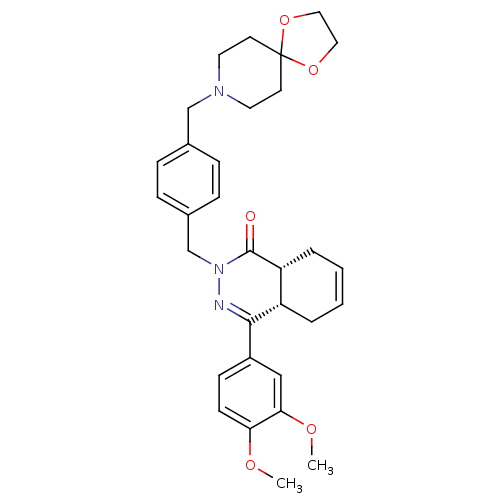 (CHEMBL596447 | cis-2-[4-(1,4-Dioxa-8-azaspiro[4.5]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304419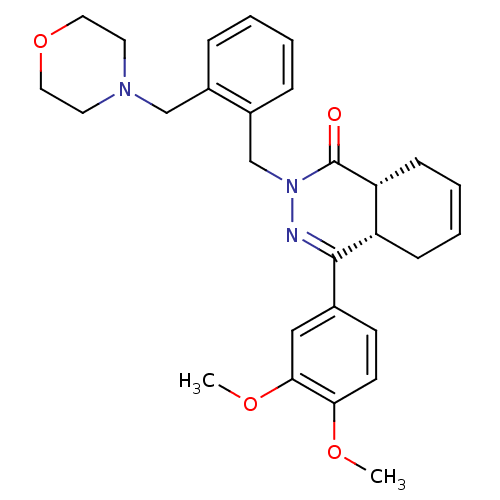 (CHEMBL592931 | cis-4-(3,4-Dimethoxyphenyl)-2-[2-(m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304418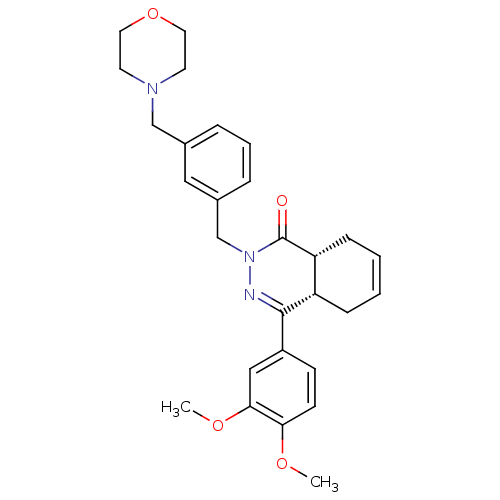 (CHEMBL596394 | cis-4-(3,4-Dimethoxyphenyl)-2-[3-(m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26732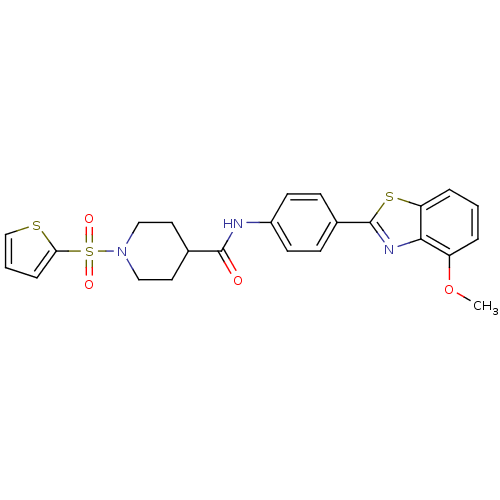 (Benzothiazole-based analogue, 1 | N-[4-(4-methoxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506948 (CHEMBL4448806) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304415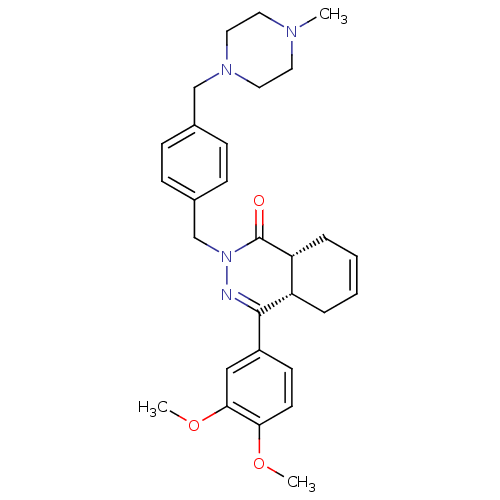 (CHEMBL595038 | cis-4-(3,4-Dimethoxyphenyl)-2-{4-[(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506933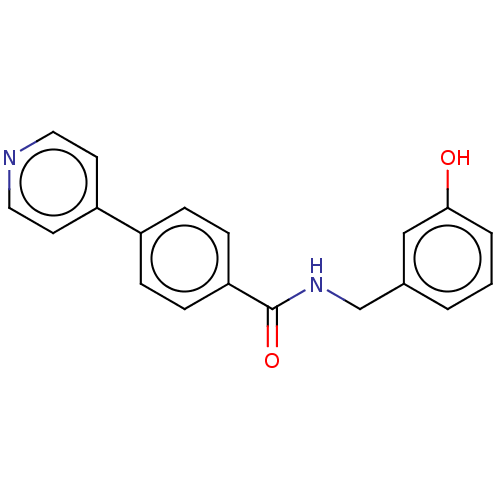 (CHEMBL4536833) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506950 (CHEMBL4434688) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506954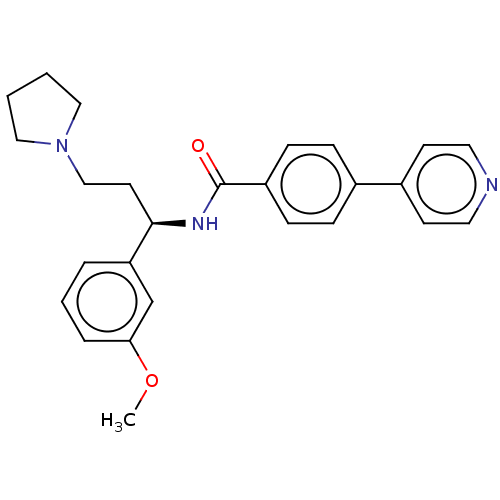 (CHEMBL4583341) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304422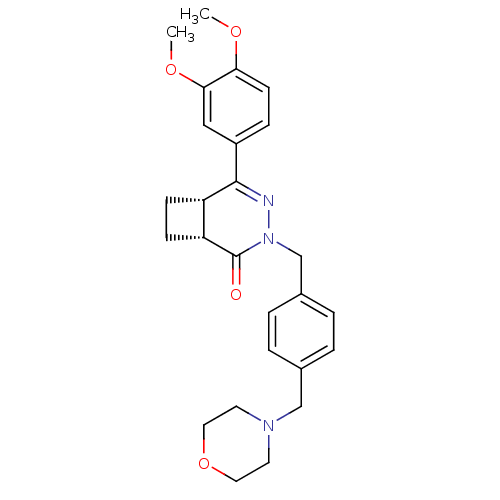 (CHEMBL605096 | cis-5-(3,4-Dimethoxyphenyl)-3-[4-(m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304409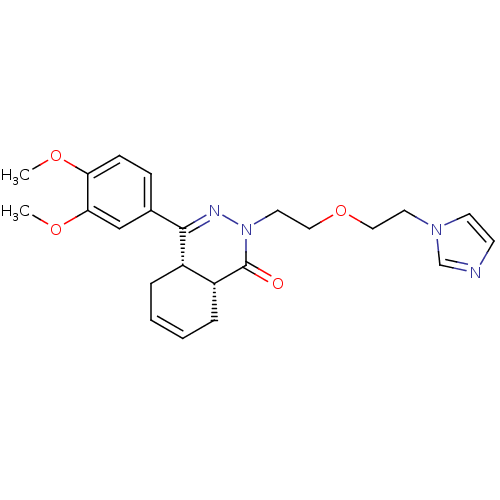 (CHEMBL595959 | cis-2-{2-[2-(1H-Imidazol-1-yl)ethox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506925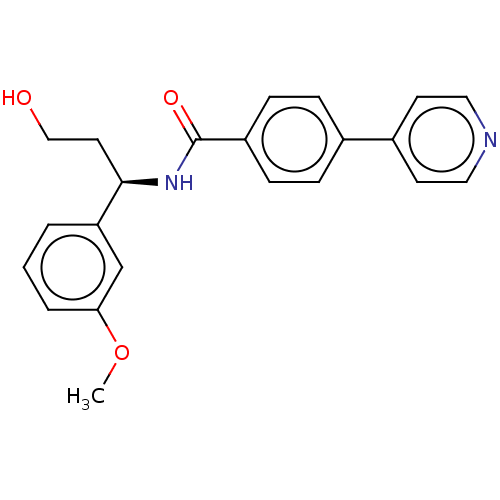 (CHEMBL4469316) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506939 (CHEMBL4459077) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304414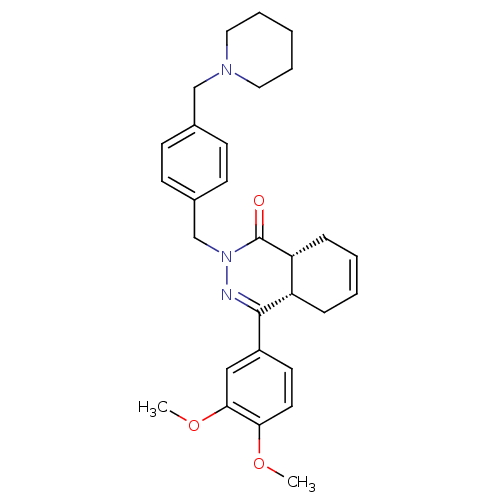 (CHEMBL603626 | cis-4-(3,4-Dimethoxyphenyl)-2-[4-(p...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304416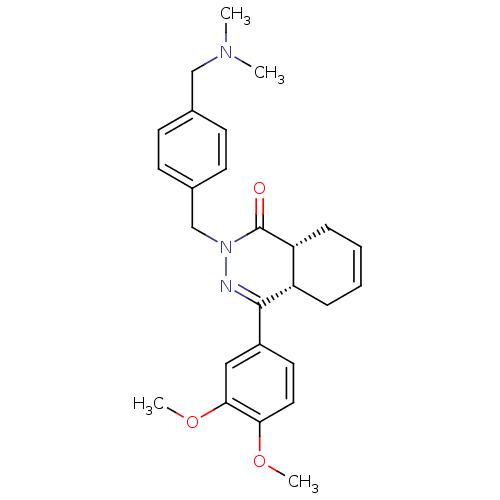 (CHEMBL595039 | cis-4-(3,4-Dimethoxyphenyl)-2-{4-[(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506946 (CHEMBL4472858) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506929 (CHEMBL4553926) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1/2 (Homo sapiens (Human)) | BDBM50506948 (CHEMBL4448806) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of ROCK1/2 in human PANC1 cells assessed as reduction in MYPT1 phosphorylation after 1 hr by ELISA | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506945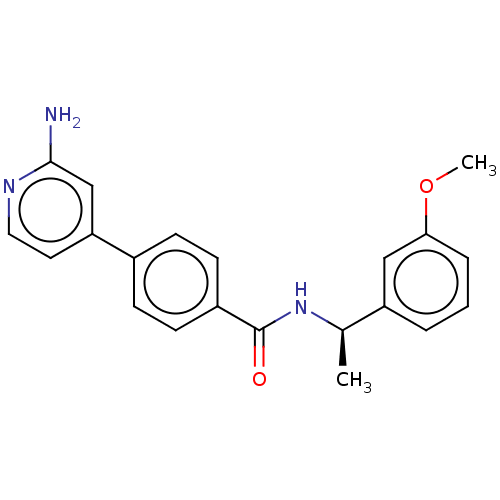 (CHEMBL4474946) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26712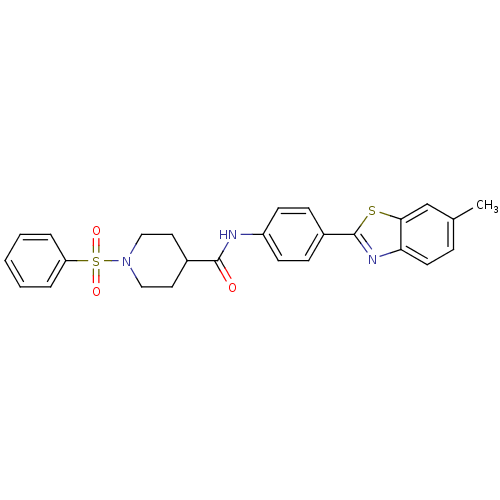 (1-(benzenesulfonyl)-N-[4-(6-methyl-1,3-benzothiazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506954 (CHEMBL4583341) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26729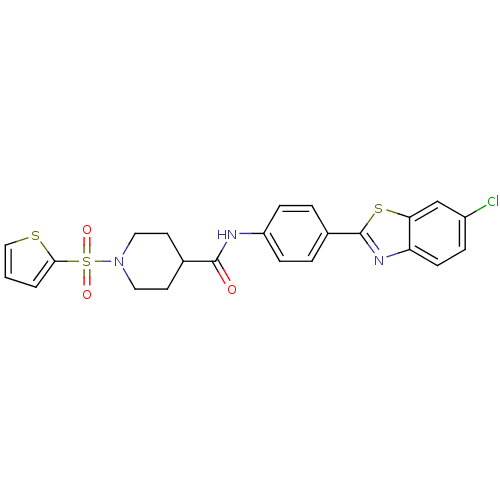 (N-[4-(6-chloro-1,3-benzothiazol-2-yl)phenyl]-1-(th...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506921 (CHEMBL4459800) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26731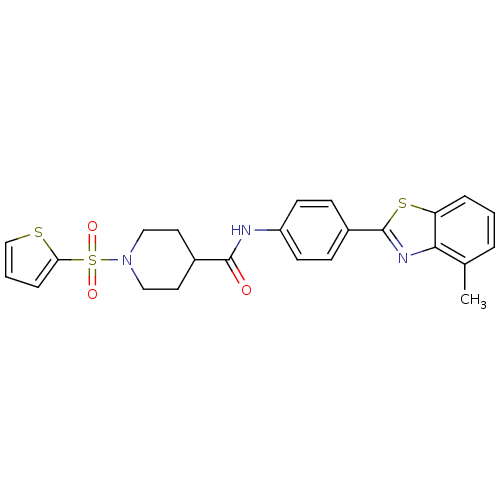 (N-[4-(4-methyl-1,3-benzothiazol-2-yl)phenyl]-1-(th...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506931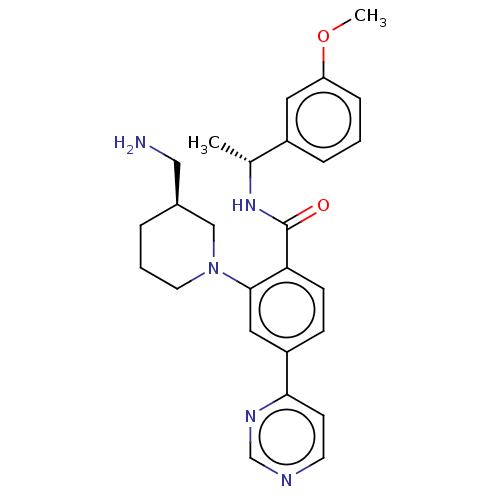 (CHEMBL4465725) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304408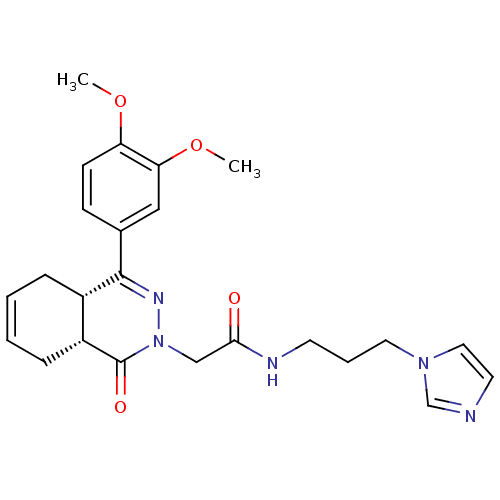 (CHEMBL605935 | N-[3-(1H-Imidazol-1-yl)propyl]-2-[c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506928 (CHEMBL4469434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26728 (N-[4-(1,3-benzothiazol-2-yl)phenyl]-1-(thiophene-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26722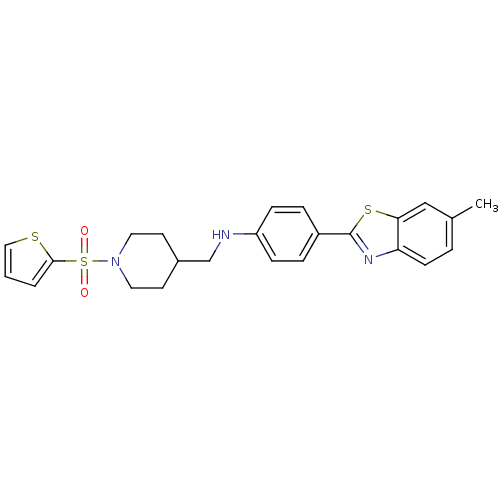 (4-(6-methyl-1,3-benzothiazol-2-yl)-N-{[1-(thiophen...