

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.200 | n/a | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18561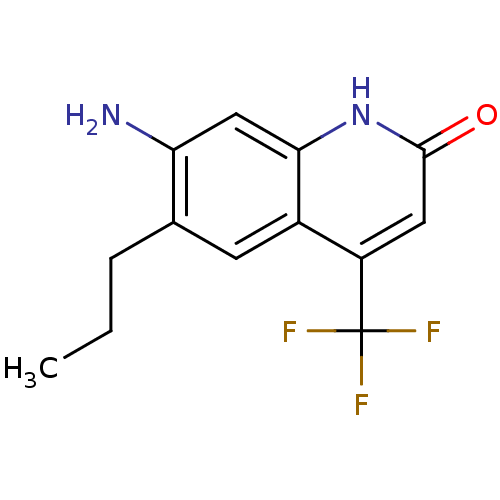 (6-alkyl, 7-amino-2-quinolinone, 11c | 7-amino-6-pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | 19 | n/a | 83 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18538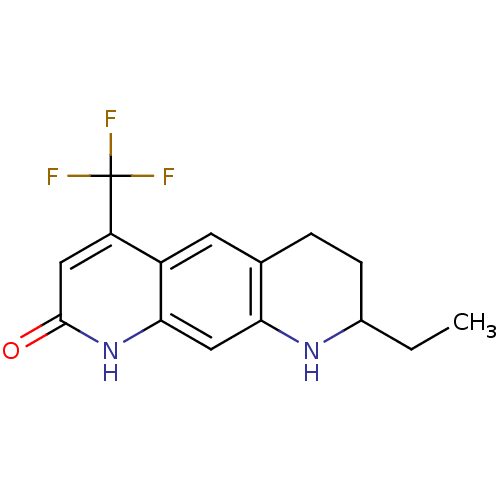 (8-ethyl-4-(trifluoromethyl)-1H,2H,6H,7H,8H,9H-pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | 14 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18562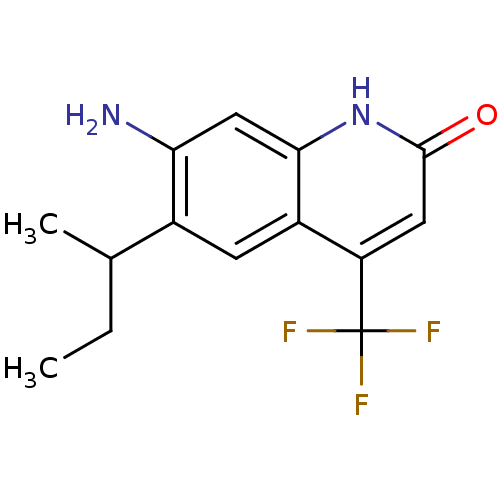 (6-alkyl, 7-amino-2-quinolinone, 11d (+/-) | 7-amin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | 14 | n/a | 65 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18552 (7-[(2,2,2-trifluoroethyl)amino]-4-(trifluoromethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -46.5 | 21 | n/a | 905 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18571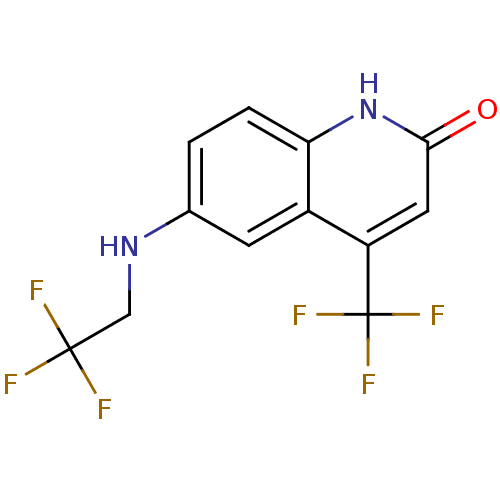 (6-[(2,2,2-trifluoroethyl)amino]-4-(trifluoromethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | 18 | n/a | 108 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18560 (6-alkyl, 7-amino-2-quinolinone, 11b | 7-amino-6-et...