

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840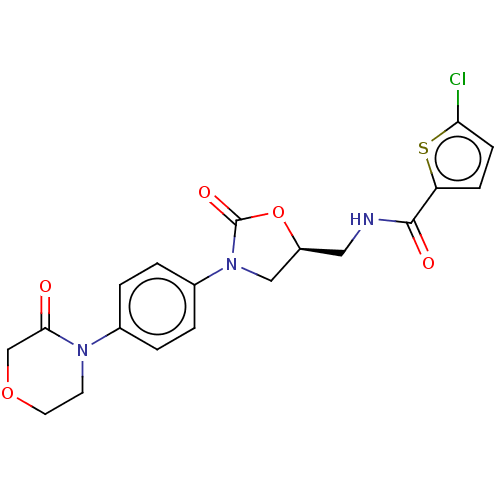 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443853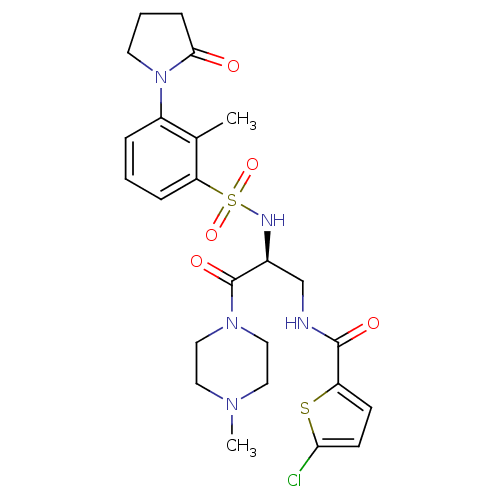 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50112086 (3-({2-[(4-Carbamimidoyl-phenylamino)-methyl]-1-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of t-PA (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443855 (CHEMBL3091517) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of plasmin (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443857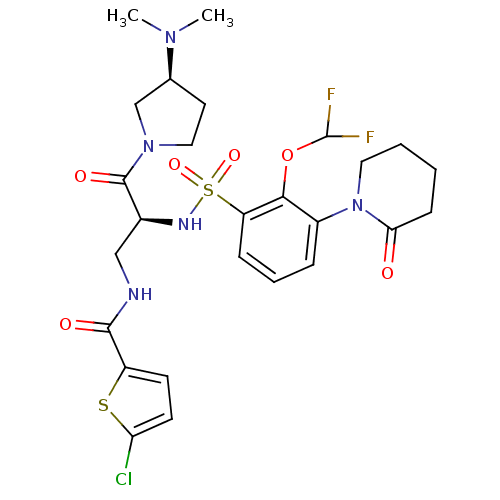 (CHEMBL3091501) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443846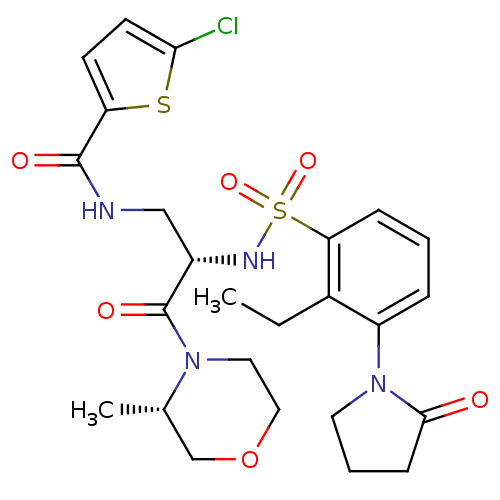 (CHEMBL3091527) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443858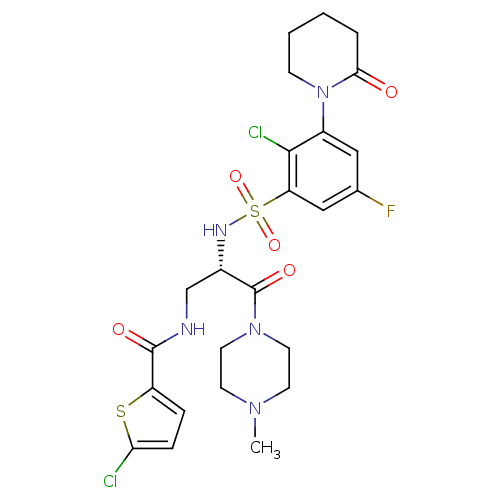 (CHEMBL3091502) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443847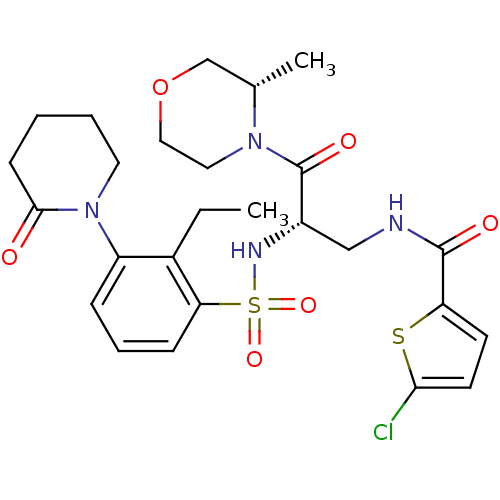 (CHEMBL3091526) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100263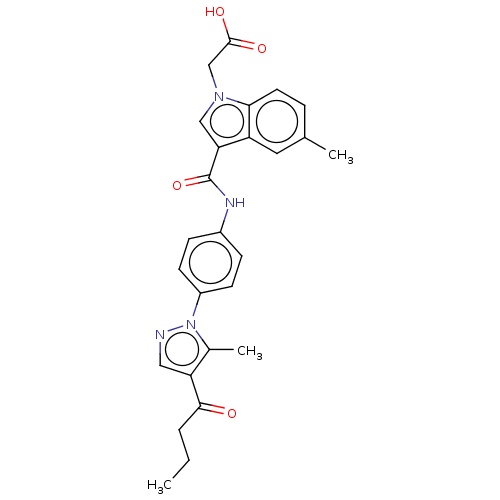 (CHEMBL3326907) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443853 (CHEMBL3091519 | US20230391761, Reference 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443861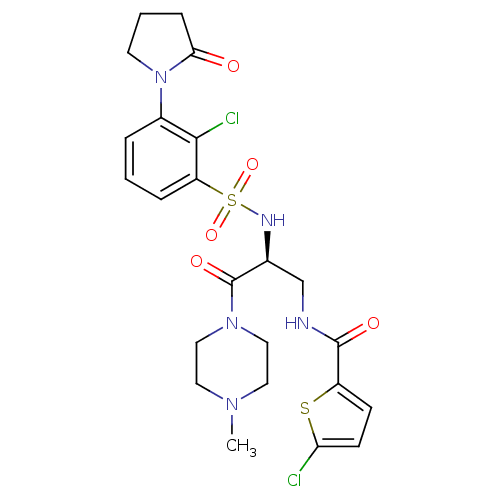 (CHEMBL3091515) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443844 (CHEMBL3091506) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443869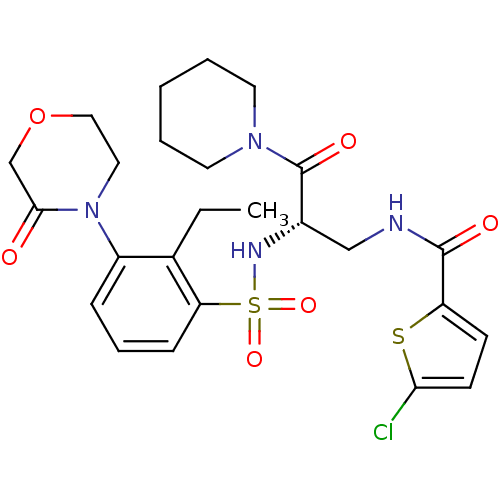 (CHEMBL3091507) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443848 (CHEMBL3091525) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443862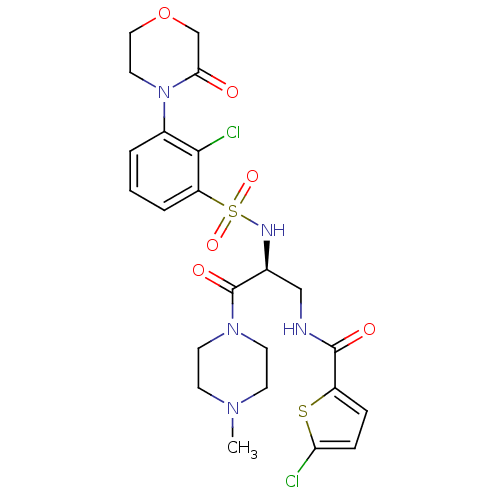 (CHEMBL3091514) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443867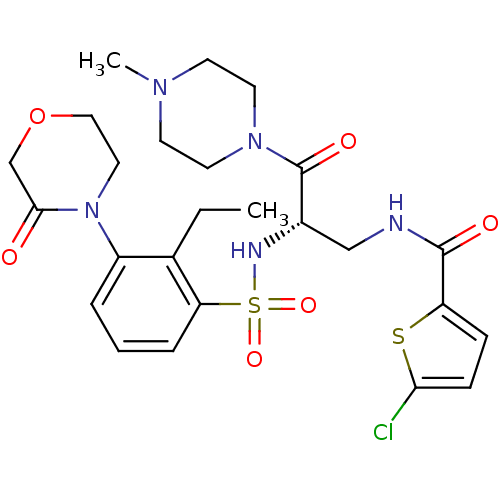 (CHEMBL3091509) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443865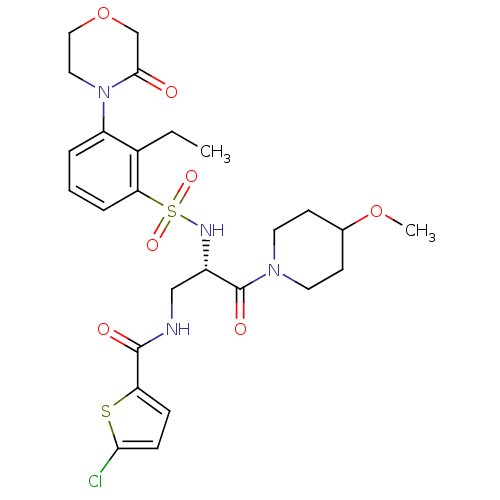 (CHEMBL3091511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM183069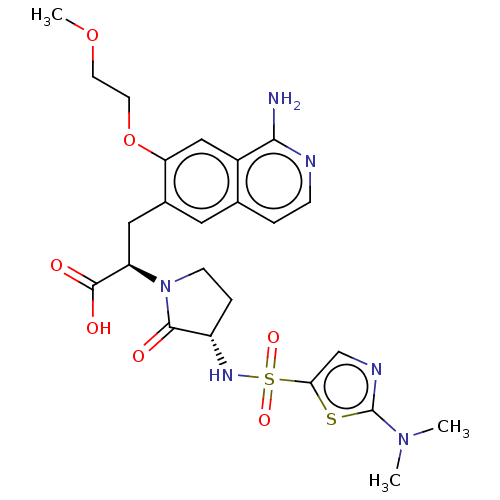 (US9150544, 103) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Sanofi