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4D (Homo sapiens (Human)) | BDBM50304421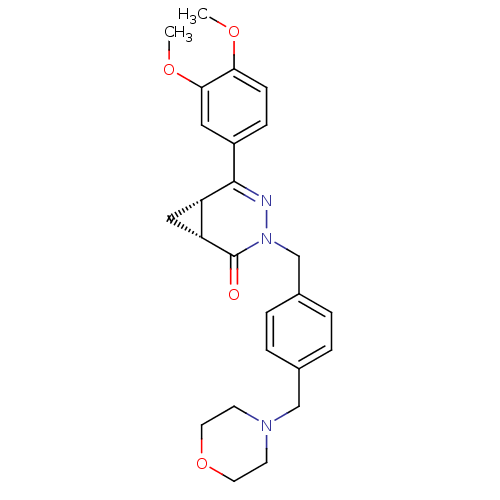 (CHEMBL603194 | cis-5-(3,4-Dimethoxyphenyl)-3-[4-(m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto 601-8550 Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE4D catalytic domain cloned from human HL60 cells assessed as inhibition of cAMP hydrolysis | Bioorg Med Chem 17: 6959-70 (2009) Article DOI: 10.1016/j.bmc.2009.08.014 BindingDB Entry DOI: 10.7270/Q2WH2Q26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506948 (CHEMBL4448806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26708 (N-[4-(6-methyl-1,3-benzothiazol-2-yl)phenyl]-1-(th...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26733 (N-{4-[4-(3-methoxyphenyl)-1,3-thiazol-2-yl]phenyl}...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506951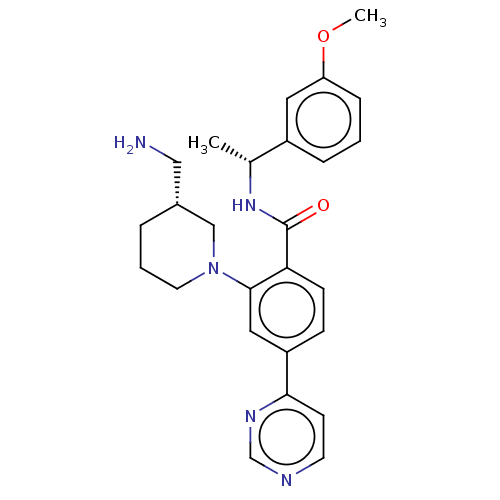 (CHEMBL4557041) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1 (Homo sapiens (Human)) | BDBM50506933 (CHEMBL4536833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of human GST-tagged catalytic ROCK1 expressed in baculovirus system using STK S2 peptide substrate