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | 23 | n/a | 593 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18542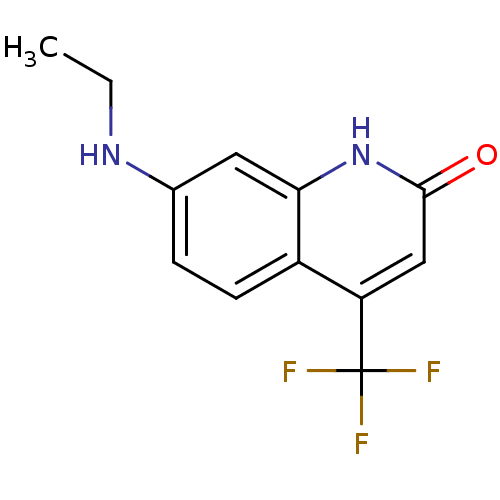 (7-(ethylamino)-4-(trifluoromethyl)-1,2-dihydroquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 29 | -44.8 | 35 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18557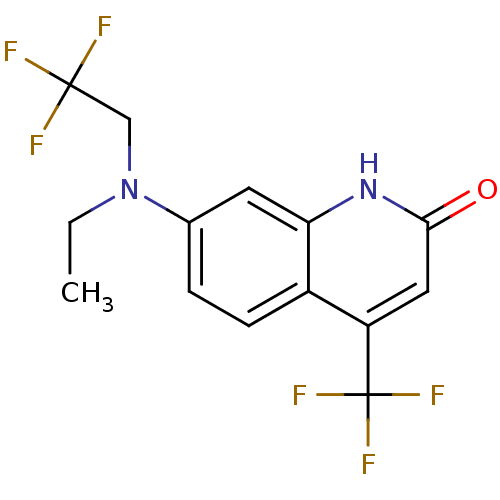 (7-[ethyl(2,2,2-trifluoroethyl)amino]-4-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -44.7 | 42 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18559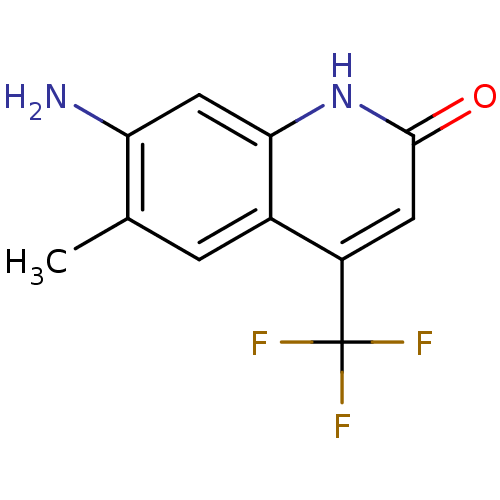 (6-alkyl, 7-amino-2-quinolinone, 11a | 7-amino-6-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18553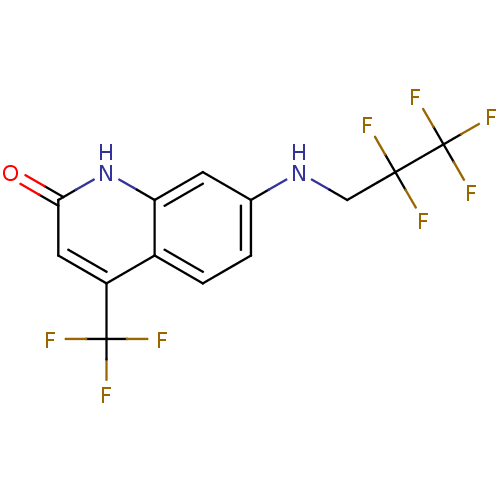 (7-[(2,2,3,3,3-pentafluoropropyl)amino]-4-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 36 | -44.2 | 58 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18539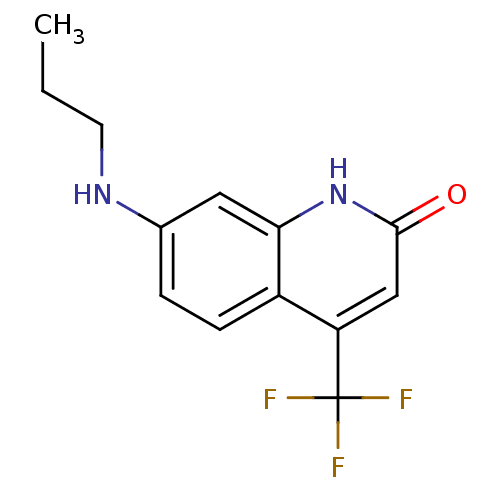 (7-(propylamino)-4-(trifluoromethyl)-1,2-dihydroqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 37 | -44.1 | 32 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18543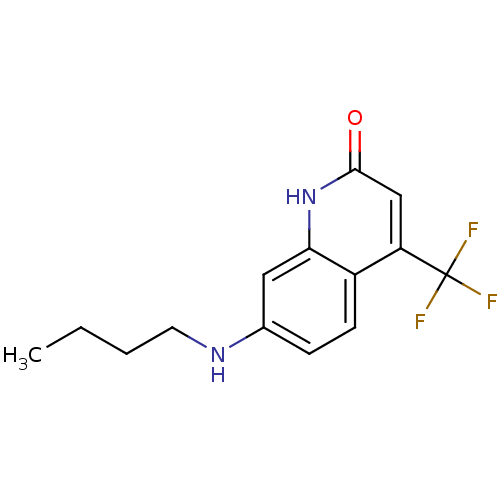 (7-(butylamino)-4-(trifluoromethyl)-1,2-dihydroquin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | -43.3 | 67 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18575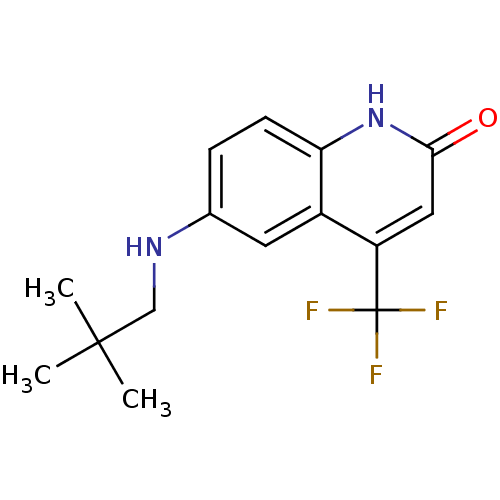 (6-[(2,2-dimethylpropyl)amino]-4-(trifluoromethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18574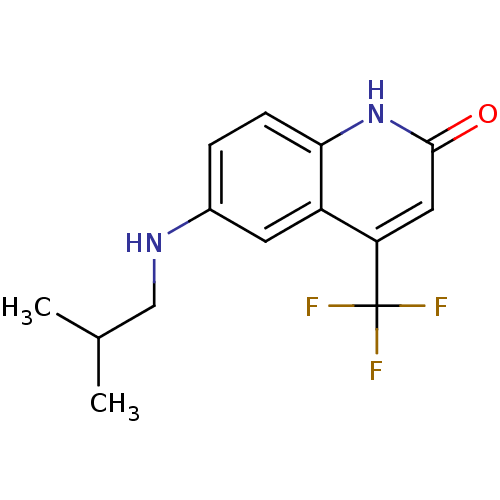 (6-[(2-methylpropyl)amino]-4-(trifluoromethyl)-1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | 22 | n/a | 404 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18545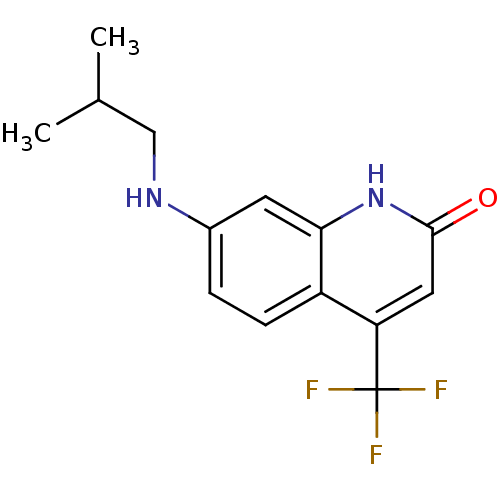 (7-[(2-methylpropyl)amino]-4-(trifluoromethyl)-1,2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 65 | -42.7 | 74 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18537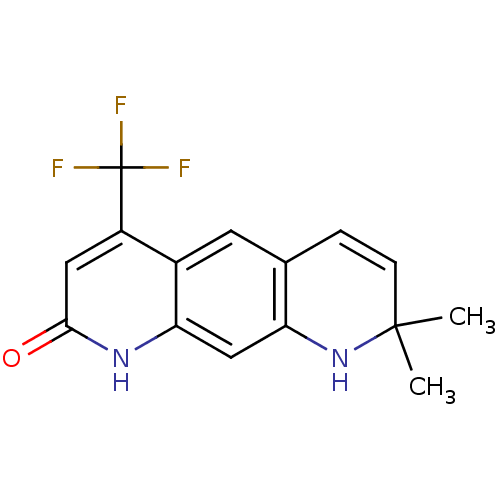 (8,8-dimethyl-4-(trifluoromethyl)-1H,2H,8H,9H-pyrid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 73 | -42.4 | 27 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18573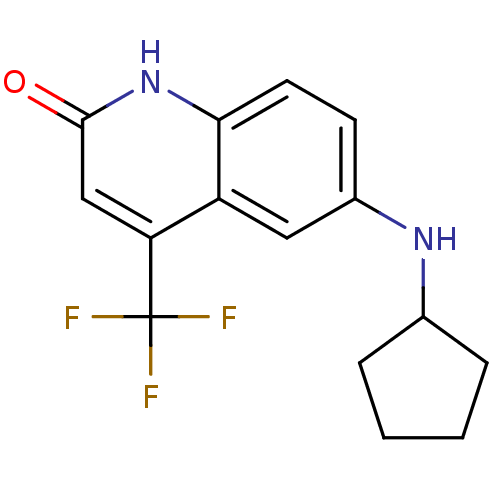 (6-(cyclopentylamino)-4-(trifluoromethyl)-1,2-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18572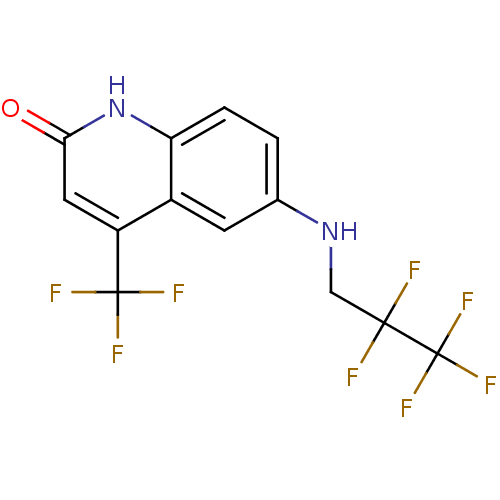 (6-[(2,2,3,3,3-pentafluoropropyl)amino]-4-(trifluor...