US Patent | Assay Description The compounds according to the invention are tested in a range of concentrations (10pM to 10 ÁM final) in assay buffer (50 mM TRIS, 100 mM NaCl, 0.1 ... | US Patent US9150544 (2015) BindingDB Entry DOI: 10.7270/Q27S7MKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443864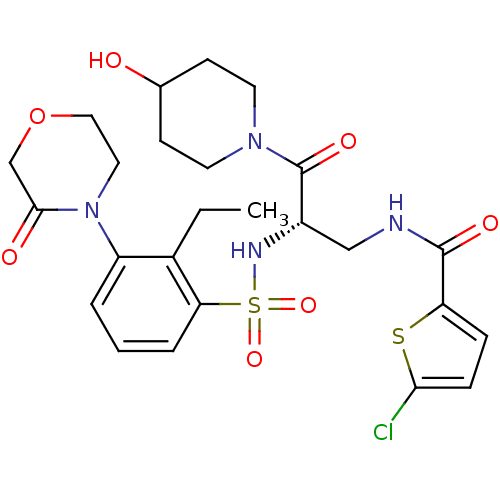 (CHEMBL3091512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188704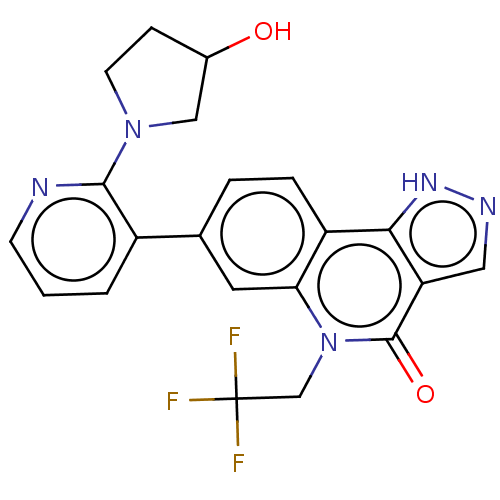 (US9169246, 246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.46 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443845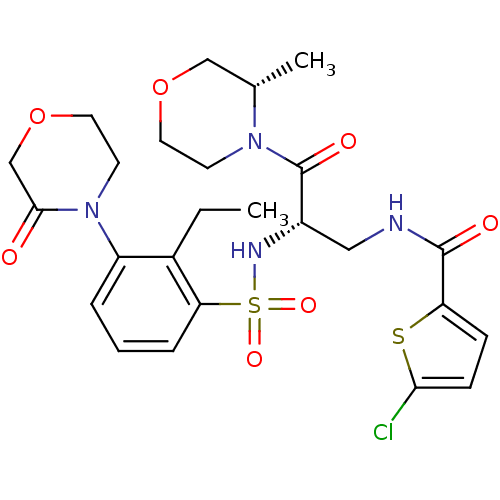 (CHEMBL3091528) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443856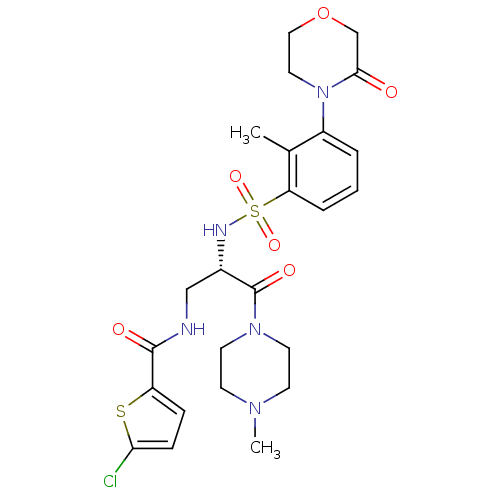 (CHEMBL3091503) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443866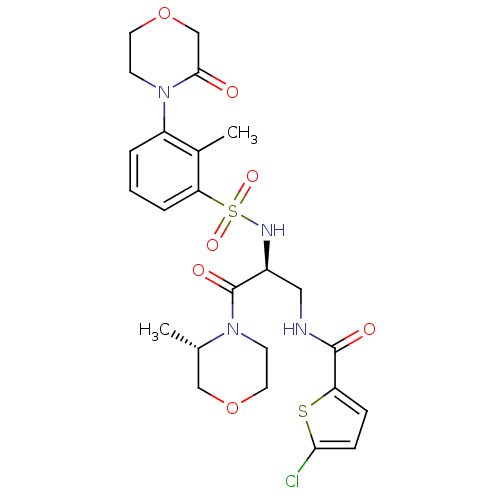 (CHEMBL3091510) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM182990 (US9150544, 24) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Sanofi US