and and 33P-ATP after 60 mins by HTRF a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506956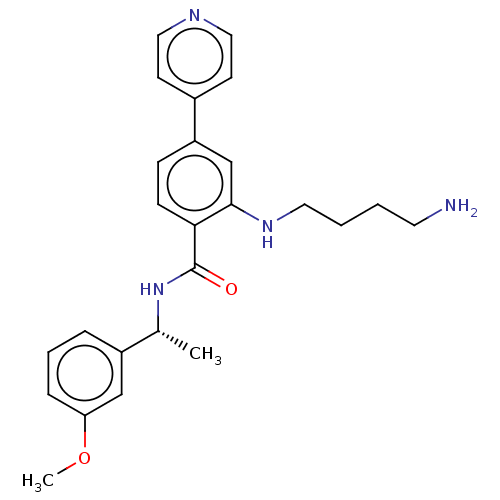 (CHEMBL4555093) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 [30-579] (Rattus norvegicus (rat)) | BDBM26724 (N-[4-(quinolin-2-yl)phenyl]-1-(thiophene-2-sulfony...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 8.0 | 22 |
Abbott Laboratories | Assay Description [3H]anandamide was incubated with membranes to produce radiolabeled ethanolamine and unlabeled arachidonic acid. Charcoal selectively binds anandamid... | J Med Chem 52: 170-80 (2009) Article DOI: 10.1021/jm801042a BindingDB Entry DOI: 10.7270/Q24B2ZMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM50506941 (CHEMBL4572198) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center Curated by ChEMBL | Assay Description Inhibition of GST-fused human recombinant ROCK2 (11 to 552 residues) expressed in Spodoptera frugiperda insect cells using STK S2 peptide substrate a... | J Med Chem 61: 11074-11100 (2018) Article DOI: 10.1021/acs.jmedchem.8b01098 BindingDB Entry DOI: 10.7270/Q2RB77XJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 247 total ) | Next | Last >> |