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | 17 | n/a | 246 | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18544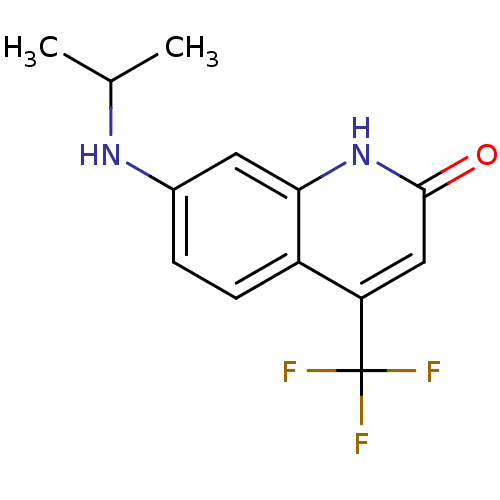 (7-(propan-2-ylamino)-4-(trifluoromethyl)-1,2-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 79 | -42.2 | 56 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18541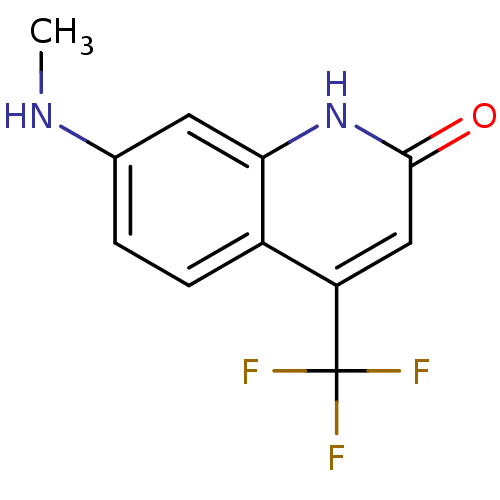 (7-(methylamino)-4-(trifluoromethyl)-1,2-dihydroqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 83 | -42.0 | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18547 (7-[(2-methylbutan-2-yl)amino]-4-(trifluoromethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 110 | -41.3 | 209 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18548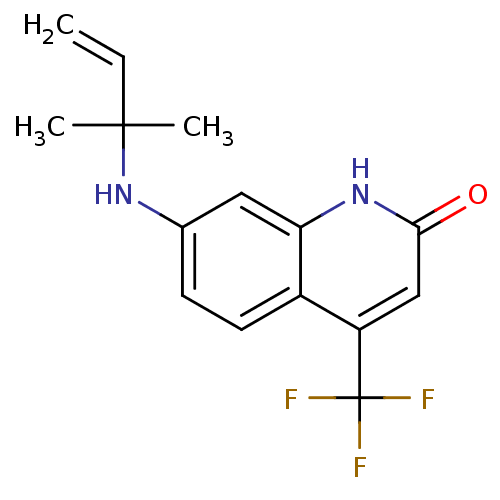 (7-[(2-methylbut-3-en-2-yl)amino]-4-(trifluoromethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 118 | -41.1 | 122 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18546 (7-[(2,2-dimethylpropyl)amino]-4-(trifluoromethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 134 | -40.8 | 98 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18525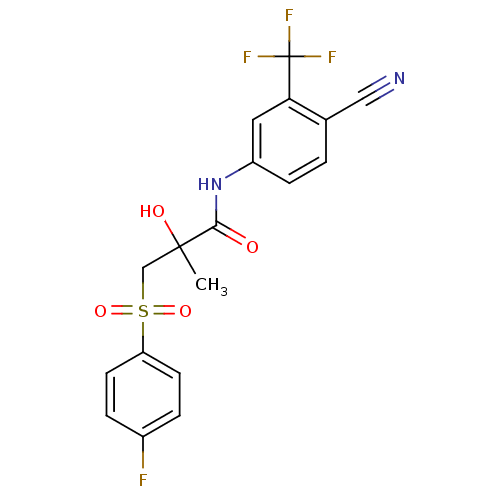 (Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 151 | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18566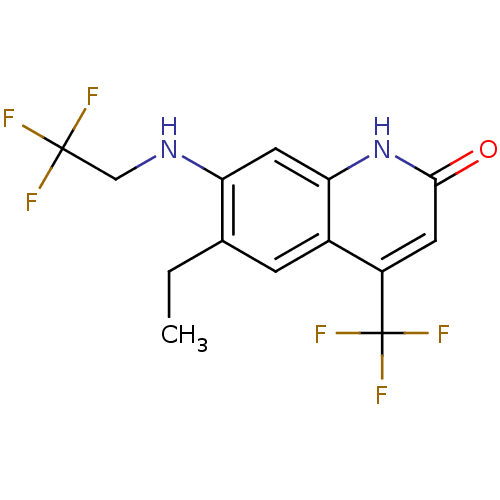 (6-alkyl, 7-alkylamino-2-quinolinone, 11h | 6-ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 157 | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18549 (7-(cyclobutylamino)-4-(trifluoromethyl)-1,2-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 161 | -40.3 | 43 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18554 (7-(benzylamino)-4-(trifluoromethyl)-1,2-dihydroqui...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 163 | -40.3 | 294 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18558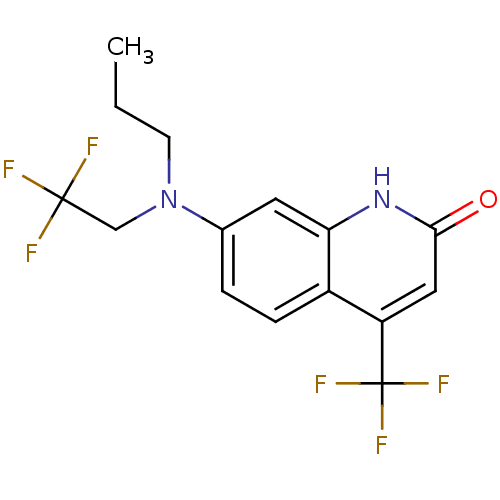 (7-[propyl(2,2,2-trifluoroethyl)amino]-4-(trifluoro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 241 | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18540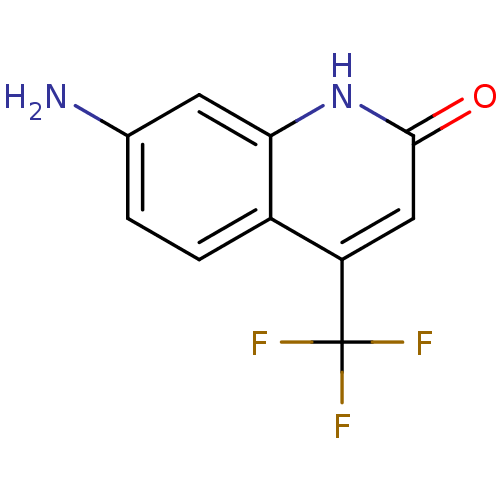 (7-amino-4-(trifluoromethyl)-1,2-dihydroquinolin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 407 | -37.9 | 28 | n/a | 1.30E+3 | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18556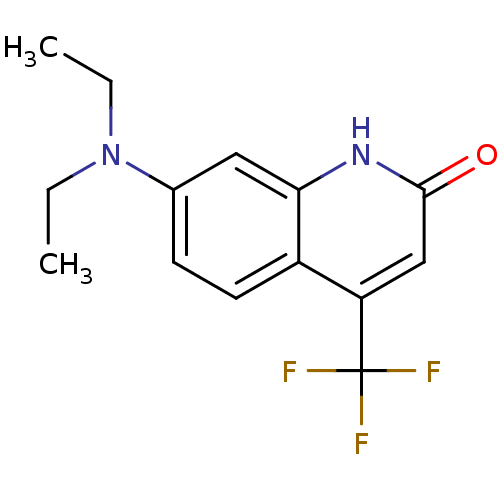 (7-(diethylamino)-4-(trifluoromethyl)-1,2-dihydroqu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 463 | -37.6 | 591 