Patent | Assay Description The compounds according to the invention are tested in a range of concentrations (10pM to 10 ÁM final) in assay buffer (50 mM TRIS, 100 mM NaCl, 0.1 ... | US Patent US9150544 (2015) BindingDB Entry DOI: 10.7270/Q27S7MKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188545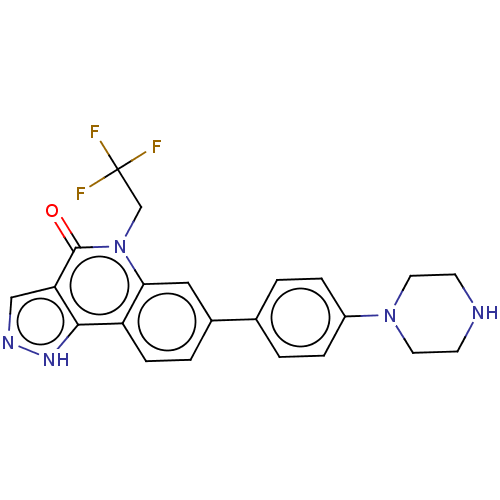 (US9169246, 30) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443860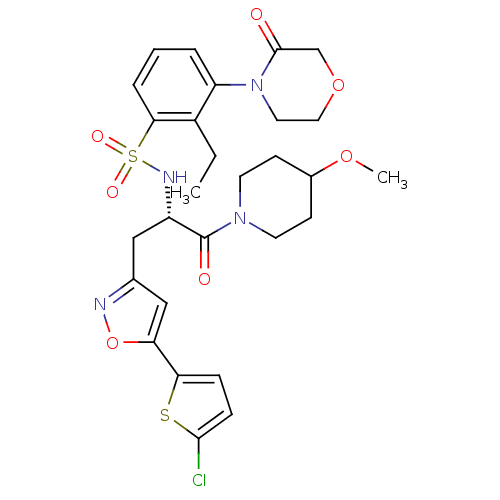 (CHEMBL3091505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50328705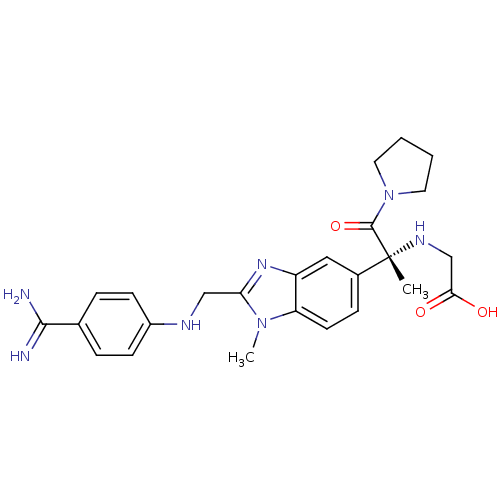 (CHEMBL1270156 | Tanogitran) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of factor-10a (unknown origin) | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443863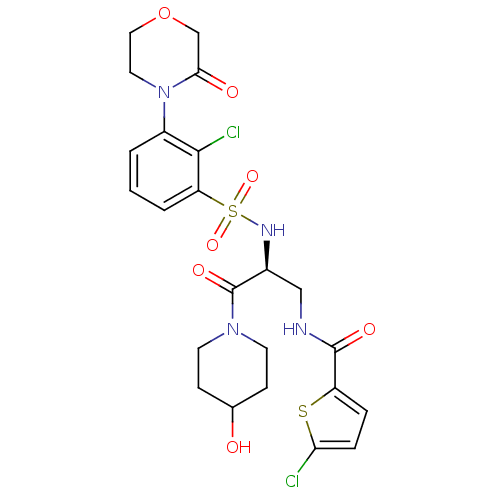 (CHEMBL3091513) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188567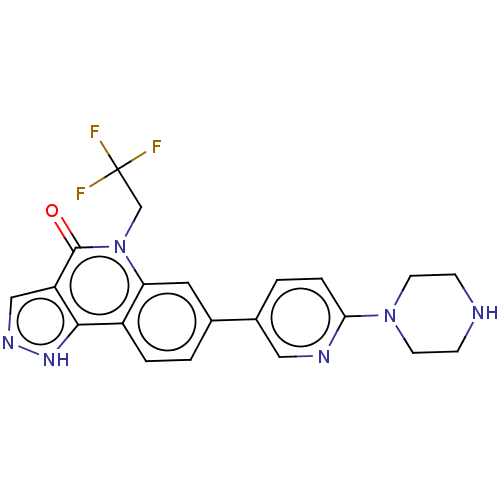 (US9169246, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.06 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443868 (CHEMBL3091508) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50443859 (CHEMBL3091504) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis R&D Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using S-2765 as substrate measured up to 20 mins by chromogenic assay | J Med Chem 56: 9441-56 (2014) Article DOI: 10.1021/jm4005835 BindingDB Entry DOI: 10.7270/Q20K2B0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188731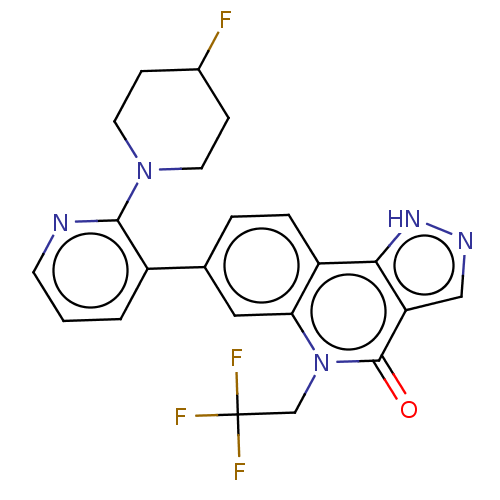 (US9169246, 273) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.45 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188706 (US9169246, 248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.87 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188541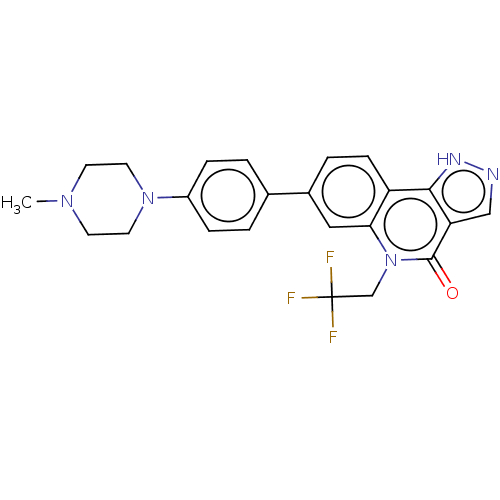 (US9169246, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.94 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM182980 (US9150544, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Sanofi US Patent | Assay Description The compounds according to the invention are tested in a range of concentrations (10pM to 10 ÁM final) in assay buffer (50 mM TRIS, 100 mM NaCl, 0.1 ... | US Patent US9150544 (2015) BindingDB Entry DOI: 10.7270/Q27S7MKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188569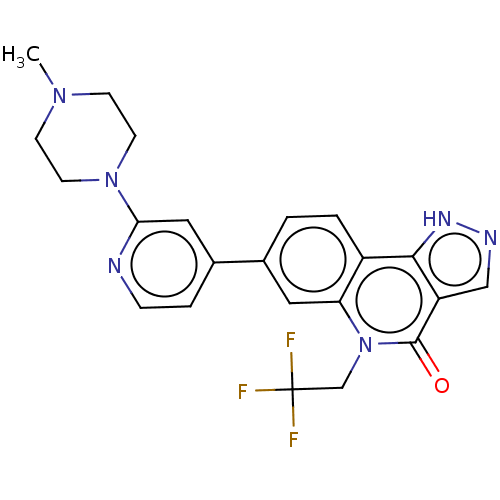 (US9169246, 68) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.12 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188707 (US9169246, 249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.19 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188621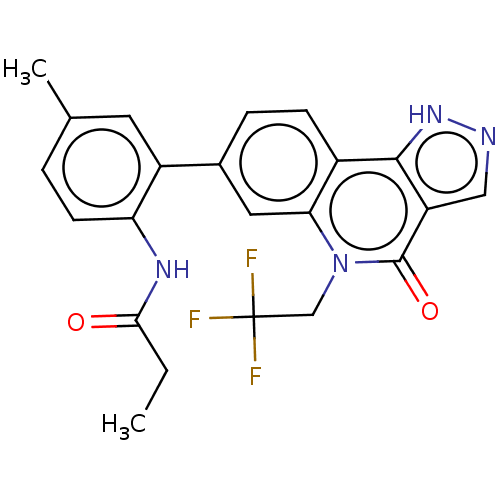 (US9169246, 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.29 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188708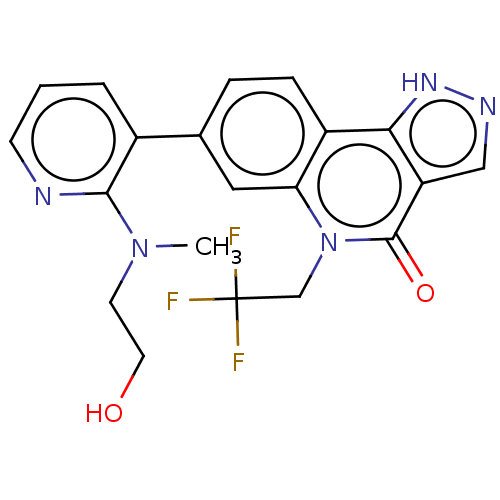 (US9169246, 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.41 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188686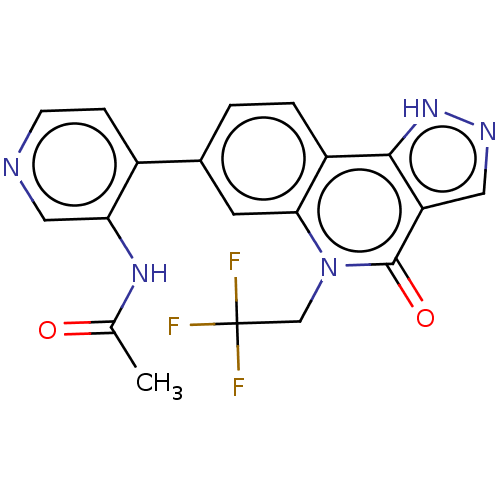 (US9169246, 226) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.68 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188659 (US9169246, 199) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188693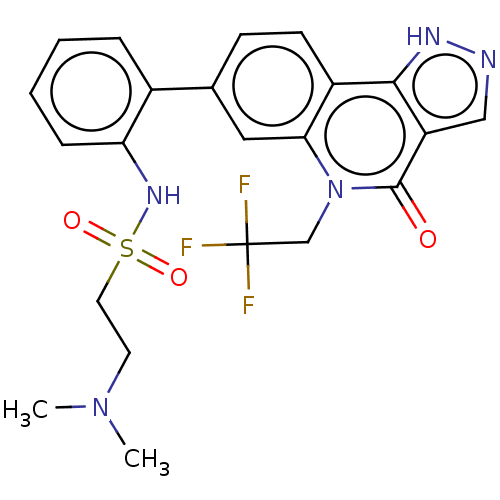 (US9169246, 235) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.99 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM183016 (US9150544, 50) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Sanofi US Patent | Assay Description The compounds according to the invention are tested in a range of concentrations (10pM to 10 ÁM final) in assay buffer (50 mM TRIS, 100 mM NaCl, 0.1 ... | US Patent US9150544 (2015) BindingDB Entry DOI: 10.7270/Q27S7MKF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50100269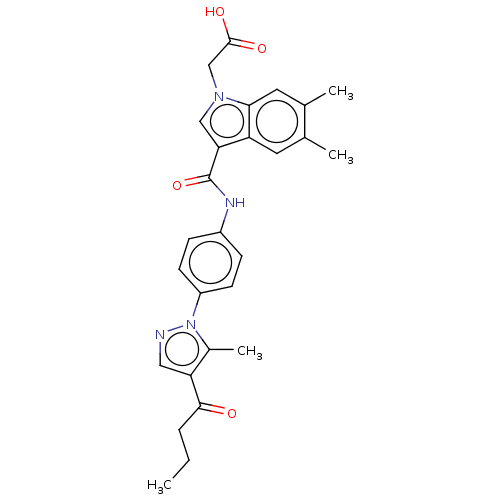 (CHEMBL3325627) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Displacement of [33P]2MeS-ADP from P2Y12 receptor (unknown origin) transfected in CHO cells after 30 mins by scintillation counting analysis | J Med Chem 57: 7293-316 (2014) Article DOI: 10.1021/jm500588w BindingDB Entry DOI: 10.7270/Q2D79D21 