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18563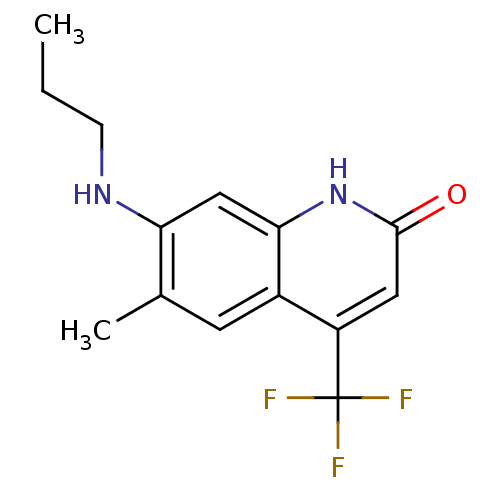 (6-alkyl, 7-alkylamino-2-quinolinone, 11e | 6-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 641 | n/a | 674 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18564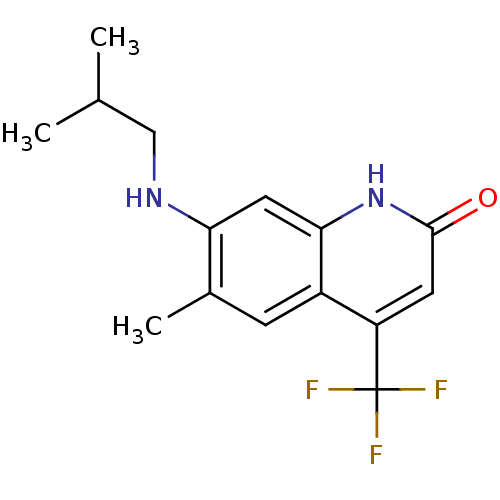 (6-alkyl, 7-alkylamino-2-quinolinone, 11f | 6-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 650 | n/a | 301 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18555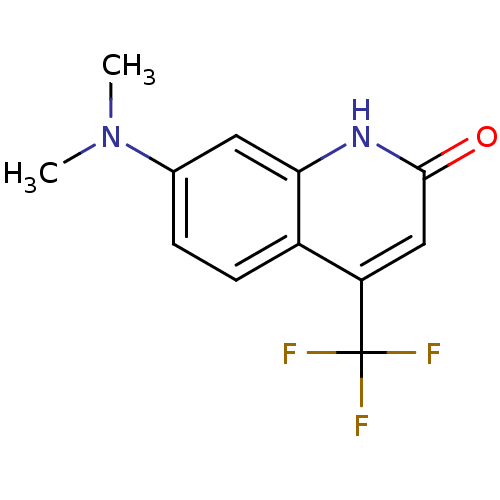 (7-(dimethylamino)-4-(trifluoromethyl)-1,2-dihydroq...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 761 | -36.3 | 1.16E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18565 (6-alkyl, 7-alkylamino-2-quinolinone, 11g | 6-ethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 869 | n/a | 504 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18550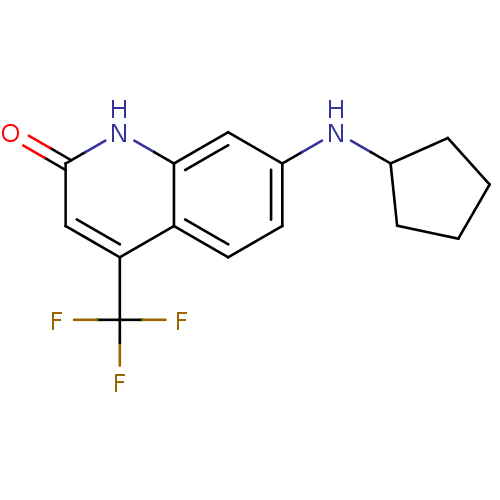 (7-(cyclopentylamino)-4-(trifluoromethyl)-1,2-dihyd...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -35.6 | 149 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18551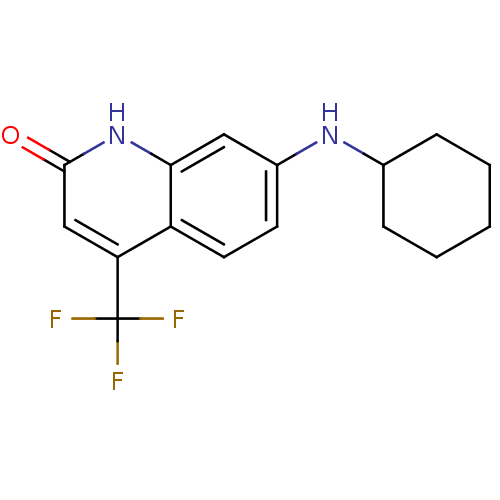 (7-(cyclohexylamino)-4-(trifluoromethyl)-1,2-dihydr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | -35.6 | 507 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Ligand Pharmaceuticals Inc. | Assay Description The compounds were evaluated in a transcriptional activation assay with hAR in a mammalian cell background (CV-1) as the primary in vitro assay. Rece... | Bioorg Med Chem Lett 17: 1523-6 (2007) Article DOI: 10.1016/j.bmcl.2007.01.007 BindingDB Entry DOI: 10.7270/Q22J6950 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50152989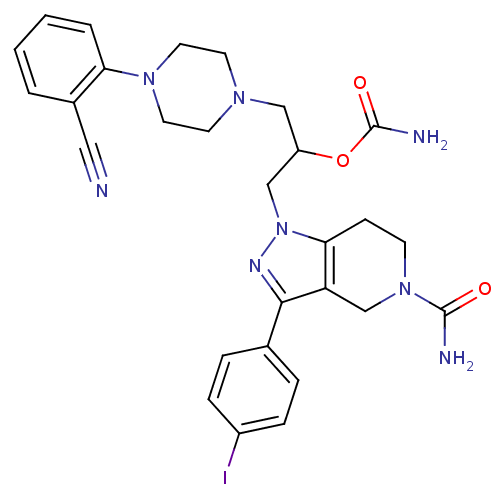 (3-(5-carbamoyl-3-(4-iodophenyl)-4,5,6,7-tetrahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50182092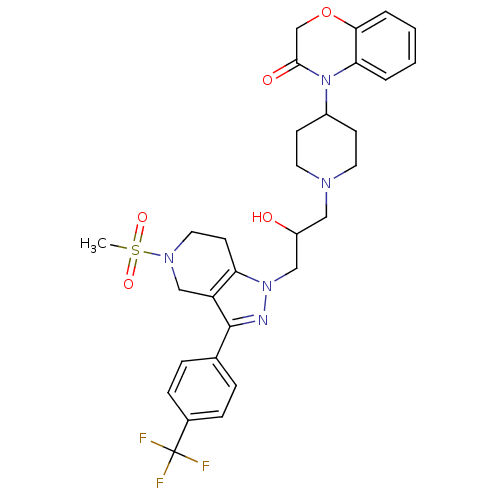 (4-(1-(2-hydroxy-3-(5-(methylsulfonyl)-3-(4-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50162828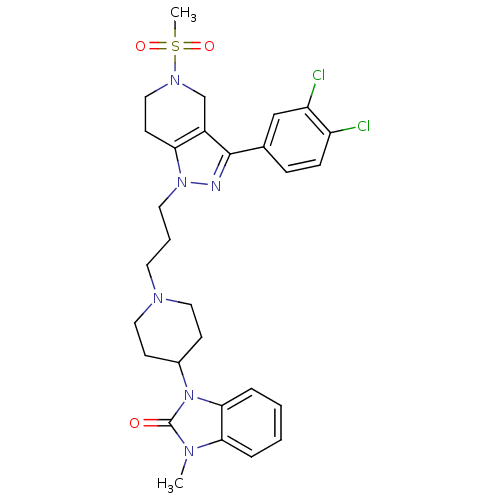 (1-(1-(3-(3-(3,4-dichlorophenyl)-5-(methylsulfonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221229 (3-(1-(2-hydroxy-3-(5-(methylsulfonyl)-3-(4-(triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221228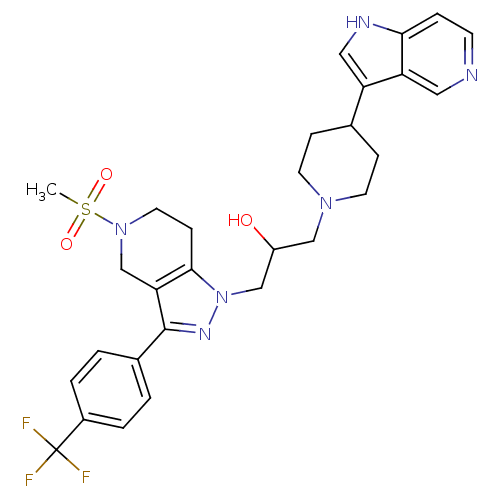 (1-(4-(1H-pyrrolo[3,2-c]pyridin-3-yl)piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of cathepsin S in human JY cells by invariant chain degradation assay | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221221 (CHEMBL397622 | ethyl 3-(1-(2-hydroxy-3-(5-(methyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221219 (1-(4-(1H-pyrrolo[2,3-b]pyridin-3-yl)piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221238 (1-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-3-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221241 (1-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-3-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221230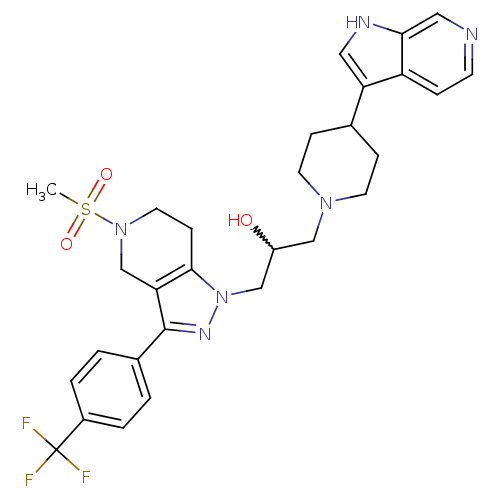 (1-(4-(1H-pyrrolo[2,3-c]pyridin-3-yl)piperidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221225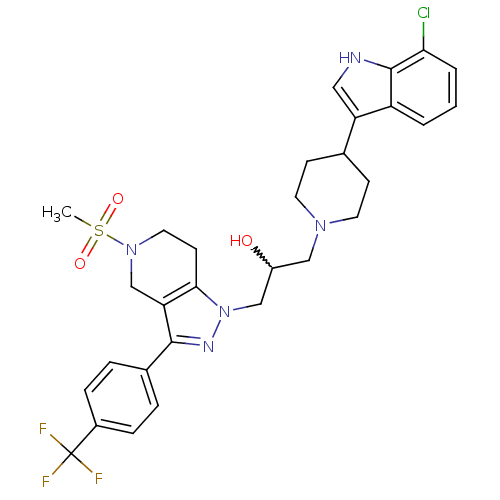 (1-(4-(7-chloro-1H-indol-3-yl)piperidin-1-yl)-3-(5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221227 (CHEMBL396600 | N-ethyl-6-fluoro-3-(1-(3-(5-(methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50221223 (1-(4-(5-chloro-2-methyl-1H-indol-3-yl)piperidin-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Inhibition of human cathepsin S | Bioorg Med Chem Lett 17: 5525-8 (2007) Article DOI: 10.1016/j.bmcl.2007.08.038 BindingDB Entry DOI: 10.7270/Q2ST7PKK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 88 total ) | Next | Last >> |