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188692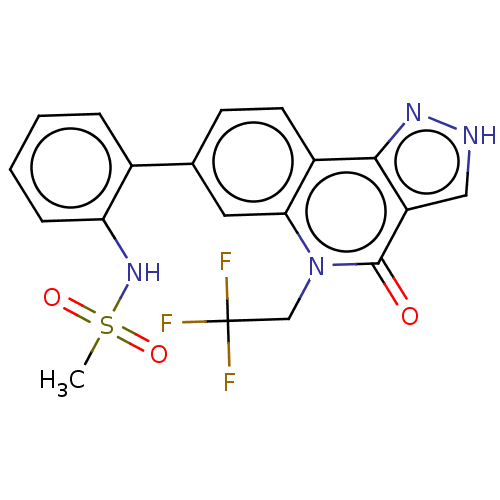 (US9169246, 234) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.33 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188709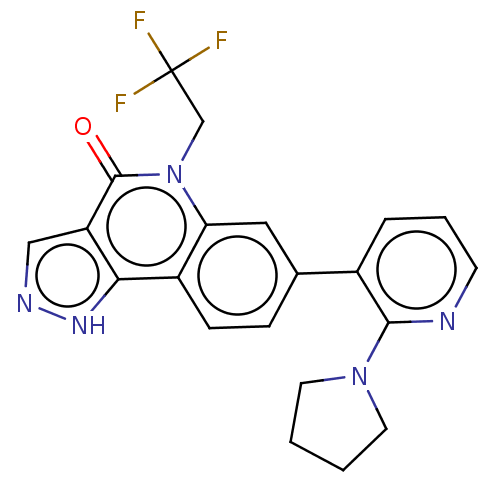 (US9169246, 251) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.34 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188571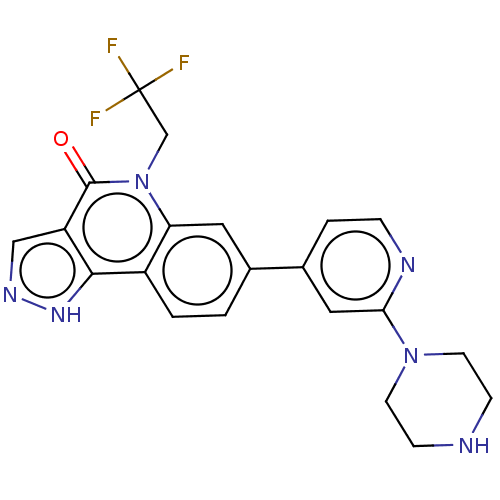 (US9169246, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.62 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Methionine aminopeptidase 2 (Homo sapiens (Human)) | BDBM188728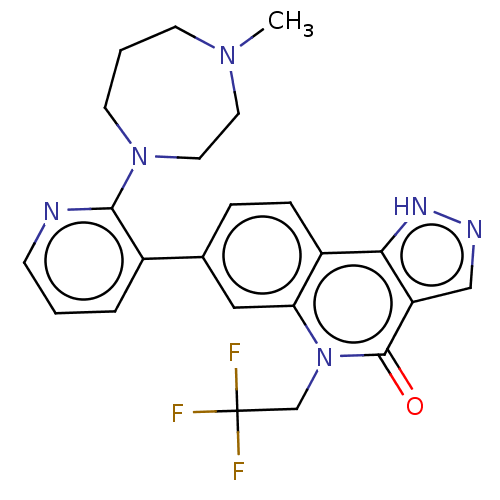 (US9169246, 270) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description The enzymatic test is a test in two steps.In a first step, it consists in placing in contact the compound according to the invention, the dialysed Me... | US Patent US9169246 (2015) BindingDB Entry DOI: 10.7270/Q2B856XZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 628 total ) | Next | Last >> |